Abstract
Adequate levels of thyroid hormone are critical during development and metamorphosis, and for maintaining metabolic homeostasis. Perchlorate, a common contaminant of water sources, inhibits thyroid function in vertebrates. We utilized threespine stickleback (Gasterosteus aculeatus) to determine if timing of perchlorate exposure during development impacts adult dermal skeletal phenotypes. Fish were exposed to water contaminated with perchlorate (30 mg/L or 100 mg/L) beginning at 0, 3, 7, 14, 21, 42, 154 or 305 days post fertilization until sexual maturity at one year of age. A reciprocal treatment moved stickleback from contaminated to clean water on the same schedule providing for different stages of initial exposure and different treatment durations. Perchlorate exposure caused concentration-dependent significant differences in growth for some bony traits. Continuous exposure initiated within the first 21 days post fertilization had the greatest effects on skeletal traits. Exposure to perchlorate at this early stage can result in small traits or abnormal skeletal morphology of adult fish which could affect predator avoidance and survival.
Keywords: Gasterosteus aculeatus, hypothyroidism, thyroid hormone, skeletal abnormality, endocrine disruption
1. INTRODUCTION
Many endocrine disrupting contaminants have deleterious effects even at low concentrations (Carr and Patiño, 2011). Understanding the effects of these compounds during critical developmental stages is necessary for risk assessment, establishing regulations, and directing remediation efforts.
Perchlorate is a widespread inorganic anion found in ground and surface waters throughout the United States and in other countries (Brandhuber et al., 2009; USEPA, 2011). Perchlorate salts occur naturally at low levels in arid regions such as the Southwestern U.S., Antarctica and the Atacama Desert in Chile (Kounaves et al., 2010; Rajagopalan et al., 2006; Rao et al., 2007; Urbansky et al., 2001). Perchlorate is highly soluble, persistent, and stable in aqueous environments (Urbansky, 2002). These properties make perchlorate mobile and available to interact with biota in surface waters. Because it is a strong oxidizer, perchlorate has been manufactured for use in solid rocket propellant, munitions and many other industrial products (Trumpolt et al., 2005). Anthropogenic sources, such as military and manufacturing sites, are responsible for most of the environmental contamination in the U.S. (Morrison et al., 2006; Trumpolt et al., 2005).
Perchlorate competitively inhibits the uptake of iodide from the bloodstream into thyroid tissue (Carr et al., 2005; Wolff, 1998). Perchlorate has a greater affinity than iodide for the sodium/iodide symporter (NIS, alias SLC5A5) located in the basolateral membrane of thyrocytes (Urbansky, 2002; Wolff, 1998). Interruption of iodide concentration into thyroid tissue can impair synthesis of thyroid hormone (TH, which includes both thyroxine [T4] and triiodothyronine [T3]), as has been demonstrated in several vertebrate animal models (Bradford, 2011; Bradford et al., 2005; Carr et al., 2005; Goleman et al., 2002; McNabb et al., 2004; Patiño and Mukhi, 2007; Pickford et al., 2005; Yu et al., 2002). If the supply of iodide is sufficiently reduced for an extended length of time (i.e., TH stores become exhausted) hypothyroidism can develop (Wolff, 1998). Appropriate levels of TH are critical to normal development, growth and metabolism in vertebrates (Choksi et al., 2003; Power et al., 2001). Individuals most at risk to the effects of perchlorate exposure are iodide deficient, and/or in early development or metamorphosis (Carr and Patiño, 2011; Leung et al., 2010; Liu and Chan, 2002; Melse-Boonstra and Jaiswal, 2010; Tietge et al., 2005; Trumbo, 2010). In addition, perchlorate may have effects independent of iodide deficiency (LaRoche et al., 1966; McDougal et al., 2011). Environmental exposure in fish occurs via the respiratory and gastrointestinal epithelia, integument, and sometimes ingested food (Furin et al., 2013). Oviparous fish embryos are exposed to contaminants in the ambient water and/or pore water of sediments that can pass through the chorion.
Disruption of TH can profoundly impact teleost development and metamorphosis (Blanton and Specker, 2007; Carr and Patiño, 2011; Leatherland, 1982; Power et al., 2001). The role of TH in skeletal development has been studied in divergent fish species including zebrafish (Danio rerio) (Brown, 1997; Shkil et al., 2012), African barbs (Labeobarbus intermedius) (Shkil et al., 2012), medaka (Oryzias latipes) (Sekimizu et al., 2007), and Japanese flounder (Paralichthys olivaceus) (Okada et al., 2005; Okada et al., 2003). Bony structures differ in their response to hypo- and hyperthyroid conditions; some fail to develop entirely, some over-develop, and others are relatively unchanged (Power et al., 2001; Shkil et al., 2012). Effects depend on species and the character of interest with many changes being permanent. Evidence suggests that variation in relative timing of development (heterochrony) and relative rate of growth (allometry) drive these adverse effects under different TH levels (Shkil et al., 2012).
Bernhardt et al. (2011) determined that chronic exposure to perchlorate reduces development of bony structures in young threespine stickleback (Gasterosteus aculeatus, hereafter: stickleback) in a concentration-dependent manner. They found that stickleback exposed to greater than 12 mg/L perchlorate exhibited phenotypic abnormalities. Of the 25 measured bony characters, 24 were significantly modified, and gross abnormalities occurred such as missing lateral plates and lack of skin pigments. Exposed fish had reduced fitness with abnormal locomotion and reproduction (Bernhardt and von Hippel, 2008; Bernhardt et al., 2006; Bernhardt et al., 2011). These results raise the question of the developmental time frame during which perchlorate exerts its effects on these phenotypes.
The current study uses controlled variations in timing and duration of perchlorate exposure to further quantify the effects of perchlorate on stickleback skeletal development. Concentration and critical developmental windows are considered in light of development of dermal skeleton features, with a focus on defensive traits already determined to be affected by perchlorate (Bernhardt et al., 2011). Based on the morphological changes with perchlorate exposure observed by Bernhardt et al. (2011), and changes in thyroid endpoints during development (Furin et al. p. XX, this issue), we hypothesized that perchlorate exposure during the first three weeks of embryonic and larval development will lead to reduced growth and smaller skeletal armor traits in adults.
2. MATERIALS AND METHODS
2.1. Experimental design
Using a static renewal experiment (i.e., partial, periodic replacement of treatment solutions (USEPA, 2002)), stickleback were exposed to one of two different concentrations of sodium perchlorate (30 and 100 mg/L) at different time points for varying durations across their development. Embryos started in either perchlorate treated water or control water. At 0, 3, 7, 14, 21, 42, 154 (5 months) or 305 (10 months) days post fertilization (dpf), fish were transferred into or out of contaminated water. Fish that began in contaminated water and subsequently moved into clean water were in the downshift (rescue) exposure regime, and those moved from clean water to contaminated water were in the upshift exposure regime (Figure 1). Fish were raised in their respective treatments until approximately one year of age when they were collected and processed for morphological analysis. Due to differential survivorship and use for other experimental endpoints, sample sizes varied (Table 1).
Figure 1.
