Abstract
Background & Aims
The neuroprotective effect of the spheroid reservoir bioartificial liver (SRBAL) was evaluated in a porcine model of drug-overdose acute liver failure (ALF).
Methods
Healthy pigs were randomized into three groups (standard therapy (ST) alone, ST + No-cell device, ST + SRBAL device) before placement of an implantable intracranial pressure (ICP) monitor and a tunneled central venous catheter. One week later, pigs received bolus infusion of the hepatotoxin D-galactosamine and were followed for up to 90 hours.
Results
At 48 hours, all animals had developed encephalopathy and biochemical changes confirming ALF; extracorporeal treatment was initiated and pigs were observed up to 90 hours after drug infusion. Pigs treated with the SRBAL, loaded with porcine hepatocyte spheroids, had improved survival (83%, n=6) compared to ST alone (0%, n=6, p=0.003) and No-cell device therapy (17%, n=6, p=0.02). Ammonia detoxification, peak levels of serum ammonia and peak ICP, and pig survival were influenced by hepatocyte cell dose, membrane pore size and duration of SRBAL treatment. Hepatocyte spheroids remained highly functional with no decline in mean oxygen consumption from initiation to completion of treatment.
Conclusions
The SRBAL improved survival in an allogeneic model of drug-overdose ALF. Survival correlated with ammonia detoxification and ICP lowering indicating that hepatocyte spheroids prevented the cerebral manifestations of ALF (brain swelling, herniation, death). Further investigation of SRBAL therapy in a clinical setting is warranted.
Keywords: liver failure, bioartificial liver, spheroid, hepatocyte, D-galactosamine
Introduction
Acute liver failure (ALF) is a severe, life-threatening medical condition that portends a poor prognosis. Mortality rate of ALF patients exceed 30% (1). The only proven therapy is liver transplantation. With the disparity between patients awaiting liver transplantation and the limited donor pool, it is crucial to consider alternative therapies. Many artificial liver devices and hybrid (cell-based) bioartificial liver (BAL) devices have been developed with a hand full making it to clinical testing (2–4). However, each device tested thus far has had limitations that prevented it from demonstrating a survival benefit when randomized to standard medical therapy of liver failure.
The therapeutic goal of a liver support device is to prevent the extra-hepatic manifestations of liver failure and improve survival, best if spontaneously without the surgical risks of liver transplantation and long term side effects of immunosuppression. Purely artificial liver support devices benefit patients through simple removal of wastes and other small molecules, while BAL systems also have the potential for metabolic detoxification and synthetic activity. Over time, it has become apparent that the complex mechanisms by which the liver ensures homeostasis are unlikely to be replaced by non-biologic detoxification alone (5). Immortalized cell lines, such as the human hepatoblastoma lines HepG2 and C3A (6), human hepatocytes (7), and porcine hepatocytes (3, 8), have been tested in BAL devices. Human cell lines are capable of expansion in vitro and production of human proteins (9); however, they have incomplete metabolic profiles compared to primary hepatocytes (10). Specifically, cell lines lack normal expression of urea cycle enzymes (11, 12) known to play a mechanistic role in the development of cerebral edema, herniation and brain death of ALF (13).
Human hepatocytes are the preferred source of cells for a BAL, but they are currently an impractical source due to limitations in both quantity and availability. Metabolic profiles of human and porcine hepatocytes are similar, and therefore porcine hepatocytes are a reasonable alternative to human hepatocytes provided safety guidelines are followed to minimize potential risks of xenozoonosis (14, 15). Potential infectious and immunological risks of porcine cells can be further reduced by the use of appropriate membranes to separate the blood and hepatocyte compartments of the BAL (16–19).
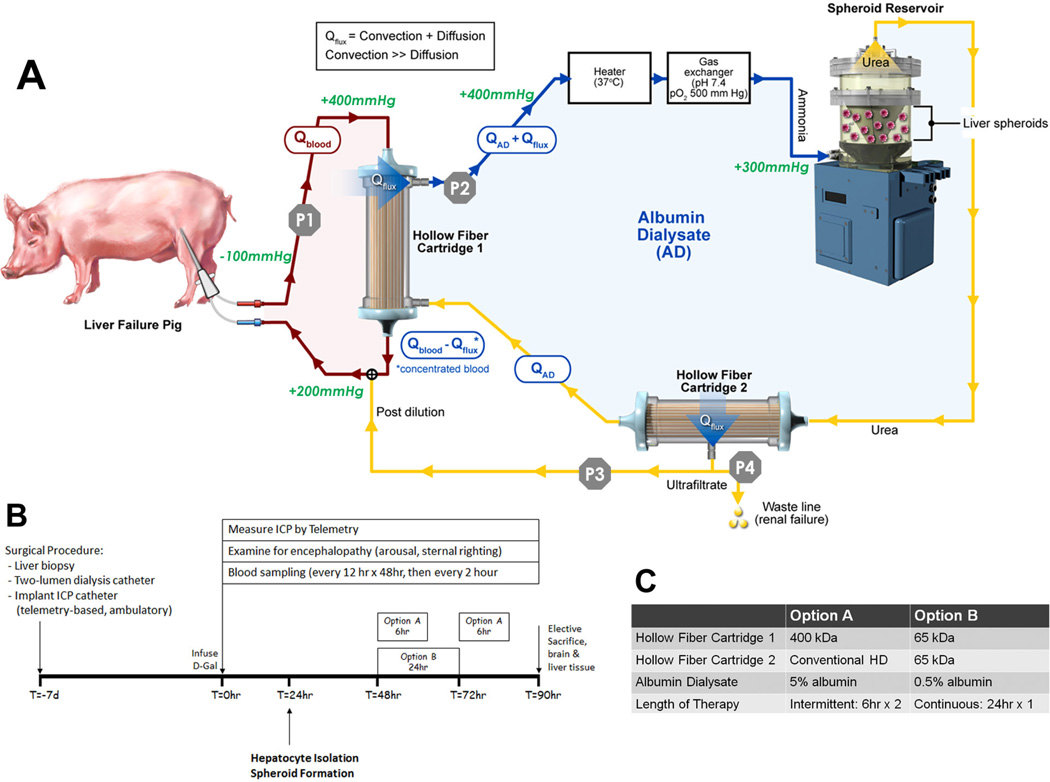
A limitation of primary hepatocytes is that they exhibit diminished functionality over time under standard culture conditions. Therefore, alternative culture systems have been proposed. A promising consideration are multicellular, three-dimensional spherical aggregates, called hepatocyte spheroids (20), formed by cell-to-cell adhesion mediated by surface molecules such as E-cadherin (21). Hepatocytes within these spheroid aggregates are polarized and form bile canaliculi (22). The spheroid structure protects hepatocytes from apoptosis caused after epithelial cells separate from their basement membrane, known as anoikis (23), and allows them to remain viable and functionally stable for days to weeks in suspension (24). Other features of hepatocyte spheroids are the simplicity of spheroid formation in rocked suspension culture, the large cell mass possible in high density cell culture, and the ease of scale-up for extracorporeal use in a BAL device such as the spheroid reservoir bioartificial liver (SRBAL) (25). The SRBAL device is illustrated schematically in Fig. 1A and in an animation (Movie S1).
Fig. 1. Experimental Design.
Panel A – Schematic of Extracorporeal Circuit including SRBAL Apparatus. Red line indicates the blood compartment, while yellow/blue line indicates the acellular albumin dialysate (AD) compartment. Flow rates in these two compartments, Qblood and QAD, along with flux across the membrane of Hollow Fiber Cartridge 1, Qflux, and waste line flow were determined by pumps P1, P2, P3, and P4. Standard flow rates for the system were Qblood = 200 mL/min, QAD = 200 mL/min, Qflux. = 50 mL/min. The waste line pump (P4) was not operated in this study (waste line flow = 0mL/min). Pressure limits are labeled in green italic font. Removal of permeable waste molecules, such as ammonia, from the blood occurred by both diffusion and convection (35). Blue lines indicate areas of high ammonia concentration in the AD circuit where total flow rate was QAD+ Qflux. The Spheroid Reservoir functioned as a suspension bioreactor with fluid entering its bottom and exiting its top. Oxygen consumption by hepatocyte spheroids was determined by the product of QAD+ Qflux multiplied by the difference in oxygen tension between inlet and outlet. An oxygenator was used to maintain the inlet oxygen tension above 500 mmHg. Exit oxygen tension averaged about 100mmHg. An outflow filter (0.2µm) prevented loss of hepatocytes from the reservoir. The semipermeable membrane of Hollow Fiber Cartridge 2 was used to maximize removal of detoxification products, such as urea, and achieve fluid balance in the AD circuit.