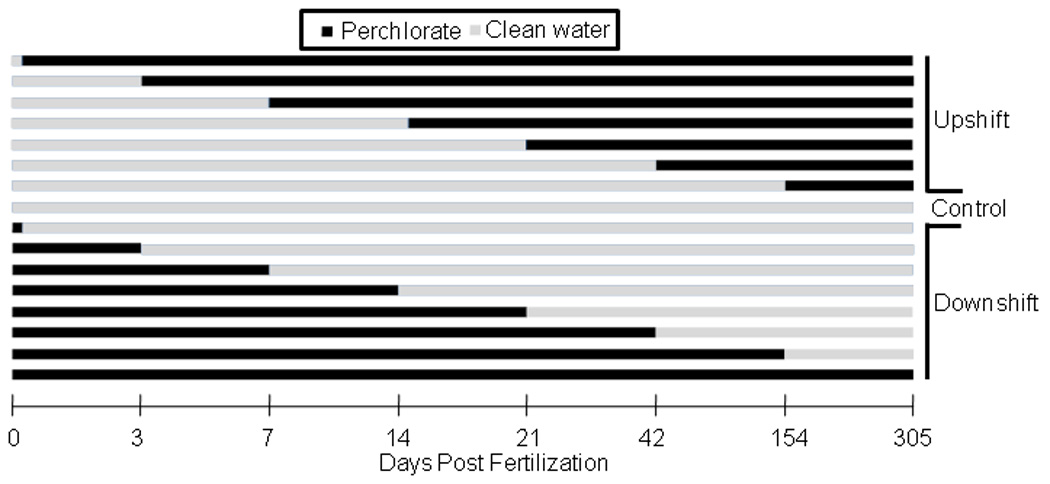
Perchlorate exposure regime. This protocol was used for both 30 and 100 mg/L exposures with three replicates at each dpf. Zero dpf downshift fish were exposed during fertilization and for 15 minutes post fertilization before their transfer to clean water.
Table 1.
Number of threespine stickleback (combining three replicates) for each measurement by perchlorate exposure treatment. Some treatments had no usable fish (NA).
| 100 mg/L |
30 mg/L |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dpf | Exposurea | SL | BD | PS | DS | PP | AP | SL | BD | PS | DS | PP | AP |
| Control | Upshift | 106 | 203 | 203 | 106 | 103 | 79 | 106 | 203 | 203 | 106 | 103 | 79 |
| Downshift | 106 | 203 | 203 | 106 | 103 | 79 | 106 | 203 | 203 | 106 | 103 | 79 | |
| Day 0 | Upshift | NA | 15 | 15 | 0 | 0 | 0 | NA | 15 | 14 | 0 | 0 | 0 |
| Downshift | 11 | 21 | 21 | 11 | 11 | 6 | 32 | 47 | 45 | 32 | 32 | 23 | |
| Day 3 | Upshift | 13 | 28 | 28 | 13 | 13 | 7 | NA | 15 | 15 | 0 | 0 | 0 |
| Downshift | 34 | 49 | 19 | 34 | 34 | 32 | 18 | 28 | 27 | 18 | 18 | 9 | |
| Day 7 | Upshift | NA | 16 | 16 | 0 | 1 | 0 | NA | 15 | 15 | 0 | 0 | 0 |
| Downshift | 25 | 40 | 40 | 25 | 25 | 20 | 23 | 38 | 37 | 23 | 23 | 22 | |
| Day 14 | Upshift | 18 | 33 | 33 | 18 | 18 | 14 | 1 | 16 | 15 | 1 | 1 | 0 |
| Downshift | 16 | 31 | 31 | 16 | 15 | 9 | 9 | 24 | 23 | 9 | 9 | 4 | |
| Day 21 | Upshift | 12 | 27 | 27 | 12 | 12 | 7 | 6 | 21 | 21 | 6 | 6 | 6 |
| Downshift | 22 | 22 | 22 | 22 | 22 | 18 | 16 | 31 | 31 | 16 | 16 | 14 | |
| Day 42 | Upshift | 11 | 21 | 20 | 11 | 11 | 6 | 11 | 26 | 26 | 12 | 12 | 11 |
| Downshift | 18 | 33 | 33 | 18 | 18 | 13 | 9 | 24 | 23 | 9 | 9 | 5 | |
| Day 154 | Upshift | 30 | 45 | 44 | 30 | 30 | 28 | 6 | 21 | 19 | 6 | 6 | 6 |
| Downshift | 17 | 31 | 31 | 17 | 17 | 13 | 5 | 20 | 18 | 5 | 5 | 0 | |
| Day 305 | Upshiftb | 6 | 21 | 21 | 6 | 6 | 0 | 18 | 33 | 33 | 18 | 18 | 16 |
Upshift exposures began in control water and were moved to contaminated water. Downshift exposures were the reciprocal of upshift exposures.
Only upshift exposure regime was performed at 305 dpf.
dpf- days post fertilization
SL- standard length
BD- body depth
PS- pelvic spine length
DS- dorsal spine length
PP- posterior plate height
AP- anterior plate height
2.2. Fish collection and experimental procedures
Stickleback were collected from Rabbit Slough, Alaska (61.534° N, 149.266° W) with un-baited wire-mesh (0.64 cm) minnow traps on 4 June, 2008. They were transported to the University of Alaska Anchorage in aerated coolers where they were kept in outdoor 1600-L pools, in de-chlorinated city water with Instant Ocean© added to 3 g/L.
On 10 June, 2008, a mass cross was performed to generate a representative study population with genetic variation randomly distributed throughout the treatments. Testes were collected from 40 males and eggs from 40 females. Egg clutches from all females, and testes from all males, were combined to randomize the genetic pool before fertilizing batches in Petri dishes (100 × 200mm) for all treatments. Sterilized reverse osmosis (RO) purified water with Instant Ocean© added to 4 g/L was used as the embryo medium. Sodium perchlorate (> 98% purity, Sigma-Aldrich, St. Louis, MO, USA) dried in an oven at 90° C before weighing, was added to produce embryo medium at 30 and 100 mg/L. Water was changed daily and dead embryos were removed. Day 0 downshift fish were fertilized in perchlorate treated water and then moved into uncontaminated water after 15 minutes. Within 3 dpf, embryos in the initial Petri dishes were divided into three replicates with approximately 100 embryos each (3 replicates X 100 embryos = 300 embryos/treatment).
Embryos were kept in an incubator held at 20 ± 0.5° C for the first ten days and then transferred to aerated 56-L aquaria (60cm×31cm×32cm) with an AZOO© multi sponge filter (65mm diameter). Water levels were adjusted in aquaria to maintain a ratio of at least 1-L water per 1cm of fish, and Bacta-pur© N3000 (IET-Aquaresearch Ltd., Quebec, Canada) was added to control nitrogenous waste. Water was changed every two weeks or as needed. Sodium perchlorate salt was not added directly to aquaria and was only used when making fresh treatment water in separate holding tanks, which was added as needed on the two week schedule. Dead fish were removed daily. RO water was added weekly to replace evaporative loss and maintain desired perchlorate concentrations. The lighting cycle mimicked the natural diurnal cycle for Anchorage, Alaska and the average water temperature during the experimental period was 13.5 ± 0.5° C. Salinity (4–5 g/L), pH (7.0–8.0) and ammonia (<1.9 mg/L total nitrogen (USEPA, 2013)) were measured with a YSI photometer model 9100 (Yellow Springs Instrument Co., Yellow Springs, OH, USA) periodically to check for abnormal levels; none were detected. Perchlorate concentrations were measured using an Acorn Ion 6 meter (Oakton Instruments, Vernon Hills, USA) with a perchlorate ISE electrode (Cole-Parmer, Vernon Hills, IL, USA). Fry (<2 months old) were fed live brine shrimp and a mixture of Golden Pearls 100 (a commercial larval food), Artemia food (both from Aquatic Ecosystems, Apopka, FL, USA), and frozen ground brine shrimp (Brine Shrimp Direct, Ogden, UT, USA). Once large enough (at approx. 50 dpf), fish were fed frozen brine shrimp daily. Perchlorate intake from food sources was assumed to be negligible.