Panel B – Experimental time line showing the sequence of events and timing of extracorporeal treatments of Option A and Option B.
Panel C – detailed summary of Option A and Option B.
The current prospective randomized controlled study was designed to serve as a preclinical pivotal trial of the SRBAL device in a porcine model of drug-overdose ALF. We refer to this study as the Recovery ALF study, or RALF study, since the ALF animals were allowed to recover after treatment. We utilized a modification of the porcine drug over-dose model originally proposed by Ho et al. (26). We hypothesized that pigs treated with the SRBAL will have improved survival associated with improved detoxification of ammonia and reduced cerebral manifestations of ALF. Our experimentation was designed to answer the following questions: 1. Is ALF in our porcine model associated with a rise in serum ammonia, cerebral edema and brain death? 2. Can hepatocytes in a hybrid extracorporeal device, such as SRBAL, lower serum ammonia and prevent the cerebral manifestation of ALF? 3. Is there a correlation between duration of treatment and response to SRBAL therapy? 4. Is there a correlation between dose of cells in the SRBAL and response to therapy? 5. Is there a correlation between porosity of the SRBAL membrane and response to SRBAL therapy? Answers to these questions are needed to establish a mechanistic role for cell-based liver support therapy and to design future clinical trials of the SRBAL.
Materials and Methods
Randomization
Upon arrival from the vendor (Manthei Hog Farm, Elk River, MN) at least two weeks prior to treatment, healthy 45kg (range 43.8 – 51.0 kg) female swine were randomized in blocks of three to one of three intervention groups: standard therapy (ST) alone, ST plus treatment with a No-cell device (ST+No-cell therapy), and ST plus treatment with a SRBAL device (ST+SRBAL therapy). All animal husbandry and procedures were performed in accordance with the guidelines set forth by the Mayo Foundation Animal Care and Use Committee.
Standard Therapy of ALF Pigs
Fig. 1B provides a time line of experimental events. Animals in all groups underwent general anesthesia (inhaled isoflurane 2–3%, induction: 5mg/kg Telazol, 2 mg/kg Xylazine, 0.01 mg/kg Glycopyrrolate) for the placement of an ambulatory intracranial pressure (ICP) monitoring device, internal jugular central venous access, and baseline liver biopsy one week prior to induction of ALF. The ambulatory ICP device (RAUMEDIC, Germany) included an intraparenchymal catheter placed through a burr hole in the pig’s right anterior skull and a subcutaneous transmitter under the right anterior scalp. This transdermal telemetry-based system allowed monitoring of ICP in pigs while ambulatory or sedated during extracorporeal therapy (ECT). A cuffed two-lumen central venous catheter (Palindrome™ 55cm, Covidien, Mansfield, MA, www.kendallhq.com) was placed in the right internal jugular vein by Seldinger technique, tunneled subcutaneously, and exited from the animal’s back. The line was locked with heparin (1000 unit/mL) to retain patency. An open liver biopsy was performed through a small midline laparotomy. Animals were recovered and then received standard animal care for one week to allow healing of incisions and to confirm health status.
One week after the catheterization procedure, animals were sedated with 0.1–0.2 mg/kg/min propofol IV through one lumen of central line while a baseline blood sample was collected through the other lumen. Pigs then received intravenous bolus infusion of 0.75 g/kg D-galactosamine (t = 0 hour) and were recovered from sedation. Animals were monitored every 6 hours for the first 24 hours and every two hours for the remainder of the study. Monitoring steps included blood draws, ICP monitoring, and clinical observations every 6 hours (every 2 hours while on SRBAL therapy). Chemical analysis (AST, INR, total bilirubin, Cr, glucose, ammonia) was performed in the Mayo Clinical Chemistry Lab. Ammonia concentrations in plasma were quantified by AMON slide assay (Product #1721869, Vitro Chemistry Products) on the Vitros 5600 Integrated System (www.orthoclinical.com). Propofol (0.1–0.2 mg/kg/min) was administered briefly via the central venous line to assure lateral decubitus positioning for all ICP measurements. Reported values of ICP (mmHg) were the average of 3–5 measurements obtained over a 2 minute interval. All blood glucose values less than 75 mg/dL were treated with 10 ml of 50% dextrose (D50). All animals received 1 L of normal saline to ensure hydration at the time of D-galactosamine infusion. Animals also received 1 L of normal saline at 24 hours post infusion of D-galactosamine.
Study Endpoints
Animals were followed up to 90 hours after D-galactosamine. Animals surviving at least 90 hours reached the primary study endpoint. Animals demonstrating Grade 4 hepatic encephalopathy (non-responsiveness to sternal rub, ear tug, or auditory stimulus) while off propofol reached a second endpoint and were removed from the study. A third study endpoint was the inability to maintain sternal positioning with an ICP >20 mmHg. All animals were euthanized by sodium pentobarbital overdose after reaching one of these study endpoints.
Tissue Examination
Immediately following death, water content of fresh brain white matter was determined by an established gravimetric method (27). A post mortem liver specimen was also obtained from each pig immediately after death. Liver tissue was formalin fixed and paraffin embedded for permanent staining by hematoxylin and eosin (H&E), as well as immunohistochemical staining with an anti-Ki67 antibody (#M7240, lot#20001030, www.dako.com). Immunohistochemical sections were treated with 3% hydrogen peroxide in ethanol for five minutes to inactivate endogenous peroxidase. Sections were pretreated with protease K (DAKO, Carpentaria, California), and endogenous pig immunoglobulins were blocked using porcine block M (Biocare Medical, Concord, California). Sections were incubated with rat anti pig primary antibody (Ki67) for 60 minutes using 1–400 dilution. Labeling of primary antibody was carried out by using the Biocare Medical kit antibody and diaminobenzidine. Sections were then counter stained using modified Schmitz hematoxylin and visualized using Axiovert 135 inverted microscope (Axioscope, Carl Zeiss, Inc., Thornwood, NJ). Images were analyzed at low (4×), medium (10–20×), and high (40×) power by a liver pathologist in a blinded fashion. A semi-quantitative system was used to measure inflammation (absent 0, present 1), zone 3 necrosis (absent 0, present 1), panlobular necrosis (absent 0, focal 1, diffuse 2), percentage necrosis (absent 0, 1–25% 1, 26–76% 2, >75% 3), and fatty changes (absent 0, present 1). A total score was assigned to post mortem liver specimens from all eighteen study animals and six liver specimens collected at baseline (ST alone × 2, ST + no device × 2, ST+SRBAL × 2). Liver Injury Score was determined from the rank order of the total score of these 24 liver specimens. Positive nuclear staining by Ki67 was quantified across ten separate visual fields at 40×; mean and standard deviation of these counts were determined.
Extracorporeal Therapy (ECT)
ECT with either the No-cell device or SRBAL device was initiated at 48 hours following D-galactosamine infusion. Light sedation during ECT therapy was limited to 0.1 mg/kg/min propofol. Animals received continuous monitoring of heart rate, blood pressure, cutaneous oximetry, and ICP during ECT. The pig was connected to the extracorporeal device, illustrated in Figure 1A and Movie Video S1, via two-lumen central venous catheter. Prior to initiation of ECT, all animals received 1 L of normal saline followed by maintenance fluid hydration with 5% Dextrose in normal saline (D5NS) at 100 mL/hour during ECT. This rate was increased as needed to a maximum rate of 500 mL/hr to maintain MAP above 50 mmHg. Norepinephrine (0.04 µg/kg/min) intravenous drip was added if MAP could not be maintained above 50 mmHg with intravenous fluids. Blood activated clotting time (ACT) was measured hourly during ECT and heparin was administered intravenously as needed to maintain ACT above 200 seconds.
Two options of No-cell therapy and SRBAL therapy were evaluated. Option A consisted of two × 6-hour treatments (48 to 54 hours, 72 to 78 hours) using a Hollow Fiber Cartridge #1 (see Fig. 1) with polysulfone fibers, 400kD nominal molecular weight cut-off (nMWCO), and 5g/dL albumin in the dialysate circuit. Option B consisted of one × 24 hour treatment (48 to 72 hours) using a Hollow Fiber Cartridge #1 with polysulfone fibers, 65kD nMWCO, and 0.5g/dL albumin in the dialysate circuit. Details of the two treatment options are summarized in Fig. 1C.
Hepatocyte Harvest
Healthy hepatocyte donor pigs were obtained from a local certified vendor (Manthei Hog Farm, Elk River, MN). Twenty-four hours prior to planned SRBAL therapy, hepatocytes were harvested according to a previously described 2-step perfusion technique (28). Viability of isolated hepatocytes was determined by trypan blue exclusion. Hepatocyte spheroids were produced by rocker technique (25). Viability of hepatocytes in 3-dimension spheroids was determined by epifluorescent microscopy (29). One donor pig was used to produce hepatocyte spheroids for each treatment. Donor animals received a high (40%) protein diet for one week prior to hepatocyte harvest based on results of a preliminary study reported in Supplemental Material 1.
Statistics
Categorical data were described by counts and percents; numerical data was described by mean (±standard deviation) or median (min, max) of the group. Statistical significance between different treatment groups were determined by the chi-square test for categorical data and Student’s T-Test for quantitative data, or if the data were heavily skewed (non-normal) then Wilcoxon Rank-Sum Test for medians. If baseline measures were available then the T-test was performed using analysis of covariance (ANCOVA), adjusting for baseline. The Pearson product-moment correlation was used to describe and test correlations, or if inspection suggested skewed data or outliers the Spearman’s rank-correlation test was used. For data recorded longitudinally the main analysis was of the end of study values at 90 hours, and last observation carry forward (LOCF) was used in case of missing values. Missing values were anticipated before starting the study in case of deaths before 90 hours.
When interpreting statistical significance we first compared the ST vs. ST+SRBAL groups to test for an effect of the SRBAL device. If this was significant we next interpreted the significance of the test comparing the ST+SRBAL and ST+No-cell groups, in order to identify the possible effect of the biological component of the device beyond its physical component alone. By only declaring statistical significance between the ST+SRBAL and ST+No-cell groups in case of significance between the SR+SRBAL vs ST groups, the process qualifies as a closed testing procedure. Therefore, when performed sequentially in this manner, both tests may be made at the nominal level while controlling for family wise error rates, without any further adjustments for p-values as otherwise may be required when making multiple comparisons (30). Further tests between groups were made in an exploratory manner and no further adjustments were made to protect the family wise error rate. Statistical significance was established when p ≤ 0.05. Analyses were made using SAS 9.3 (SAS Institute Inc., Cary, NC, USA) and Excel 2011 Version 14.4.6 (www.microsoft.com).
Results
Experimental Overview
The sequence of experimental events is provided in Fig. 1B. A total of 21 pigs were instrumented with a tunneled hemodialysis catheter and implantable ICP monitor. Seven days later, pigs received intravenous D-galactosamine at T=0 hr. Two animals developed rapidly progressing ALF and reached a study endpoint of Grade 4 coma prior to the initiation of treatment at 48 hours. One animal developed a Staphylococcus aureus line sepsis with febrile seizure requiring early euthanasia. These three animals were excluded from further analysis as potential benefit of therapy could not be assessed. This resulted in a total of 18 animals randomized to three treatment groups: ST alone (n=6), ST+No-cell therapy (n=6), ST+SRBAL therapy (n=6). Animals in the No-cell therapy and SRBAL therapy groups were further subdivided based on configuration of the extracorporeal circuit, frequency and duration of therapy (Option A- two × 6-hour treatments, n=3; Option B- one × 24-hour treatment, n=3), defined in Fig. 1C. Group assignments were made prior to infusion of hepatotoxic drug.
Degree of Liver Failure at Initiation of Treatment
All 18 study animals had normal biochemical profiles prior to administration of D-galactosamine. Forty-eight hours later, all pigs demonstrated laboratory values consistent with ALF (Table 1). Each of the three treatment groups had comparable AST (p=0.90), total bilirubin (p=0.77), INR (p=0.56), creatinine (p=0.89), and plasma ammonia (p=0.64) at initiation of therapy. Fifteen of the 18 pigs required dextrose supplementation for blood glucose levels below 75 mg/dL prior to 48 hours. All animals showed signs of Grade I to II encephalopathy consisting of lethargy, unsteady gait, and loss of appetite at 48 hours. Fluctuations in mean levels of AST, total bilirubin, and creatinine after initiation of therapy are shown in Supplemental Material 2.
Table 1.
Median (min – max) Lab Values Prior to Extracorporeal Therapy (ECT), T=48 hrs
| Treatment Group | AST (U/L) |
Ammonia (ug/dL) |
INR | Total Bilirubin (mg/dL) |
Creatinine (mg/dL) |
ICP (mmHg) |
Glucose Requirement |
|---|---|---|---|---|---|---|---|
| ST (n=6) |
2024 (315–5528) |
267 (28–581) |
9.6 (6.2–15.3) |
4.1 (2.8–7.3) |
0.9 (0.7–1.3) |
4 (3–6) |
100% (6/6) |
| ST+No-cell BAL (n=6) |
2632 (274–7485) |
336 (73–800) |
10.3 (4.2–15.3) |
5.1 (3.5–8.5) |
0.9 (0.7–1.1) |
6 (2–20) |
67% (4/6) |
| ST+SRBAL (n=6) |
2187 (482–6890) |
432 (213–1200) |
8.1 (2.0–15.3) |
4.6 (2.2–10.3) |
1.0 (0.8–1.1) |
7 (2–16) |
83% (5/6) |
Fluid and Vasopressor Support
All animals received intravenous hydration at t=0hr (1 liter), t=24 hr (1 liter), and during treatment of hypoglycemia. In addition, a continuous infusion of crystalloid solution was provided during ECT treatments. As expected, total fluid administration (mean±SD) was lower in the ST group (2,757±518mL) compared to the ST+No-cell BAL group (5,603±1,006mL, p<0.001) and ST+SRBAL group (5,785±830, p<0.001). Animals received similar total fluid support under Option A (5,968±967mL) and Option B (5,420±779mL, p=0.305); this observation was noteworthy since treatment duration of Option B was 100% longer than Option A. None of the animals in the ST+SRBAL group required vasopressor therapy during extracorporeal treatment compared to 5 of 6 pigs in the ST+No-cell BAL group (p=0.003).
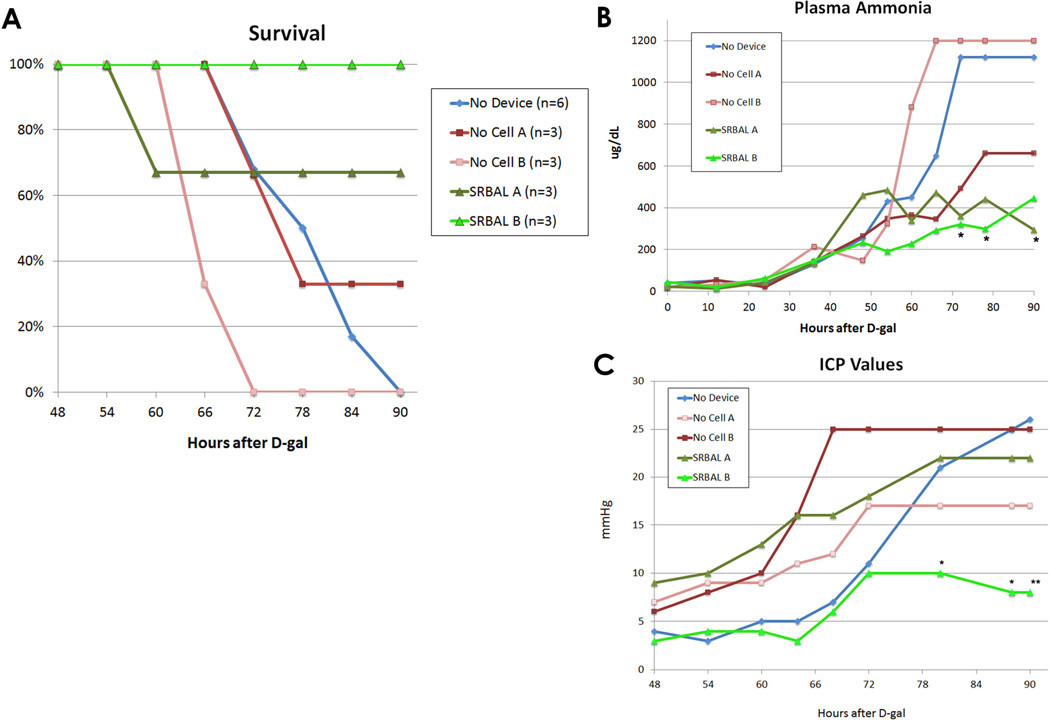
Survival
Animals treated with ST+SRBAL device had improved survival (5/6, 83% surviving to the 90 hour study endpoint) compared to ST alone (0/6, 0%, p=0.003) or the ST+ No-cell therapy (1/6, 17%, p=0.021) groups. All three ALF animals randomized to SRBAL Option B survived to the 90hr end point (Fig 2A). The single early mortality in the SRBAL group (Option A) had exhibited signs of advanced ALF with Grade IV encephalopathy and corresponding biochemical abnormalities (AST 6890 U/L, ammonia >1200 µg/dL, INR 8.4) at the onset of therapy (t=48hr); only 59 grams of hepatocyte spheroids were present during the single 6 hour treatment of that animal.