At the age of 12 months, fish were euthanized with an overdose of tricane methanesulfonate (MS-222; Argent Chemical Laboratories, Richmond, WA, USA) and fixed in 10% neutral buffered formalin. After 14–20 days, fish were thoroughly rinsed and transferred to 70% undenatured ethanol for storage. Mineralized tissue was stained using alizarin red S. Digital calipers (Fowler High precision, Newton, Massachusetts, USA) were used to measure: standard length (SL), body depth (BD), length of 2nd dorsal spine (DS), length of left and right pelvic spines (PS), height of plate anterior to the plate aligning with the ascending branch of the pelvic girdle (AP), and height of the plate dorsal to the anal spine (PP; Figure 2). The number of lateral plates (LP) was also recorded. Both left and right sides of bilateral characters were measured and added together. One individual researcher measured all fish in this study to maintain consistency.
Figure 2.
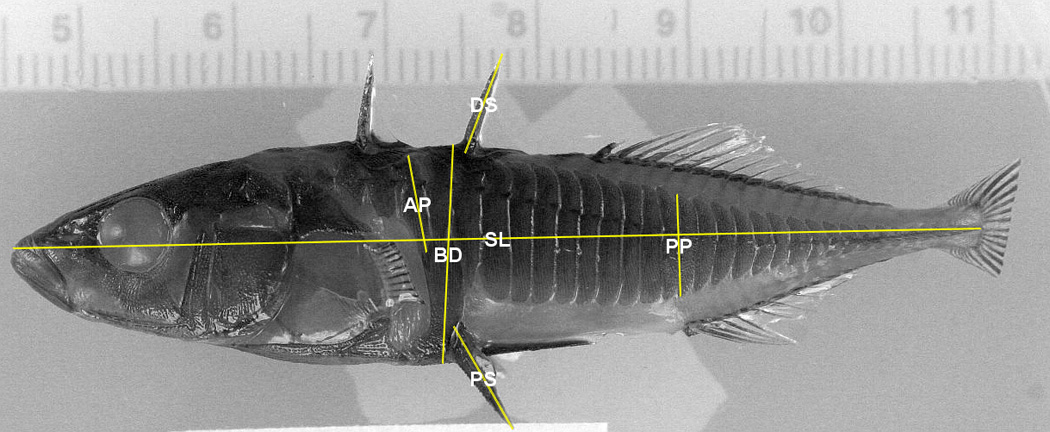
Morphometric measurements made on threespine stickleback. Photo courtesy of Jeff Colgren (UAA). Pectoral and caudal fins have been clipped on this individual.
2.3. Statistical analysis
Morphometric (continuously distributed) characters were size corrected to control for covariation of character size and body size (Bernhardt et al., 2011; von Hippel and Weigner, 2004). For each treatment, all bony characters were regressed against SL and the residuals, slope and Y intercept were calculated. The y value for each character for each treatment was then calculated using the regression equation with x set as the global mean of SL. This y value was added to the residuals to standardize all characters to a fish of the same SL. When the regression for a given treatment was non-significant it was discarded (∼5% of measurements), and size correction was calculated using a larger pool (within concentrations) of individuals.
Concordance was determined from 30 (10 each from control, 30 and 100 mg/L) randomly chosen individuals that were measured blindly a second time at least two days after the initial measurement (Palmer, 1994). A concordance coefficient was calculated for morphometric characters by dividing the smaller value by the larger value (von Hippel, 2000). In addition, for the three bilateral morphometric traits, a two-way mixed model ANOVA - with side (fixed) and individual (random) as factors and trait as the dependent variable - was utilized to test for directional asymmetry and to determine if measurement error (ME) contributed significantly to between-side variance (Palmer, 1994).
Normality of dependent variables was determined by visually inspecting plotted data and using the Shapiro-Wilk test. In most cases, due to small or uneven sample sizes, non-parametric tests were used with significance set at α<0.05. Parametric tests were used only when all assumptions were met. Density effects were evaluated for control fish by regressing fish density in aquaria against trait values. Measurements of bony characters were done on preserved fish, but ∼ 50% of the SL, PS and BD measurements were conducted on digital images of anesthetized fish using ImageJ (Rasband, 1997–2013). A t-test comparing the measurements on live fish vs. preserved fish was used to determine if the methods resulted in significantly different results. Mortality during the first 15 dpf and survivorship at 374 dpf was compared between perchlorate concentrations and controls using ANOVA.
After size adjusting, treatments were compared using a Kruskal-Wallis test and non-parametric Tukey multiple contrast test. Upshift and downshift exposure regimes were analyzed separately and compared to controls. A Hotellings T2 test was used to test for differences between upshift and downshift exposure regimes at each dpf across all measured traits. Mann-Whitney U tests (with Bonferroni correction) were then used to test for differences between upshift and downshift for each character at each dpf. Directional asymmetry of bilateral morphometric traits was assessed using plots and a t-test (with Bonferroni correction) to determine if R-L was different from zero. Fluctuating asymmetry was assessed using |R-L| for each trait value and differences between treatments determined using a non-parametric Levene’s test (Palmer, 1994).
3. RESULTS
3.1. Density, validation, mortality and asymmetry
Mean perchlorate concentrations in aquaria were maintained within the target concentration range for the duration of the experiment (Figure 3). Fish density in aquaria was statistically associated with two of the five traits measured. After size correction, BD and PS were negatively correlated with the number of fish per tank (slope = −0.029, t = −3.443, p < 0.001; slope = −0.047, t = −2.514, p =0.013, respectively). These effects were primarily driven by two aquaria with only one or two surviving fish. Overall, fish density varied little (mean ± SE: 17±0.56 fish/tank on 19 June 2009 (time point near collection)), and DS, AP and PP were not significantly affected by density, so we did not account for density in the following analyses. Neither mortality in the first 15 dpf nor survivorship at one year of age significantly differed between controls and fish exposed to different concentrations of perchlorate (One-way ANOVA: F(1,109) = 0.48, p = 0.49; F(1,109) = 0.60, p = 0.44, respectively).
Figure 3.
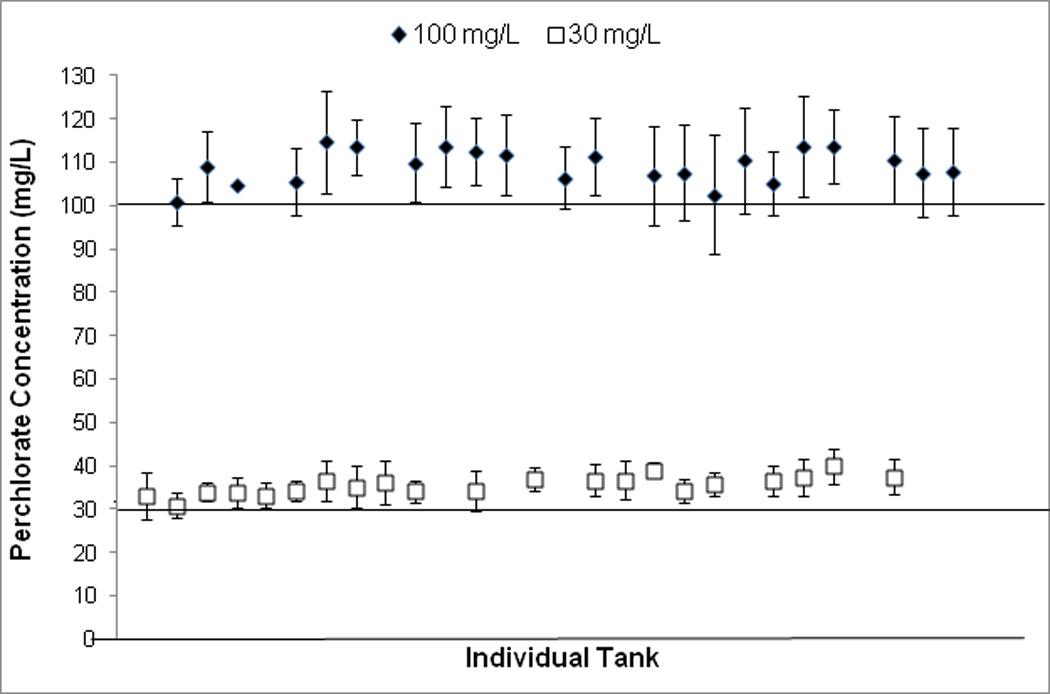
Mean ± SE perchlorate concentrations in individual aquaria over the course of the experiment (July 12, 2008- July 1, 2009). Each point represents one aquarium.