Fig. 2. SRBAL Therapy improved Survival, Plasma Ammonia, Intracranial Pressure (ICP).
Panel A – Survival curves of all five treatment conditions. Survival at 90 hours of pigs treated with the SRBAL (Option A and Option B) was significantly improved (83%, n=6) compared to the two control groups, No-cell device therapy (17%, n=6, p=0.01) and ST alone (0%, n=6, p=0.004) groups. 100% survival was observed under conditions of SRBAL Option B.
Panel B - Median values of plasma ammonia for each of the five treatment conditions are shown. Pigs treated with the SRBAL (both Option A and Option B) had stabilization in plasma ammonia during therapy, beginning at 48 hr, (* indicates p<0.05 compared to the ST control group at the corresponding time point).
Panel C - Median ICP values for pigs in each of the five treatment conditions are shown. Pigs treated with the SRBAL Option B had lower final ICP compared to the ST group (** p<0.005). The difference in ICP of pigs in SRBAL Option B and ST control groups was significant from 80–90 hours (* p<0.05).
Length of SRBAL therapy
Of the six animals treated with the SRBAL, three underwent intermittent therapy (Option A, two 6 hour treatments initiated 24 hours apart) and three underwent continuous therapy for 24 hours (Option B). The impact of length of therapy and total cell dose of hepatocyte spheroids on major outcome variables is summarized in Table 2. There were no clinically detectable adverse events associated with either treatment option. However, the three pigs that underwent continuous SRBAL Option B therapy had lower peak ICP (26.0±7.5mmHg vs. 12.0±2.6mHg, p=0.039) and tended to lower final ICP (22.2±10.8 mmHg vs. 7.0±2.8mHg, p=0.077) compared to the animals treated under intermittent SRBAL Option A therapy. All pigs treated under SRBAL Option B survived to the 90 hr end point and they had significantly lower serum ammonia levels after treatment (T=72hr) compared to corresponding control animals treated with the No-cell Option B device (303±95 vs. 1135±113 µg/dL, p<0.001).
Table 2.
RALF Summary - Impact of Cell Dose and Treatment Duration on Outcome
| Group | # | Treatment Conditions | NH3.72hr | Bili.72hr | Cr.72hr | AST.peak | ICP.peak | Survival | Study | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total Cell Dose (gm) | Duration (hrs) | µg/dL | mg/dL | mg/dL | U/L | mmHg | hours | Endpoint | ||
| ST | 1 | 0 | 0 | NA | 4.7 | 4.1 | 2342 | NA | 74 | Stage 4 HE |
| ST | 2 | 0 | 0 | 1200 | 7.2 | 1.9 | 5528 | NA | 72 | Stage 4 HE |
| ST | 3 | 0 | 0 | 1120 | 5.4 | 0.6 | 2229 | 23 | 79 | ICP |
| ST | 4 | 0 | 0 | 582 | 6.2 | 0.7 | 675 | 30 | 80 | ICP |
| ST | 5 | 0 | 0 | 1200 | 20.9 | 1.8 | 1850 | 21 | 70 | Stage 4 HE |
| ST | 6 | 0 | 0 | 419 | 4.0 | 0.6 | 585 | 28 | 88 | ICP |
| ST+NC (Option A) | 1 | 0 | 10 ‡ | 493 | 5.1 | 0.9 | 2075 | 58 | 77 | ICP |
| ST+NC (Option A) | 2 | 0 | 12 | 274 | 5.9 | 1.0 | 1155 | 18 | 90 | Survival |
| ST+NC (Option A) | 3 | 0 | 6 ‡ | 876 | 5.8 | 1.6 | 2730 | 21 | 70 | Stage 4 HE |
| ST+NC (Option B) | 1 | 0 | 17 ‡ | 1200 | 4.5 | 1.9 | 2207 | 22 | 66 | Stage 4 HE |
| ST+NC (Option B) | 2 | 0 | 14 ‡ | 1200 | 9.9 | 0.9 | 7485 | 47 | 62 | ICP |
| ST+NC (Option B) | 3 | 0 | 22 ‡ | 1004 | 2.4 | 1.8 | 1021 | 25 | 70 | ICP |
| ST+SRBAL (Option A) | 1 | 149 | 12 | 246 | 5.7 | 0.8 | 3243 | 18 | 90 | Survival |
| ST+SRBAL (Option A) | 2 | 59 | 6 ‡ | 867 | 5.8 | 0.9 | 6890 | 33 | 57 | ICP |
| ST+SRBAL (Option A) | 3 | 346 | 11 £ | 359 | 4.6 | 0.8 | 895 | 27 | 90 | Survival |
| ST+SRBAL (Option B) | 1 | 138 | 24 | 321 | 4.8 | 0.8 | 1081 | 11 | 90 | Survival |
| ST+SRBAL (Option B) | 2 | 94 | 24 | 387 | 2.1 | 0.9 | 567 | 15 | 90 | Survival |
| ST+SRBAL (Option B) | 3 | 166 | 24 | 200 | 3.7 | 0.9 | 1203 | 10 | 90 | Survival |
Legend:
indicates treatment duration shortened due to early end point of animal;
indicates treatment duration shortened for technical reason.
Stage 4 HE - non-responsiveness to sternal rub, ear tug, or auditory stimulus
ICP - inability to maintain sternal positioning with an ICP >20 mmHg
Ammonia Detoxification
Plasma levels of ammonia throughout the study are stratified by group and summarized in Fig. 2B. Pigs in the ST+SRBAL group demonstrated stable plasma ammonia levels during the treatment interval compared to the two control groups. At 78 hours (end of all ECT therapies), the average (mean±ST) plasma ammonia level of ST+SRBAL animals was 468±229 µg/dL compared to ST alone (938±325 µg/dL, p=0.018) and ST+No-cell BAL (866±363 µg/dL, p=0.024) groups. Only three animals, ST+SRBAL Option A (n=2) and ST+SRBAL Option B (n=1), had lower plasma levels of ammonia at 78 hours vs. 48 hours. The rate of rise in plasma ammonia between 48 to 78 hours in the ST alone group (+23.6±11.1 µg/dL/hr) and ST+No-cell BAL group (+20.8±13.1 µg/dL/hr) were similar (p=0.70). In contrast, plasma ammonia levels of ST+SRBAL animals rose at a rate of 2.0±7.1 µg/dL/hr (p=0.002, p= 0.011, respectively). Following completion of SRBAL therapy, plasma ammonia levels of treated pigs rose at a rate of 5.1±15.0 µg/dL/hr. Animals in SRBAL treatment Options A and Option B groups both received a significant detoxification benefit of therapy as their plasma ammonia levels remained lower than corresponding control groups throughout the treatment interval (Supplemental Material 3).
Intracranial Pressure and Brain Edema
All animals exhibited an increase in intracranial pressure (ICP) following administration of D-galactosamine. Average ICP was similar in all three groups at T=48 hr, the initiation of therapy (p=0.44). After 48 hours, animals treated with ST+SRBAL Option B demonstrated progressively lower ICP compared to the other groups (Fig. 2C). The final ICP of animals in ST+SRBAL Option B (7.0±2.8mmHg) was significantly lower than ST group (25.0±4.4mmHg, p=0.005). All animals euthanized prior to the 90 hour study endpoint exhibited clinical signs suggestive of cerebral edema and herniation including posturing of the extremities, loss of brainstem reflexes, and a progressive rise in ICP followed by a modest decline. Water content (%) of white matter was determined as a direct measure of brain edema at study endpoint: ST alone (81.76%, n=1), ST+NC therapy (81.90±0.82%, n=4), ST+SRBAL (80.69±1.33%, n=4). Positive correlations were observed between brain %H20 and both final ICP and peak ICP, as shown in Supplemental Material 4. Of animals treated under Option B conditions, significantly lower brain water content was observed with SRBAL therapy (ST+NC, 82.31±0.69% vs. ST+SRBAL, 80.11±0.77%, p=0.033).