The measurements taken on digital photos vs. direct measurements on fish resulted in no significant differences in trait values with the exception of SL (SL, t = 23.04, df = 563, p < 0.0001; BD, t = 0.83, df = 980, p = 0.409; PS, t = 1.69, df = 901.45, p = 0.091). SL was expected to differ between fish measured with alternate methods due to the different collection times of samples for each measurement type (i.e., fish measured physically were older) and all other measurements were size corrected. The mean concordance of repeated measurements (n = 30) for all traits ranged from 0.84–0.98 (Table 2). The nested ANOVA (side within individual) revealed that the measurement error was sufficiently large to mask detection of fluctuating asymmetry in this study (Table 3; see also Supplementary Data).
Table 2.
Concordance of morphometric measurements of threespine stickleback. The smaller value was divided the by the larger value of each trait measurement and then averaged over all individuals to determine the strength of correlation between repeated measures; n = 30.
| Trait | Concordance |
|---|---|
| SL | 0.98 |
| Body Depth | 0.96 |
| Dorsal Spine | 0.90 |
| Pelvic Spine L | 0.94 |
| Pelvic Spine R | 0.94 |
| Posterior Plate L | 0.84 |
| Posterior Plate R | 0.86 |
| Anterior Plate L | 0.94 |
| Anterior Plate R | 0.94 |
Table 3.
Two-way mixed model ANOVA of Sides (fixed) by Individual (random). A significant Side X Individual P value would indicate measurement error variance is sufficiently smaller than between side variance to allow detection of trait asymmetry (Palmer, 1994). A significant Side P value indicates directional asymmetry. n = 30
| Side |
Individual |
Side X Individual |
||||
|---|---|---|---|---|---|---|
| Trait | F | P | F | P | F | P |
| Pelvic Spine | 2.151 | 0.153 | 23.433 | <0.001 | 1.176 | 0.292 |
| Posterior Plate | 1.681 | 0.198 | 3.424 | <0.001 | 0.469 | 0.986 |
| Anterior Plate | 9.981 | 0.004 | 14.4 | <0.001 | 1.32 | 0.181 |
3.2. Concentration and timing of exposure
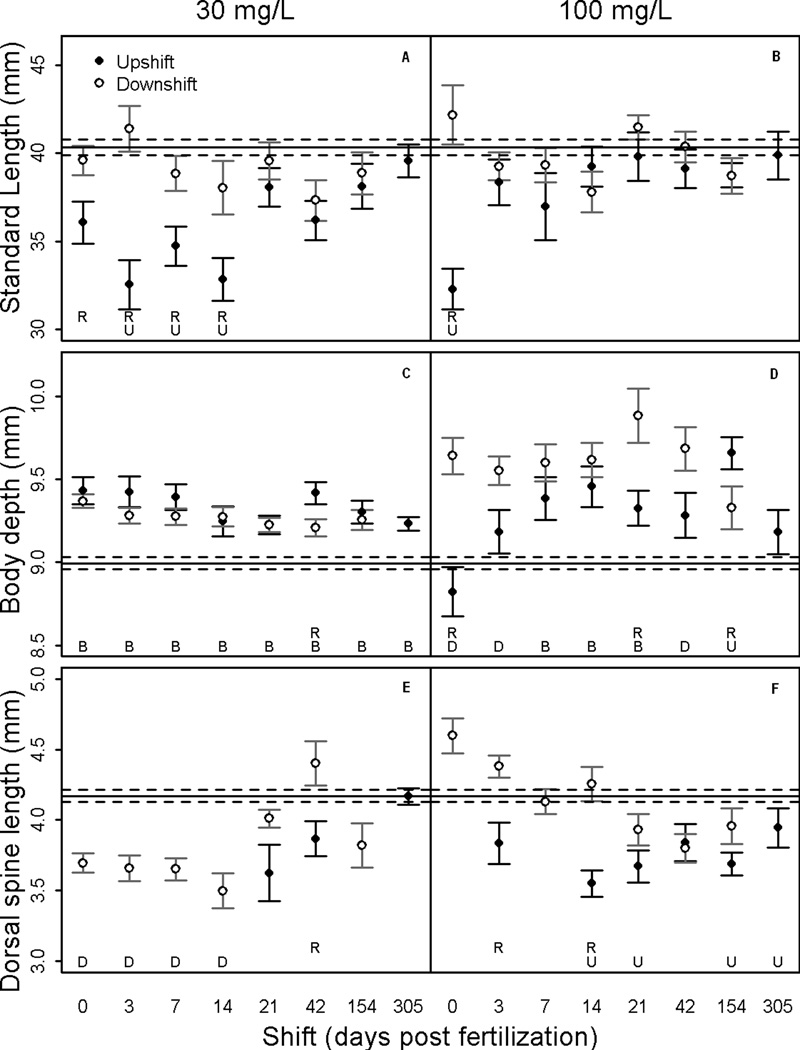
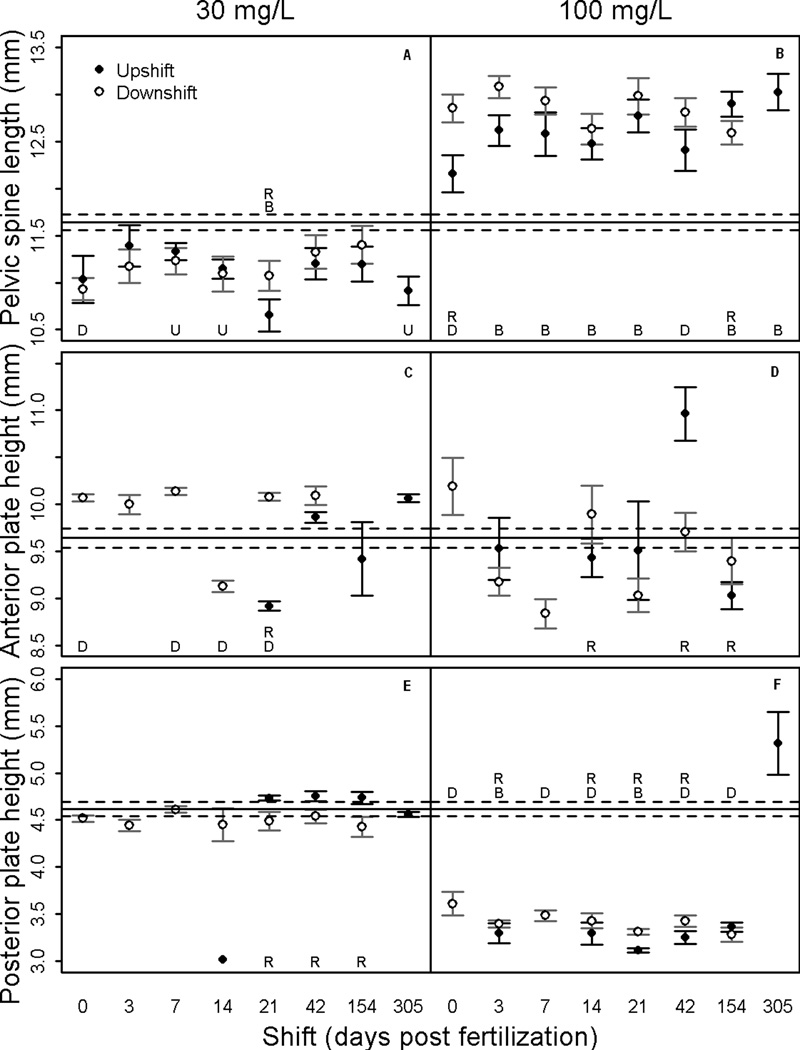
The traits measured in this study varied in their response to perchlorate exposure. SL, PS and PP showed significant trends (Figures 4 & 5). The measurements for BD, DS and AP showed a weak trend or no response to perchlorate exposure (Figures 4 & 5). Overall, concentration of perchlorate was more important than differential timing of exposure for disrupting development of skeletal traits.