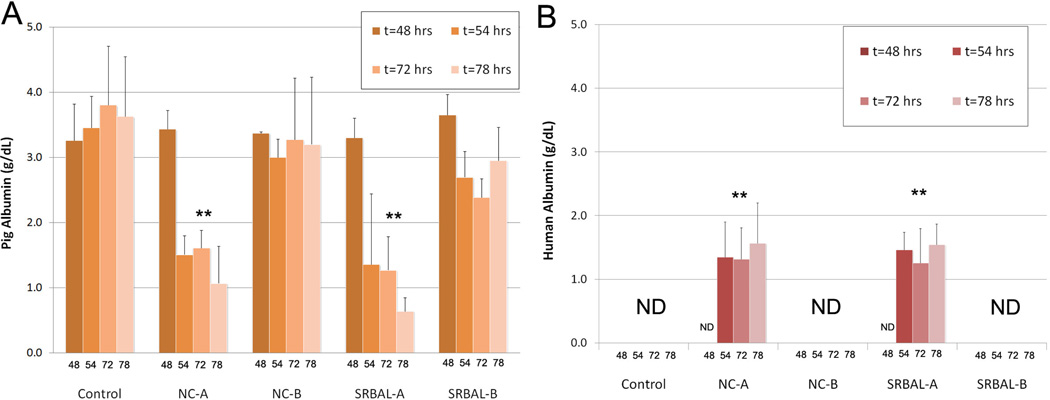
Membrane Porosity and Levels of Plasma Albumin
Plasma samples gathered during therapy were analyzed to quantify the impact of membrane porosity on levels of porcine albumin and human albumin (from the albumin dialysate circuit) during ECT. Figure 3A demonstrates relative stable levels of porcine albumin in no device control animals and animals treated under Option B conditions (65 kD membrane). In contrast, animals treated under Option A conditions (400 kD membrane) experienced a significant decline in plasma levels of porcine albumin after onset of ECT therapy. As expected, significant levels of human albumin crossed the 400 kD membrane from the dialysate circuit to the pig blood during Option A conditions. Levels of human albumin remained <0.1 g/dL through ECT during Option B conditions.
Fig. 3. Porcine and Human Albumin in Pig Plasma.
Panels A – Levels of porcine albumin declined significantly in pig blood during ECT therapy under Option A conditions. Bars equal mean values, error bars equal standard deviation. ** indicates p<0.05 vs. corresponding 48hr time point.
Panels B – Levels of human albumin rose significantly in pig blood during ECT therapy under Option A conditions. Bars equal mean values, error bars equal standard deviation. ** indicates p<0.05 vs. corresponding 48hr time point. ND indicates level less than 0.1g/dL.
Reactive Oxygen Species (ROS) and Pro-Inflammatory Cytokines
Plasma samples were gathered to quantify oxidative stress (ROS) and levels of proinflammatory cytokines (IL1β, IL6, IL18, TNFα) during development of ALF and ECT therapy. Animals in the ECT groups demonstrated a decrease in ROS during the course of therapy while animals in ST alone group had progressive rise in oxidative stress during the same time period (Supplemental Material 5). A rise in levels of three pro-inflammatory cytokines (IL1β, IL6, IL18) was observed during the development of ALF (Supplemental Material 6). However, the magnitude of these levels was wide and did not correlate with outcome or response to therapy. Levels of TNFα were low and generally below the detection limits of the assay.
ALF Liver Histology
As expected, slides of liver tissue stained for H&E and KI-67 differed significantly at baseline (7 days before infusion of hepatotoxin) compared to final endpoint in all groups. No evidence of liver pathology was observed at baseline, while a blinded pathologist identified significant levels of liver injury and mitotic activity by in each of the three ALF groups compared to baseline (p<0.0001, see Table 3). Representative liver histology at normal baseline and ALF endpoints are provided in Fig. 4. All ALF liver tissue demonstrated prominent inflammation. Over 75% of ALF liver specimens showed fatty changes not observed at baseline. Hemorrhagic necrosis of varying levels of severity was observed in all ALF specimens, as well as regenerative hepatocytes, apoptotic hepatocytes, and bile duct proliferation. Zone 3 necrosis was observed in 78% of liver specimens. Diffuse pan-lobular necrosis was observed in 33% of specimens, most often in the ST alone group. The mean level of liver injury was greatest in the ST alone group and least in the SRBAL group (p=0.048). Staining of the regenerative marker Ki67 increased significantly in all three ALF groups compared to baseline measurements (p<0.0001). A trend with direct correlation was observed between Ki67 score and survival duration; however, this trend did not reach statistical significance (p=0.096). Similarly, a trend with indirect correlation was observed between liver injury score and survival duration, and again this trend was not significant (p=0.201).
Table 3.
Liver Histology
| Time Point - Group | Liver Injury Score | Regenerative Index |
|---|---|---|
| Baseline - All Groups | 3.5 ± 1.9 # | 2.4 ± 1.8 # |
| Death – ST alone | 18.5 ± 3.9 | 6.8 ± 6.6 |
| Death – ST+No-cell BAL | 14.8 ± 6.4 | 15.0 ± 11.0 |
| Death – ST+SRBAL | 12.5 ± 5.2 ** | 12.2 ± 10.8 |
Legend
Liver Injury Score of H&E stained sections
Regenerative Index quantified by Ki-67 positive nuclei per 40× high power field.
p<0.0001, measurements at baseline vs. death
p<0.05, measurements at death (ST alone vs. ST+SRBAL)
Fig. 4. Liver Histology.
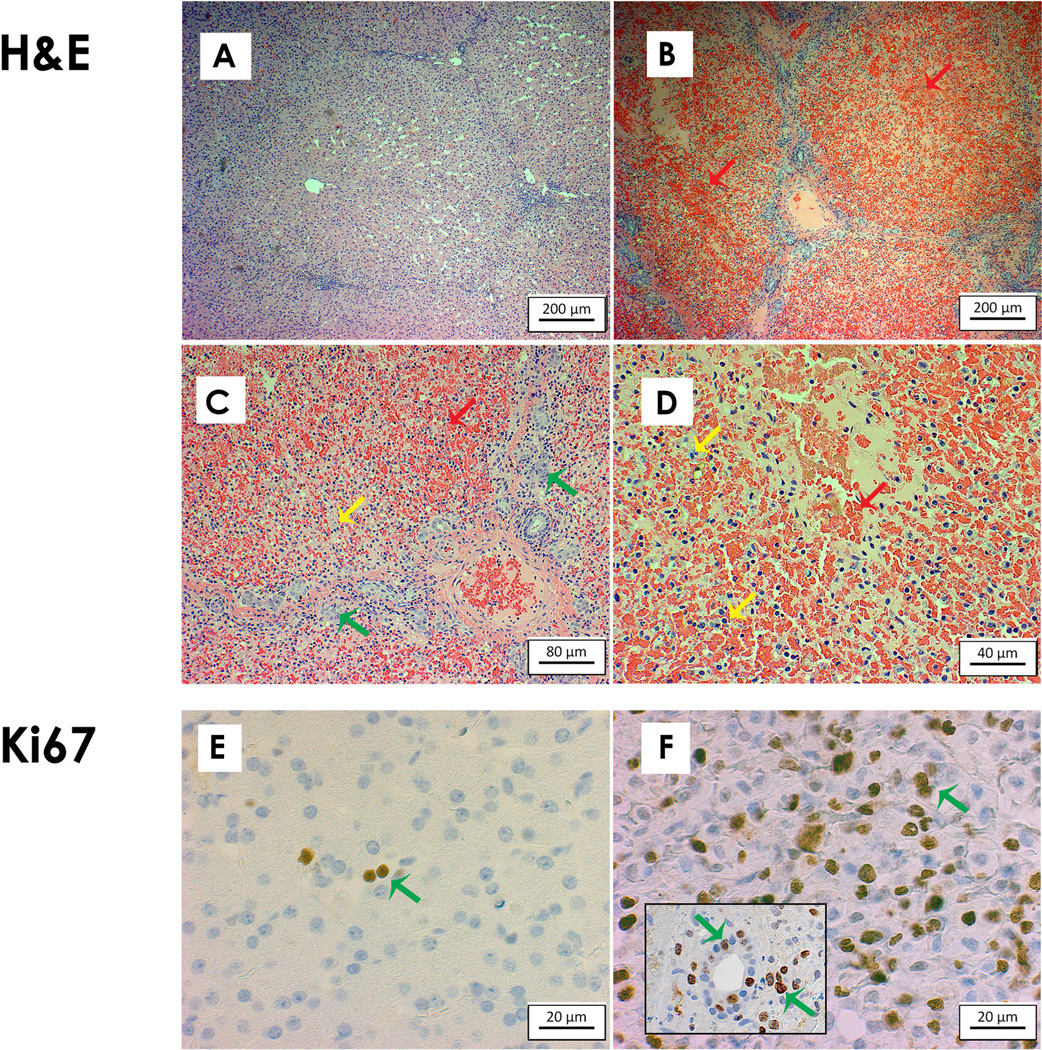
Panels A/E – normal liver at baseline stained with H&E/Ki67.