Figure 4.
Mean ± SE of standardized trait lengths of threespine stickleback in each treatment. Upshift = fish began in clean water and were transferred to contaminated water. Downshift = reciprocal of upshift. Significant differences at α<0.05 indicated by: (U) between upshift and control, (D) between downshift and control, (R) between upshift and downshift regimes. The solid horizontal line is the control mean and the dashed lines are the SE around the control mean.
Figure 5.
Mean ± SE trait lengths of threespine stickleback in each treatment. Upshift = fish began in clean water and were transferred to contaminated water. Downshift = reciprocal of upshift. Significant differences at α<0.05 indicated by: (U) between upshift and control, (D) between downshift and control, (R) between upshift and downshift regimes. The solid horizontal line is the control mean and the dashed lines are the SE around the control mean.
Body size measurements (SL and BD) significantly differed between perchlorate treated and control fish (F = 18.63(2,373), p < 0.0001 and F = 36.27(2,367), p < 0.0001, respectively). Mean SL was reduced compared to controls for fish in the 30 mg/L upshift exposure regime that were continuously exposed starting in the first two weeks post fertilization (Figure 4A). Only fish exposed beginning at 0 dpf in the 100 mg/L upshift regime had significantly reduced SL (Figure 4B). The mean SL for all downshift treatments was equivalent to controls regardless of perchlorate concentration. There was not a concentration-dependent effect on SL, except with respect to the window of effect during early upshift treatments.
Compared to controls, mean BD increased in almost all treatments and variance in BD was greater in the 100 mg/L exposed fish than in the 30 mg/L exposed fish (Figure 4C & D). Stickleback in the 100 mg/L upshift exposure regime had deeper bodies than control fish in the 7, 14, 21 and 154 dpf treatments but not in 0, 3, 42 and 305 dpf treatments (Figure 4C). All fish exposed to 100 mg/L perchlorate in the downshift regime had deeper bodies than control fish, with the exception of the 154 dpf treatment. Concentration-dependent trends were not present for BD, but the 100 mg/L downshift regime suggested a critical window between 0 and 154 dpf (Figure 4D).
The mean DS was generally reduced with exposure to both concentrations of perchlorate (Figure 4E & F). The only discernible temporal pattern was that as the date of rescue (downshift) increased, mean DS tended to shorten in 100 mg/L exposures. Neither concentration of perchlorate nor timing of exposure definitively affected DS.
The mean PS increased in all 100 mg/L exposures and decreased in all 30 mg/L exposures (Figure 5A & B). Timing of exposure was not important to PS growth.
The mean response of the AP was variable, but tended to increase with 30 mg/L exposure in the downshift exposure regime and variance in AP was greater for 100 mg/L treatments than for 30 mg/L treatments (Figure 5C & D). Neither perchlorate concentration nor timing of exposure had definitive affects on AP.
The mean PP was reduced in all 100 mg/L exposures with the exception of the 305 dpf upshift treatment (Figure 5F). The 30 mg/L concentration did not affect PP phenotype. The 100 mg/L upshift regime suggests that PP can be repressed if perchlorate exposure occurs anytime from fertilization to 154 dpf.
Upshift and downshift exposure regimes differed across all measured bony characters on 0, 3, 14 and 21 dpf for 100 mg/L exposure (T2 = 13.0, p < 0.01; T2 = 3.92, p < 0.01; T2 = 11.07, p < 0.001; T2 = 11.36, p < 0.001, respectively) and 3 and 21 dpf for 30 mg/L exposure (T2 = 6.18, p < 0.01; T2 = 57.18, p < 0.001, respectively). The 100 mg/L concentration had a greater effect on dermal bone development than the 30 mg/L concentration, consistent with an expected concentration and response relationship.
3.3. Gross abnormalities
With respect to observed gross abnormalities, three control fish were missing all keel plates on the left side. One control fish was missing all plates on the right side including keel plates, but was normal on the left side. In 30 mg/L perchlorate contaminated water, two individuals were missing keel plates on the left side and one individual was missing a left PS. In 100 mg/L perchlorate contaminated water, one individual had a total of 20 plates on the left and only 4 on the right, another individual was missing all keel plates on the right side and another fish was missing a left PS. Transparency, defined as the lack of dermal pigment and lateral plates, was not observed. The frequency of gross abnormalities found in this study were concluded to be similar to that of natural populations because no perchlorate treatment effects were observed.
4. DISCUSSION
The upshift/downshift experimental was designed to detect sensitive windows during development in which perchlorate exposure has the greatest effect on stickleback gross morphology. The expected result was a concentration-response reduction in growth and skeletal development with perchlorate exposure early in development having the greatest effect. Results showed that sodium perchlorate exposure at environmentally relevant levels (Carr and Patiño, 2011; Theodorakis et al., 2006a; Theodorakis et al., 2006b) affects gross morphology of stickleback, but with differential responses depending on the specific character measured. The results reported here are part of a larger study that included measurements of thyroid tissue histomorphology and thyroid hormone levels in whole body homogenates of stickleback from the same experimental groups. Whole body TH levels were not affected by perchlorate exposure but thyroid tissue histomorphology was altered and sensitive developmental windows were detected (Furin et al., p. XX, this issue). Our results showed that timing of exposure is likely important in the development of some skeletal characters as well.
Different concentrations of perchlorate and the timing and duration of exposure affected fish growth measured as SL and BD. Chronic exposure to 30 mg/L beginning in the first 14 dpf caused fish to have a reduced SL, while in the 100 mg/L exposure group, only those fish exposed beginning on 0 dpf had significantly reduced SL (upshift; Figure 4B). This result is consistent with other studies in which growth was reduced when fish were chronically exposed to perchlorate (Bernhardt et al., 2011; Crane et al., 2005; Liu et al., 2008; Mukhi et al., 2007; Schmidt et al., 2012). There may be a threshold concentration that triggers a physiological change and a compensatory mechanism could explain the smaller window of sensitivity at 100 mg/L (e.g., perchlorate uptake mechanisms might become saturated or fish may eliminate perchlorate at a greater rate when exposed to higher concentrations (Furin et al., 2013)).
SL was significantly affected by continuous exposure to 30 mg/L perchlorate if it began during the first two weeks post fertilization. In contrast, fish rescued within 14 dpf showed no effect of perchlorate on SL. Therefore, the effect of perchlorate on SL is likely not driven by exposure only during early development. Compensatory growth in which early deficits are made up for later in development could mask those early effects, but were not measured in the current study. Under natural conditions, reduced growth would have fitness consequences and the timing and duration of perchlorate exposure should be considered when evaluating risk at contaminated sites.
Most exposure windows in both 30 mg/L and 100 mg/L produced fish with deeper bodies than controls (upshift and downshift; Figure 4C & D). BD increased in all exposure treatments except when exposure began on 0 dpf in 100 mg/L. Being the longest duration of exposure, this is a puzzling result and further study is needed to verify these findings. Hyperplasia due to adenomatous goiter could contribute to a deeper BD measurement and has been demonstrated in previous studies (Honma et al., 1977). BD increased with delayed exposure in 100 mg/L upshift treatments through 14 dpf, but was consistently high for all exposures in 30 mg/L (Figure 4C & D). Body size metrics of stickleback responded to perchlorate differently depending on exposure concentration and timing of exposure.