Panels B/C/D – ALF liver histology stained with H&E at increasing magnification demonstrates extensive hemorrhagic necrosis (red arrows), bile duct proliferation (green arrows), and inflammation (yellow arrows).
Panel F – Ki67 staining was increased from baseline indicating a strong proliferative response (green arrows) of both hepatocytes and bile duct cells (see inset) to acute liver injury.
Scale bars indicate relative size as marked.
Spheroid Inoculum
A total of eight donor pigs (weight range, 13.1–24.4kg) were sacrificed to procure hepatocytes and produce spheroids for eight SRBAL treatments as summarized in Table 4. Hepatocyte viability after all isolations exceeded 90% (96.4±2.9%); inoculated spheroid mass ranged from 59–228 grams (mean ± SD, 119±57g) in these eight SRBAL treatments. Albumin production ranged from 8.7 – 28.3 (17.7±7.7) µg/hr/gm during spheroid formation.
Table 4.
Parameters used to characterize hepatocyte isolation and spheroid formation.
| SRBAL Harvest # (animal.treatment) |
Donor Pig Weight (kg) |
Hepatocyte Viability (%) |
Isolated Hepatocyte Mass (g) |
Average Spheroid Diameter ‡ (µm) |
Spheroid Inoculate Mass (g) |
Spheroid Yield (%) |
|---|---|---|---|---|---|---|
| #1 - Option A(1.1) | 16.3 | 99 | 79 | 75 | 63 | 80% |
| #2 - Option A(1.2) | 16.5 | 98 | 125 | 69 | 86 | 69% |
| #3 - Option A(2.1) | 13.1 | 97 | 173 | 83 | 59 | 34% |
| #4 - Option A(3.1) | 18.0 | 95 | 316 | 59 | 228 | 72% |
| #5 - Option A(3.2) | 19.0 | 90 | 156 | 61 | 118 | 76% |
| #6 - Option B(1.1) | 24.4 | 98 | 306 | 60 | 138 | 45% |
| #7 - Option B(2.1) | 20.8 | 98 | 253 | 90 | 94 | 37% |
| #8 - Option B(3.1) | 22.6 | 96 | 327 | 79 | 166 | 51% |
| Average | 18.8 | 96.4 | 216.9 | 72.0 | 119.0 | 58% |
Spheroid diameter was determined by Coulter counter method immediately prior to inoculation of spheroids into the treatment reservoir.
The six animals treated with the SRBAL were dichotomized based on average spheroid inoculum during treatments. Pigs receiving SRBAL therapy with >100g of hepatocyte spheroids (n=3) had lower peak plasma concentrations of ammonia (384±80 vs. 729±123 µg/dL, p=0.015) compared to three pigs receiving therapy at lower (<100g) cell dose. Ammonia peaks tended to occur later (t=86±7hr vs. 75±16hr, p=0.34) if animals were treated with a higher inoculum of spheroids compared to a lower inoculum. Pigs treated with the larger inoculum of hepatocytes also tended toward lower peak ICP compared to treatments with a lower dose of hepatocytes (13.0±4.4. vs. 25.0±9.2 mmHg, p=0.10). Of pigs undergoing SRBAL therapy, the highest peak ICP was observed at the lowest cell dose (59 gram).
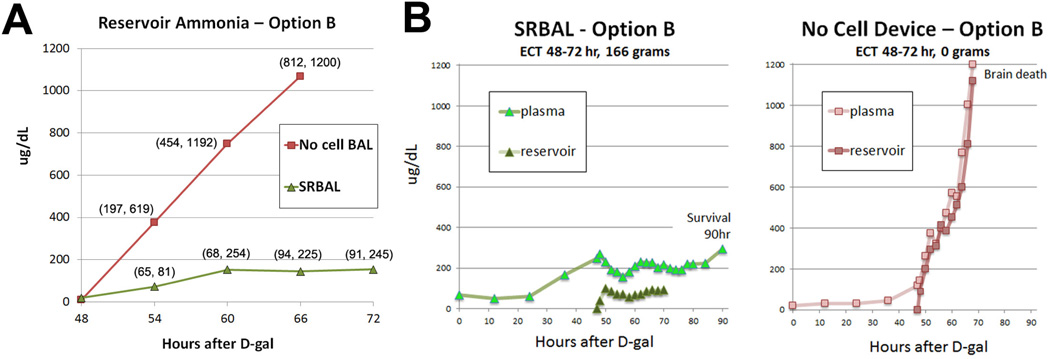
SRBAL Performance
Ammonia levels within the reservoir compartment were measured every 2 hours during ECT. When the reservoir contained hepatocyte spheroids under Option B conditions, the ammonia levels remained stable during ECT, increasing at a slow rate of 4.4 µg/dL/hr from 48 to 72 hr (Fig. 5A). In contrast, when no cells were present in the reservoir under Option B conditions, ammonia levels rose at a rapid rate of 57.5 µg/dL/hr (p=0.02) during ECT. The impact of hepatocyte spheroids during ECT was most obvious when concentrations of plasma ammonia and reservoir ammonia, obtained simultaneously, were compared under similar time frames (Figure 5B). Functionality of hepatocyte spheroids was also assessed by measuring oxygen consumption within the reservoir during ECT. The oxygen consumption rate by spheroid hepatocytes remained stable throughout the course of SRBAL treatments with a mean (±SD) starting value of 4.59(±2.24) mmolO2/hr vs. 4.77(±2.33) mmolO2/hr at the end of SRBAL therapy.
Fig. 5. Ammonia Metabolism by Hepatocyte Spheroids.
Panel A – Ammonia levels in the reservoir compartment during ECT – Option B conditions. Rate of ammonia detoxification was increased significantly (p=0.003) by spheroid hepatocytes in SRBAL compared to No-cell conditions (Option B conditions, n=3 treatments per group). Markers indicate median values (min, max) at each time point.
Panel B –Plasma and reservoir levels of ammonia, obtained simultaneously, from representative animals treated under cellular (SRBAL Option B, left) and no cell (ST+No cell Device, right) device configurations. Ammonia concentration remained stable in the reservoir of the device loaded with 166 grams of hepatocyte spheroids and the plasma of its treatment animal. In contrast, under the same time frame and similar starting conditions at t=48hrs, ammonia concentrations rose sharply in the no-cell reservoir and plasma of its treatment animal until reaching a study endpoint (brain death). Both devices started treatment with minimal detectable ammonia in the reservoir circuit (t=48hr).
Functional assessment - Post SRBAL therapy
Following therapy, the reservoir and AD circuit were pumped and media was sampled from the reservoir to evaluate ammonia clearance by spheroid hepatocytes in the absence of ammonia influx from ALF pigs. All concentrations of ammonia declined within the spheroid reservoir during post-treatment interval. The median rate of ammonia clearance was 449 µg/hr, but ranged from 37 µg/hr (corresponding to the lowest inoculated cell dose of 59g) to a maximum clearance of 903 µg/hr.
Discussion
The RALF study was designed as a preclinical pivotal evaluation of the SRBAL device in a porcine model of ALF. Placement of a tunneled dialysis catheter and implantation of an ICP catheter one week prior to administration of a potent hepatotoxin, D-galactosamine, allowed us to carefully study the relationship of plasma ammonia to cerebral edema formation in an ambulatory model of ALF, and in the setting of ECT. More importantly, the RALF study is novel in successfully establishing efficacy of primary mammalian hepatocytes in the treatment of ALF in terms of the hyperammonemia-cerebral edema-brain herniation axis. All animals enrolled in this study were housed in general swine housing and allowed free access to nutrition, water, and ambulation. Light sedation was only used for measurement of ICP and during ECT. Furthermore, the implantable ICP device allowed for continuous monitoring of the animal’s ICP, an indirect but accurate measurement of brain swelling, without need for invasive interventions. After sacrifice, a direct correlation was observed between percent water content of fresh brain and both peak and final ICP.
Five scientific questions were posed in designing the RALF study. Our first two questions, regarding the animal model and the role of SRBAL therapy in preventing the hyperammonemia-cerebral edema-brain herniation axis of ALF, were answered convincingly. Namely, SRBAL therapy improved the survival of ALF pigs by detoxifying waste molecules, including ammonia, and ultimately by preventing cerebral herniation and brain death. ROS and representative pro-inflammatory cytokines were observed to rise during progression of ALF, but these markers were not predictive of outcome. Our data suggests that hyperammonemia plays a strong role in the pathogenesis of brain edema in a porcine model of drug-overdose ALF; but the interaction between ammonia, inflammation, and oxidative stress requires further study.