The PS demonstrated a concentration-dependent response, with an increase in mean spine length in 100 mg/L and a decrease in 30 mg/L treatments, as compared to control fish (Figure 5A & B). Perchlorate exposure at 250 mg/L has previously been shown to inhibit pelvic fin development in zebrafish (Brown, 1997). This result was observed for PS in the present study for 30 mg/L but not 100 mg/L exposures, suggesting possible compensatory mechanisms at the higher concentration. Similarly, TH deficiency causes abnormal pectoral fin development in Japanese flounder (Okada et al., 2003) and African barbs (Shkil et al., 2012) and prevents normal caudal fin regeneration in medaka (Sekimizu et al., 2007). Brown (1997) observed larger paired fins in 65 dpf zebrafish exposed to potassium perchlorate with late rescue (56 dpf) using T4. Also, Shkil et al. (2012) observed some early pectoral girdle developmental effects of hypothyroidism in zebrafish and African barbs, but the adult morphology was minimally affected. T4 treatment generally causes premature differentiation and abnormalities in pectoral fins of zebrafish (Brown, 1997), barbs (Shkil et al., 2012; Smirnov et al., 2012), goldfish (Carassius auraius) (Reddy and Lam, 1992a), chum salmon (Onchorhyncus keta) (Dales and Hoar, 1954), and tilapia (Oreochromis mossambicus) (Reddy and Lam, 1992b). These studies demonstrate that paired fin development is at least partially controlled by TH and perchlorate exposure may disrupt this process.
The DS in 100 mg/L downshift treatments tended to be longer with earlier rescue (Figure 4F). In contrast, when transferred out of perchlorate during the first two weeks, the DS in 30 mg/L treatments was significantly shorter than in control fish. This finding suggests either that the onset or the rate of character development is altered with perchlorate exposure during the embryonic stage. The difference in concentration response could be that the 100 mg/L early rescue resulted in either early differentiation or compensatory growth as seen in the PS. Further study is needed to determine the underlying mechanisms.
An unexpected result was that in some stickleback, the adult phenotype for BD, PS, DS, PP and AP was altered when perchlorate exposure occurred only during the first 3–7 dpf. Given that the embryo’s source of T4 and T3 is maternal at this early stage of development (Power et al., 2001), and the expression of NIS and formation of functioning thyroid follicles begins sometime between 8 and 11 dpf (A. Petersen, J. Postlethwait, W. A. Cresko, C. L. Buck and F. A. von Hippel, unpublished data), hypothyroidism via perchlorate is likely not driving these effects. Perchlorate may alter the timing and/or rate of development of some traits through non-thyroidal pathways as has been demonstrated in gene expression studies (McDougal et al., 2011). NIS is present in non-thyroidal tissues of vertebrates, such as mammary glands, the gastric mucosa, salivary glands and kidneys (Josefsson et al., 2002). Different effects of perchlorate, such as induction of osmotic or oxidative stress, should also be investigated.
Taken together, our results show that perchlorate exposure from ambient water causes abnormal development of the stickleback dermal skeleton. Overall, 100 mg/L exposures affected bony characters to a greater degree and with more variance around the mean than 30 mg/L exposures. Comparing all characters, the upshift and downshift exposure regimes differed significantly from each other for four different dpf in 100 mg/L and for two in 30 mg/L treatments, both within the first 21 dpf. This result suggests that higher concentrations of perchlorate cause a greater degree of abnormal development and the first 21 dpf represent a critical developmental window for disruption of skeletal traits. Many developmental milestones occur within this window (e.g., pigmentation, heart beating, fin development, etc.), including functioning thyroid tissue (Swarup, 1958b). Concentration of perchlorate is important in relation to skeletal development in threespine stickleback as previously demonstrated (Bernhardt et al., 2011) with some possibility for non-thyroid driven responses.
TH deficiency and TH excess both have effects on individual skeletal structures in fish, including over- or under-development (Okada et al., 2003; Shkil et al., 2012). Shkil et al. (Shkil et al., 2012) concluded that for barbs and zebrafish, skeletal elements that originate early, such as the first skull bones, and ossification of most vertebral centra, are not TH dependent but that some elements arising later, including neural arches and spines in the vertebral column and fin rays in dorsal and anal fins, are TH dependent. Assuming that the main toxic effect of perchlorate is hypothyroidism caused by disruption of TH homeostasis, this study corroborates earlier conclusions from other fish studies for some traits in stickleback. The DS (100 mg/L), PS (30 mg/L) and PP (100 mg/L) were all reduced (Figures 4 & 5) and develop late in stickleback ontogeny (Bell, 1981; Swarup, 1958a). The late development conclusion is confounded, however, by toxicant concentration, as seen in PS (Figure 5) and PP (Figure 4), and the potential for compensatory growth, which has been documented for skeletal structures with T4 rescue (Brown, 1997). Additionally, plasma TH levels are correlated with metamorphosis, season, migration, salinity and sexual maturity in fish (Blanton and Specker, 2007; Carr and Patiño, 2011; Peter, 2011), and hence thyroid-disrupting compounds such as perchlorate may have different complex interactions at different times during development (Brown, 1997; Carr and Patiño, 2011). Furthermore, non-thyroidal effects of perchlorate may be important.
Future work should focus on the first 21 dpf when perchlorate exposure has its greatest apparent effect. Choosing skeletal characters that develop across different stages of ontogeny would provide information on the influence of perchlorate at those particular stages. Age of fish at collection, specific characters measured and timing/duration of perchlorate exposure should be carefully considered when designing experiments to evaluate hypothalamus-pituitary-thyroid axis effects. Including a positive control such as iodide deficiency or another thyrotoxicant with a different mode of action could help to distinguish direct from indirect mechanisms of perchlorate on skeletal development. Finally, given the wide range of phenotypes induced by perchlorate exposure, future work should examine molecular mechanisms that lead to different outcomes.
5. Conclusion
This study demonstrates the existence of complex relationships involved with perchlorate-induced disruption of TH homeostasis and its influence on skeletal development in stickleback. TH does not have a blanket effect on rate of development (Shkil et al., 2012). Rather, differences in the rate of development and/or induction period that cause skeletal abnormalities likely depend on the particular skeletal element, concentration of perchlorate, and duration and timing of exposure. Chronic perchlorate exposure beginning early in development has the greatest potential for causing deleterious effects on fitness related to growth and skeletal morphology in stickleback.
Supplementary Material
Highlights.
Perchlorate causes concentration-dependent differences in growth of bony traits
Chronic perchlorate exposure beginning early in development has the greatest effect
Developmental disruption caused by perchlorate impacts traits related to fitness
Acknowledgments
The authors thank T. Villafranca, M. Sherbick, L. Smayda, E. Kittel, and L. Matthews for laboratory support. Thanks to R. Bernhardt and J. Willacker for advice and discussion. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM103395. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding was also provided by NIH grant number 1R01ES017039. Fish were collected under Alaska Department of Fish and Game permit SF-2008-019, and all research protocols were approved by the UAA Institutional Animal Care and Use Committee (protocol 2007vonhi1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christoff G. Furin, Email: bfurin@gmail.com.
John Postlethwait, Email: jpostle@uoneuro.uoregon.edu.
C. Loren Buck, Email: clbuck@uaa.alaska.edu.
William A. Cresko, Email: wcresko@uoregon.edu.
Todd M. O’Hara, Email: tmohara@alaska.edu.
REFERENCES
- Bell MA. Lateral plate polymorphism and ontogeny of the complete plate morph of threespine sticklebacks (Gasterosteus aculeatus) Evolution. 1981;35:67–74. doi: 10.1111/j.1558-5646.1981.tb04859.x. [DOI] [PubMed] [Google Scholar]
- Bernhardt RR, von Hippel FA. Chronic perchlorate exposure impairs stickleback reproductive behaviour and swimming performance. Behaviour. 2008;145:527–559. doi: 10.1163/156853908792451511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt RR, von Hippel FA, Cresko WA. Perchlorate induces hermaphroditism in threespine sticklebacks. Environ. Toxicol. Chem. 2006;25:2087–2096. doi: 10.1897/05-454r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt RR, von Hippel FA, O’Hara TM. Chronic perchlorate exposure causes morphological abnormalities in developing stickleback. Environ. Toxicol. Chem. 2011;30:1468–1478. doi: 10.1002/etc.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton ML, Specker JL. The hypothalamic-pituitary-thyroid (HPT) axis in fish and its role in fish development and reproduction. Crit. Rev. Toxicol. 2007;37:97. doi: 10.1080/10408440601123529. [DOI] [PubMed] [Google Scholar]
- Bradford CM. Perchlorate uptake and effects on thyroid function in fish. Texas Tech University; 2011. [Google Scholar]
- Bradford CM, Rinchard J, Carr JA, Theodorakis C. Perchlorate affects thyroid function in eastern mosquitofish (Gambusia holbrooki) at environmentally relevant concentrations. Environ. Sci. Technol. 2005;39:5190–5195. doi: 10.1021/es0484505. [DOI] [PubMed] [Google Scholar]
- Brandhuber P, Clark S, Morley K. A review of perchlorate occurrence in public drinking water systems. J. Am. Water Works Assoc. 2009;101:63–73. [Google Scholar]
- Brown DD. The role of thyroid hormone in zebrafish and axolotl development. Proc. Natl. Acad. Sci. USA. 1997;94:13011–13016. doi: 10.1073/pnas.94.24.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr JA, McNabb FMA, Smith EE. Thyroid function and mechanisms of perchlorate action in vertebrates. In: Kendall R, Smith PN, editors. Perchlorate Ecotoxicology. Pensacola, FL, USA: SETAC Press; 2005. pp. 45–77. [Google Scholar]
- Carr JA, Patiño R. The hypothalamus-pituitary-thyroid axis in teleosts and amphibians: endocrine disruption and its consequences to natural populations. Gen. Comp. Endocrinol. 2011;170:299–312. doi: 10.1016/j.ygcen.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Choksi NY, Jahnke GD, St Hilaire C, Shelby M. Role of thyroid hormones in human and laboratory animal reproductive health. Birth Defects Res. B: Dev. Reprod. Toxicol. 2003;68:479–491. doi: 10.1002/bdrb.10045. [DOI] [PubMed] [Google Scholar]
- Crane HM, Pickford DB, Hutchinson TH, Brown JA. Effects of ammonium perchlorate on thyroid function in developing fathead minnows, Pimephales promelas . Environ. Health Perspect. 2005;113:396–401. doi: 10.1289/ehp.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S, Hoar WS. Effects of thyroxine and thiourea on the early development of chum salmon (Oncorhyncus keta) Can. J. Zool. 1954;32:244–251. [Google Scholar]
- Furin CG, von Hippel FA, Hagedorn B, O’Hara TM. Perchlorate trophic transfer increases tissue concentrations above ambient water exposure alone in a predatory fish. J. Toxicol. Environ. Health, A. 2013;76:1072–1084. doi: 10.1080/15287394.2013.836693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goleman WL, Carr JA, Anderson TA. Environmentally relevant concentrations of ammonium perchlorate inhibit thyroid function and alter sex ratios in developing Xenopus laevis . Environ. Toxicol. Chem. 2002;21:590–597. [PubMed] [Google Scholar]
- Honma Y, Shioda S, Yoshie S. Changes in the thyroid gland associated with the diadromous migration of the threespine stickleback, Gasterosteus aculeatus . Jap. J. Ichthyol. 1977;24:17–25. [Google Scholar]
- Josefsson M, Grunditz T, Ohlsson T, Ekblad E. Sodium/iodide-symporter: distribution in different mammals and role in entero-thyroid circulation of iodide. Acta Physiol. Scand. 2002;175:129–137. doi: 10.1046/j.1365-201X.2002.00968.x. [DOI] [PubMed] [Google Scholar]
- Kounaves SP, Stroble ST, Anderson RM, Moore Q, Catling DC, Douglas S, McKay CP, Ming DW, Smith PH, Tamppari LK, Zent AP. Discovery of natural perchlorate in the antarctic dry valleys and Its global implications. Environ. Sci. Technol. 2010;44:2360–2364. doi: 10.1021/es9033606. [DOI] [PubMed] [Google Scholar]
- LaRoche G, Woodall AN, Johnson CL, Halver JE. Thyroid function in the rainbow trout (Salmo gairdnerii): II. Effects of thyroidectomy on the development of young fish. Gen. Comp. Endocrinol. 1966;6:249–266. doi: 10.1016/s0016-6480(66)80013-8. [DOI] [PubMed] [Google Scholar]
- Leatherland JF. Environmental physiology of the teleostean thyroid gland: a review. Environ. Biol. Fishes. 1982;7:83–110. [Google Scholar]
- Leung AM, Pearce EN, Braverman LE. Perchlorate, iodine and the thyroid. Best Pract Res Clin Endocrinol Metab. 2010;24:133–141. doi: 10.1016/j.beem.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Gentles A, Theodorakis CW. Arsenate and perchlorate toxicity, growth effects, and thyroid histopathology in hypothyroid zebrafish Danio rerio . Chemosphere. 2008;71:1369–1376. doi: 10.1016/j.chemosphere.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Liu YW, Chan WK. Thyroid hormones are important for embryonic to larval transitory phase in zebrafish. Differentiation. 2002;70:36. doi: 10.1046/j.1432-0436.2002.700104.x. [DOI] [PubMed] [Google Scholar]
- McDougal JN, Jones KL, Fatuyi B, Gray KJ, Blount BC, Valentín-Blasini L, Fisher JW. The Effects of Perchlorate on Thyroidal Gene Expression are Different from the Effects of Iodide Deficiency. J. Toxicol. Environ. Health, A. 2011;74:917–926. doi: 10.1080/15287394.2011.573740. [DOI] [PubMed] [Google Scholar]
- McNabb FMA, Larsen CT, Pooler PS. Ammonium perchlorate effects on thyroid function and growth in bobwhite quail chicks. Environ. Toxicol. Chem. 2004;23:997–1003. doi: 10.1897/03-362. [DOI] [PubMed] [Google Scholar]
- Melse-Boonstra A, Jaiswal N. Iodine deficiency in pregnancy, infancy and childhood and its consequences for brain development. Best Pract Res Clin Endocrinol Metab. 2010;24:29–38. doi: 10.1016/j.beem.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Morrison RD, Vavricka EA, Duncan PB. Perchlorate. In: Morrison RD, Murphy BL, editors. Environmental Forensics: Contaminant Specific Guide. Burlington, MA, USA: Elsevier Inc; 2006. pp. 167–185. [Google Scholar]
- Mukhi S, Torres L, Patiño R. Effects of larval-juvenile treatment with perchlorate and co-treatment with thyroxine on zebrafish sex ratios. Gen. Comp. Endocrinol. 2007;150:486–494. doi: 10.1016/j.ygcen.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Okada N, Morita T, Tanaka M, Tagawa M. Thyroid hormone deficiency in abnormal larvae of the Japanese flounder Paralichthys olivaceus . Fish. Sci. 2005;71:107–114. [Google Scholar]