A third question addressed the importance of intermittent vs. continuous SRBAL therapy in severe ALF. Animals undergoing continuous SRBAL therapy received greater benefit than those undergoing intermittent therapy as evidenced by lower peak ICP and lower final ICP measurements. As a result, we intend to employ continuous therapy into the design of future clinical trials of SRBAL therapy. Though not evaluated in the current study, the spheroid reservoir configuration has been designed to easily allow exchange of a new inoculum of hepatocyte spheroids if their metabolic activity declines or change is required for some other reason.
Our fourth study question was in regards to influence of cell dose on efficacy of SRBAL therapy. A prior randomized study in a porcine devascularization model of ALF suggested benefit of acellular (albumin dialysis) therapy in attenuating the rise in intracranial pressure without significant alteration in levels of blood ammonia (31). In contrast, the neuroprotective benefit of SRBAL therapy was impacted by the inoculum of hepatocyte spheroids, especially hepatocytes from donor pigs fed a high protein diet. The beneficial effect of dietary protein on ureagenesis of donor pig hepatocytes is consistent with prior observations in rats (32) and humans (33). Of note, the single early death within the SRBAL treatment group indicated that a low hepatocyte cell dose of 59 grams was insufficient to support an ALF pig with severe liver injury and advanced hepatic encephalopathy at the onset of therapy. Doses of hepatocyte spheroids exceeding 200 gm per treatment will be evaluated in future clinical trials.
Question 5 addressed the influence of membrane porosity on efficacy of SRBAL therapy. Two membrane options were evaluated. Option A used a relatively large pore membrane (400kD nMWCO) for blood separation, while Option B used a tighter membrane with 65 kD nMWCO. These two options were selected since membranes of similar porosity are components of cell-free artificial liver support systems in current clinical practice (4, 34). Based on our prior bench studies (35), we expected that Option A would facilitate greater detoxification and therefore less time would be required for treatment than Option B. We observed similar rates of ammonia detoxification by SRBAL Options A and B. However, animals treated under Option A required more fluids during treatment and these animals were more prone to elevated ICP after therapy, possibly due to albumin shifts across the larger membrane. The one premature death in the SRBAL group was a brain herniation occurring shortly after the animal’s first treatment with the larger pore membrane (Option A). In contrast, all three SRBAL pigs treated under Option B conditions remained both hemodynamically and neurologically stable and successfully reached the 90 hour time point. Another advantage of the tighter membrane was the significant cost savings of using a lower concentration of albumin (0.5g/dL vs. 5.0g/dL) in the AD circuit. For these multiple reasons, future clinical studies will be conducted using SRBAL configured to Option B.
Significant levels of liver injury were confirmed at post mortem examination after bolus administration of 0.75 mg/kg D-galactosamine. This dose of drug resulted in severe liver injury in all animals and signs of liver regeneration in most animals. In fact, liver histology and improved ammonia detoxification after SRBAL therapy suggest that the native liver was capable of recovery. A single 24-hour treatment and 90 hours of follow-up were insufficient to carefully address this possibility. The mean rise of 13% in oxygen consumption suggested that the ALF milieu was not cytotoxic to hepatocyte spheroids under study conditions. Longer duration of both therapy and follow-up are needed and will be major areas of focus in future trials of SRBAL therapy.
At least two significant challenges were overcome to achieve successful completion of the RALF study. The first challenge involved the biologic variability of pigs in the response to hepatotoxin and the response of hepatocytes in donor livers to collagenase digestion followed by spheroid formation. For example, peak AST of study pigs ranged from 567 to 7485 U/L and two other pigs were excluded as they had already reached a study endpoint before 48 hours. This variability was addressed in part by the prospective randomized design of the RALF study. The variability in spheroid yield, which ranged from 59 to 228 grams per harvest, likely contributed to the single death in the SRBAL treatment group after a 6-hr treatment of only 59 grams of cells. Low cell yields were not related to technical problems as our methods are well developed. Rather, lot-to-lot variability in collagenase activity was a problem that is known to impact harvest cell yield and spheroid formation. To address this problem in future clinical applications of the SRBAL device, we intend to purchase large lots of collagenase and form hepatocyte spheroids from multiple donor livers to ensure that reservoir inoculations meet minimum cutoffs. Once in the SRBAL reservoir, spheroid functionality was stable for at least 24 hours.
The second challenge involved the expense of studies, both in terms of manpower and finances. Our 90-hour endpoint was selected for a practical reason. Namely, D-galactosamine was administered on Monday and animals were followed around the clock until Friday (90 hours) per our IACUC guidelines. Because of the significant expenses of large animal studies, Option A and Option B conditions were limited to three animals each and multiple variables were combined per Option. Fortunately, the benefits of SRBAL therapy proved significant and answers to our five research questions were possible.
In summary, the SRBAL represents a promising new treatment of ALF. Specifically, primary porcine hepatocytes cultured as spheroid aggregates offer both a readily available supply of donor cells and a sufficiently large cell dose for the treatment of ALF in large animals and humans. Unlike many previous cell-based therapies, the SRBAL maintains functionality of primary hepatocytes in the range of normal liver physiology, even at the high cell density required for human therapy. In particular, SRBAL demonstrated ammonia clearance at vastly higher levels than have been reported by earlier generations of BAL devices. Further randomized clinical investigation of SRBAL therapy appears warranted to determine whether its higher functionality provides a significant therapeutic benefit to individuals with ALF, ideally avoiding the need for liver transplantation.
Supplementary Material
Summary.
The RALF study is the first study of its kind to successfully establish efficacy of a liver support device composed of primary mammalian hepatocytes in a large animal recovery model of acute liver failure and in terms of the hyperammonemia-cerebral edema-brain herniation axis.
Acknowledgments
Covidien generously provided the Palindrome™ dual lumen dialysis catheters as a gift. Raumedic generously loaned us the telemetry device for ambulatory ICP monitoring. Mayo Division of Engineering and RanD-Biotech (Medolla, Italy) provided technical and manufacturing support of the ECT used in these studies. We thank Ibrahim Waaeys and Geoffrey Gerardin for expert care of ALF animals.
Funding: Financial support of this research was provided by NIH (RO1 DK056733, RO1 DK056733-S1, RO1 DK056733-S2), Wallace H. Coulter Foundation, Marriot Foundation, Darwin Deason Family Foundation, and Mayo Foundation.
Abbreviations
- AD
albumin dialysate
- ALF
acute liver failure
- BAL
bioartificial liver
- ECT
extracorporeal therapy
- ICP
intracranial pressure
- RALF
recovery acute liver failure
- ROS
reactive oxygen species
- SRBAL
spheroid reservoir bioartificial liver
- ST
standard therapy
- NC
No-cell
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: SLN is the inventor of two issued U.S. patents related to the SRBAL (#7,160,719; #8,785,117). Mayo Foundation owns the rights to these patents. Neither patent has been licensed.
Author contributions
JMG assisted in conducting experiments, collecting data and writing the manuscript.
SAM assisted in conducting experiments, collecting data and writing the manuscript.
BR assisted in conducting experiments and collecting data.
TM performed the blinded examination of liver pathology and assisted in writing the manuscript.
WKK performed the statistical analysis of study data and assisted in writing the manuscript.
FE analyzed blood samples (albumin analysis) and writing the manuscript.
RDH analyzed blood samples (cytokine analysis) and writing the manuscript.
BA assisted in study design, conducting experiments, collecting data and writing the manuscript.
HH assisted in conducting experiments, collecting data, and writing the manuscript.
CFR analyzed blood samples (ROS assay) and writing the manuscript.
SLN study design, supervised experiments, collected data, assisted in writing the manuscript.
References
- 1.Lee WM, Squires RH, Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008;47:1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kjaergard L, Liu J, Als-Nielsen B, al e. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA. 2003;289:217–222. doi: 10.1001/jama.289.2.217. [DOI] [PubMed] [Google Scholar]
- 3.Demetriou AA, Brown RS, Jr, Busuttil RW, Fair J, McGuire BM, Rosenthal P, Am Esch JS, 2nd, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239:660–667. doi: 10.1097/01.sla.0000124298.74199.e5. discussion 667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saliba F, Camus C, Durand F, Mathurin P, Letierce A, Delafosse B, Barange K, et al. Albumin dialysis with a noncell artificial liver support device in patients with acute liver failure: a randomized controlled trial. Ann Intern Med. 2013;159:522–531. doi: 10.7326/0003-4819-159-8-201310150-00005. [DOI] [PubMed] [Google Scholar]
- 5.Yu Y, Fisher JE, Lillegard JB, Rodysill B, Amiot B, Nyberg SL. Cell therapies for liver diseases. Liver transplantation. 2012;18:9–21. doi: 10.1002/lt.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly J, Dalington GJ. Modulation of liver specific phenotype in the human hepatoblastoma line Hep G2. In Vitro Cell Dev Biol. 1989;25:217–222. doi: 10.1007/BF02626182. [DOI] [PubMed] [Google Scholar]
- 7.Sauer IM, Zeilinger K, Obermayer N, Pless G, Grunwald A, Pascher A, Mieder T, et al. Primary human liver cells as source for modular extracorporeal liver support--a preliminary report. Int J Artif Organs. 2002;25:1001–1005. doi: 10.1177/039139880202501015. [DOI] [PubMed] [Google Scholar]
- 8.Sauer I, Kardassis D, Zeillinger K, Pascher A, Gruenwald A, Pless G, Irgang M, et al. Clinical extracorporeal hybrid liver support - phase 1 study with primary porcine liver cells. Xenotransplantation. 2003;10:460–469. doi: 10.1034/j.1399-3089.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 9.Knowles B, Howe C, Aden D. Human hepatocellular carcinoma cell lines sectrete the major plasma proteins and Hepatitis B surface antigen. Science. 1980;209:497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- 10.Nyberg SL, Remmel RP, Mann HJ, Peshwa MV, Hu WS, Cerra FB. Primary hepatocytes outperform Hep G2 cells as the source of biotransformation functions in a bioartificial liver. Ann Surg. 1994;220:59–67. [PMC free article] [PubMed] [Google Scholar]
- 11.Mavri-Damelin D, Damelin LH, Eaton S, Rees M, Selden C, Hodgson HJ. Cells for bioartificial liver devices: the human hepatoma-derived cell line C3A produces urea but does not detoxify ammonia. Biotechnol Bioeng. 2008;99:644–651. doi: 10.1002/bit.21599. [DOI] [PubMed] [Google Scholar]
- 12.Mavri-Damelin D, Eaton S, Damelin LH, Rees M, Hodgson HJ, Selden C. Ornithine transcarbamylase and arginase I deficiency are responsible for diminished urea cycle function in the human hepatoblastoma cell line HepG2. Int J Biochem Cell Biol. 2007;39:555–564. doi: 10.1016/j.biocel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Clemmesen J, Larsen F, Kondrug J, Hansen B, Ott P. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology. 1999;29:648–653. doi: 10.1002/hep.510290309. [DOI] [PubMed] [Google Scholar]
- 14.Paradis K, Langjord G, Long Z, Heneine W, Sandstrom P, Switzer W, Chapman L, et al. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. Science. 1999;285:1236–1241. doi: 10.1126/science.285.5431.1236. [DOI] [PubMed] [Google Scholar]
- 15.Onions DE, Witt CJ. Xenotransplantation: an overview of microbiological risks and potentials for risk management. Rev Sci Tech. 2000;19:289–301. doi: 10.20506/rst.19.1.1216. [DOI] [PubMed] [Google Scholar]
- 16.Nyberg SL, Hibbs JR, Hardin JA, Germer JJ, Persing DH. Transfer of porcine endogenous retrovirus across hollow fiber membranes: significance to a bioartificial liver. Transplantation. 1999;67:1251–1255. doi: 10.1097/00007890-199905150-00009. [DOI] [PubMed] [Google Scholar]
- 17.Morsiani E, Pazzi P, Puviani A, Brogli M, Valieri L, Gorini P, Scoletta P, et al. Early experience with a porcine hepatocyte-based bioartificial liver in acute hepatic failure patients. Int J Art Organs. 2002;25:192–202. doi: 10.1177/039139880202500305. [DOI] [PubMed] [Google Scholar]
- 18.Nyberg SL, Yagi T, Matsushita T, Hardin J, Grande JP, Gibson LE, Platt JL. Membrane barrier of a porcine hepatocyte bioartificial liver. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2003;9:298–305. doi: 10.1053/jlts.2003.50024. [DOI] [PubMed] [Google Scholar]
- 19.Matsushita T, Amiot B, Hardin J, Platt JL, Nyberg SL. Membrane pore size impacts performance of a xenogeneic bioartificial liver. Transplantation. 2003;76:1299–1305. doi: 10.1097/01.TP.0000080067.79190.3C. [DOI] [PubMed] [Google Scholar]
- 20.Landry J, Bernier D, Ouellet C, Goyette R, Marceau N. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;101:914–923. doi: 10.1083/jcb.101.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luebke-Wheeler J, Yee L, Nedredal G, Amiot B, Nyberg S. E-cadherin protects primary hepatocytes spheroids from cell death by caspase-independent mechanism. Cell Transplantation. 2010;18:1281–1287. doi: 10.3727/096368909X474258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu-Absi S, Friend J, Hansen L, Hu W-S. Structural polarity and functional bile canaliculi in rat hepatocytes spheroids. Exp Cell Res. 2002;274:56–67. doi: 10.1006/excr.2001.5467. [DOI] [PubMed] [Google Scholar]
- 23.Chiarugi P, Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76:1352–1364. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Brophy CM, Luebke-Wheeler JL, Amiot BP, Khan H, Remmel RP, Rinaldo P, Nyberg SL. Rat hepatocyte spheroids formed by rocked technique maintain differentiated hepatocyte gene expression and function. Hepatology. 2009;49:578–586. doi: 10.1002/hep.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyberg SL, Hardin J, Amiot B, Argikar UA, Remmel RP, Rinaldo P. Rapid, large-scale formation of porcine hepatocyte spheroids in a novel spheroid reservoir bioartificial liver. Liver Transpl. 2005;11:901–910. doi: 10.1002/lt.20446. [DOI] [PubMed] [Google Scholar]
- 26.Ho D, Fan S, Woo Y, Zhang Z, Lau C, Wong J. Selective plasma filtration for treatment of fulminant hepatic failure induced by D-galactosamine in a pig model. Gut. 2002;50:869–876. doi: 10.1136/gut.50.6.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marmarou A, Poll W, Shulman K, Bhagavan H. A simple gravimetric technique for measurement of cerebral edema. J Neurosurg. 1978;49:530–537. doi: 10.3171/jns.1978.49.4.0530. [DOI] [PubMed] [Google Scholar]
- 28.Sielaff TD, Hu MY, Sridhar R, Groehler K, Olson D, Mann HJ, Remmel RP, et al. A technique for porcine hepatocyte harvest and description of differentiated metabolic functions in static culture. Transplantation. 1995;59:1459–1463. doi: 10.1097/00007890-199505270-00017. [DOI] [PubMed] [Google Scholar]
- 29.Lillegard JB, Fisher JE, Nedredal G, Luebke-Wheeler J, Bao J, Wang W, Amoit B, et al. Normal atmospheric oxygen tension and the use of antioxidants improve hepatocyte spheroid viability and function. J Cell Physiol. 2011;226:2987–2996. doi: 10.1002/jcp.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcus R, Peritz E, Gabriel K. On closed testing procedures with special reference to ordered analyses of variance. Biometrika. 1976;63:655–660. [Google Scholar]
- 31.Sen S, Rose C, Ytrebo L, Davies N, Nedredal G, Drevland S, Kjonno M, et al. Effect of albumin dialysis on intracranial pressure in pigs with acute liver failure: a randomized study. Crit Care Med. 2006;34:158–164. doi: 10.1097/01.ccm.0000196203.39832.3c. [DOI] [PubMed] [Google Scholar]
- 32.Petersen K, Vilstrup H, Tygstrup N. Effect of dietary protein on the capacity of urea synthesis in rats. Hormone Metab Res. 1990;22:612–615. doi: 10.1055/s-2007-1004985. [DOI] [PubMed] [Google Scholar]
- 33.Hamberg O, Nielson K, Vilstrup H. Effects of increase in protein intake on hepatic efficacy for urea synthesis in healthy subjects and in patients with cirrhosis. J Hepat. 1992;14:237–143. doi: 10.1016/0168-8278(92)90164-k. [DOI] [PubMed] [Google Scholar]
- 34.Kribben A, Gerken G, Haag S, Herget-Rosenthal S, Treichel U, Betz C, Sarrazin C, et al. Effects of Fractionated Plasma Separation and Adsorption on survival in patients with Acute-on-Chronic Liver Failure. Gastroenterology. 2012;142:782–789. doi: 10.1053/j.gastro.2011.12.056. [DOI] [PubMed] [Google Scholar]
- 35.Nedredal GI, Amiot BP, Nyberg P, Luebke-Wheeler J, Lillegard JB, McKenzie TJ, Nyberg SL. Optimization of mass transfer for toxin removal and immunoprotection of hepatocytes in a bioartificial liver. Biotechnol Bioeng. 2009;104:995–1003. doi: 10.1002/bit.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.