- Okada N, Tanaka M, Tagawa M. Bone development during metamorphosis of the Japanese flounder (Paralichthys olivaceus): Differential responses to thyroid hormone. In: Browman HI, Skiftesvik AB, editors. The Big Fish Bang. Proceedings of the 26th Annual Larval Fish Conference; Bergen, Norway. Institure of Marine Research; 2003. pp. 177–187. [Google Scholar]
- Palmer AR. Fluctuating asymmetry analyses: a primer. In: Markow T, editor. Developmental Instability: Its Origins and Evolutionary Implications. Netherlands: Springer; 1994. pp. 335–364. [Google Scholar]
- Patiño R, Mukhi S. Effects of prolonged exposure to perchlorate on thyroid and reproductive function in zebrafish. Toxicol. Sci. 2007;96:246–254. doi: 10.1093/toxsci/kfm001. [DOI] [PubMed] [Google Scholar]
- Peter MCS. The role of thyroid hormones in stress response of fish. Gen. Comp. Endocrinol. 2011;172:198–210. doi: 10.1016/j.ygcen.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Pickford DB, Crane HM, Hutchinson TH, Brown JA. Effects of ammonium perchlorate on thyroid function in developing fathead minnows, Pimephales promelas . Environ. Health Perspect. 2005;113:396–401. doi: 10.1289/ehp.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power DM, Llewellyn L, Faustino M, Nowell MA, Björnsson BT, Einarsdottir IE, Canario AVM, Sweeney GE. Thyroid hormones in growth and development of fish. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2001;130:447–459. doi: 10.1016/s1532-0456(01)00271-x. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Anderson TA, Fahlquist L, Rainwater KA, Ridley M, Jackson WA. Widespread presence of naturally occurring perchlorate in high plains of Texas and New Mexico. Environ. Sci. Technol. 2006;40:3156–3162. doi: 10.1021/es052155i. [DOI] [PubMed] [Google Scholar]
- Rao B, Anderson TA, Orris GJ, Rainwater KA, Rajagopalan S, Sandvig RM, Scanlon BR, Stonestrom DA, Walvoord MA, Jackson WA. Widespread natural perchlorate in unsaturated zones of the southwest United States. Environ. Sci. Technol. 2007;41:4522–4528. doi: 10.1021/es062853i. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. U. S. National Institutes of Health. Bethesda, Maryland, USA: 1997–2013. http://imagej.nih.gov/ij/ [Google Scholar]
- Reddy PK, Lam TJ. Effect of thyroid hormones on morphogenesis and growth of larvae and fry of telescopic-eye black goldfish, Carassius auratus . Aquaculture. 1992a;107:383–394. [Google Scholar]
- Reddy PK, Lam TJ. Role of thyroid hormones in tilapia larvae (Oreochromis mossambicus): I. Effects of the hormones and an antithyroid drug on yolk absorption, growth and development. Fish Physiol. Biochem. 1992b;9:473–485. doi: 10.1007/BF02274228. [DOI] [PubMed] [Google Scholar]
- Schmidt F, Schnurr S, Wolf R, Braunbeck T. Effects of the anti-thyroidal compound potassium-perchlorate on the thyroid system of the zebrafish. Aquat. Toxicol. 2012;109:47–58. doi: 10.1016/j.aquatox.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Sekimizu K, Tagawa M, Takeda H. Defective fin regeneration in medaka fish (Oryzias latipes) with hypothyroidism. Zoolog. Sci. 2007;24:693–699. doi: 10.2108/zsj.24.693. [DOI] [PubMed] [Google Scholar]
- Shkil FN, Kapitanova DV, Borisov VB, Abdissa B, Smirnov SV. Thyroid hormone in skeletal development of cyprinids: Effects and morphological consequences. J. Appl. Ichthyol. 2012;28:398–405. [Google Scholar]
- Smirnov SV, Kapitanova DV, Borisov VB, Abdissa B, Shkil FN. Lake Tana large barbs diversity: Developmental and hormonal bases. J. Ichthyol. 2012;52:861–880. [Google Scholar]
- Swarup H. Stages in the development of stickleback Gasterosteus aculeatus (L.) J. Embryol. Exp. Morphol. 1958a;6:373–383. [PubMed] [Google Scholar]
- Swarup H. Stages in the development of the stickleback Gasterosteus aculeatus (L.) J. Embryol. Exp. Morphol. 1958b;6:373–383. [PubMed] [Google Scholar]
- Theodorakis C, Rinchard J, Anderson T, Liu F, Park JW, Costa F, McDaniel L, Kendall R, Waters A. Perchlorate in fish from a contaminated site in east-central Texas. Environ. Pollut. 2006a;139:59–69. doi: 10.1016/j.envpol.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Theodorakis C, Rinchard J, Carr J, Park J-W, McDaniel L, Liu F, Wages M. Thyroid Endocrine Disruption in Stonerollers and Cricket Frogs from Perchlorate-Contaminated Streams in East-Central Texas. Ecotoxicology. 2006b;15:31–50. doi: 10.1007/s10646-005-0040-6. [DOI] [PubMed] [Google Scholar]
- Tietge JE, Holcombe GW, Flynn KM, Kosian PA, Korte JJ, Anderson LE, Wolf DC, Degitz SJ. Metamorphic inhibition of Xenopus laevis by sodium perchlorate: Effects on development and thyroid histology. Environ. Toxicol. Chem. 2005;24:926–933. doi: 10.1897/04-105r.1. [DOI] [PubMed] [Google Scholar]
- Trumbo PR. Perchlorate consumption, iodine status, and thyroid function. Nutr. Rev. 2010;68:62–66. doi: 10.1111/j.1753-4887.2009.00260.x. [DOI] [PubMed] [Google Scholar]
- Trumpolt CW, Crain M, Cullison GD, Flanagan SJP, Siegel L, Lathrop S. Perchlorate: Sources, uses, and occurrences in the environment. Remediation. 2005;16:65–89. [Google Scholar]
- Urbansky ET. Perchlorate as an environmental contaminant. Environ. Sci. Pollut. Res. 2002;9:187–192. doi: 10.1007/BF02987487. [DOI] [PubMed] [Google Scholar]
- Urbansky ET, Brown SK, Magnuson ML, Kelty CA. Perchlorate levels in samples of sodium nitrate fertilizer derived from Chilean caliche. Environ. Pollut. 2001;112:299–302. doi: 10.1016/s0269-7491(00)00132-9. [DOI] [PubMed] [Google Scholar]
- USEPA. Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. 5th ed. Washington, DC, USA: 2002. [Google Scholar]
- USEPA. Drinking water: Regulatory determination on perchlorate. Fed. Regist. 2011;76:7762–7767. [Google Scholar]
- USEPA. Fed. Regist. Washington, DC, USA: 2013. Final aquatic life ambient water quality criteria for ammonia - freshwater 2013, in: USEPA (Ed.) pp. 52192–52194. [Google Scholar]
- von Hippel F. Vigorously courting male sticklebacks are poor fathers. Acta Ethol. 2000;2:83–89. [Google Scholar]
- von Hippel F, Weigner H. Sympatric anadromous-resident pairs of threespine stickleback species in young lakes and streams at the Bering Glacier, Alaska. Behaviour. 2004;141:1441–1464. [Google Scholar]
- Wolff J. Perchlorate and the thyroid gland. Pharmacol. Rev. 1998;50:89–105. [PubMed] [Google Scholar]
- Yu KO, Narayanan L, Mattie DR, Godfrey RJ, Todd PN, Sterner TR, Mahle DA, Lumpkin MH, Fisher JW. The pharmacokinetics of perchlorate and its effect on the hypothalamus-pituitary-thyroid axis in the male rat. Toxicol. Appl. Pharmacol. 2002;182:148–159. doi: 10.1006/taap.2002.9432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.