Abstract
Background
Quality of the parent-child relationship is a robust predictor of behavioral and emotional health for children and adolescents; the application to physical health is less clear.
Methods
We investigated the links between observed parent-child relationship quality in an interaction task and antibody response to meningococcal conjugate vaccine in a longitudinal study of 164 ambulatory 10-11 year-old children; additional analyses examine associations with cortisol reactivity, BMI, and somatic illness.
Results
Observed negative/conflict behavior in the interaction task predicted a less robust antibody response to meningococcal serotype C vaccine in the child over a 6 month-period, after controlling for socio-economic and other covariates. Observer rated interaction conflict also predicted increased cortisol reactivity following the interaction task and higher BMI, but these factors did not account for the link between relationship quality and antibody response.
Conclusions
The results begin to document the degree to which a major source of child stress exposure, parent-child relationship conflict, is associated with altered immune system development in children, and may constitute an important public health consideration.
Keywords: parent-child interaction, observation, antibody response, meningococcal conjugate vaccine, stress
Decades of research document reliable links between parent-child relationship quality and an array of short- and long-term behavioral health outcomes in children, including psychiatric symptoms, relationship quality with peers, and educational and occupational success (Cui, Durtschi, Donnellan, Lorenz, & Conger, 2010; Davidov & Grusec, 2006; DeGarmo, Forgatch, & Martinez, 1999; O'Connor, 2002; Patterson, Forgatch, & Degarmo, 2010). These findings have shaped the development and dissemination of interventions to improve parent-child relationship quality (Scott et al., 2010; Webster-Stratton, Reid, & Hammond, 2004). The extent to which parent-child relationship quality – and interventions that target parent-child relationship quality – might also influence children's physical health is much less clear. Research of that kind is needed to identify potential sources of the wide variation in children's physical health, and to evaluate the broader clinical and public health implications of family-focused interventions. The current study of a diverse sample of pre-adolescent children contributes to this line of research by assessing the links between observed parent-child relationship quality and antibody response to immunization; additional analyses examine other biomarkers of health that are important outcomes and potential mediators of the antibody response, namely, cortisol reactivity, BMI, and somatic illness.
The hypothesis that (early) stress exposure compromises the function of multiple biological systems and may lead, in some cases, to illness is supported by numerous animal investigations (Lubach, Coe, & Ershler, 1995) and studies of adults (Lehman, Taylor, Kiefe, & Seeman, 2009; Taylor, Lehman, Kiefe, & Seeman, 2006). These findings converge with the well-documented social class gradients of health, in which social class, used as a proxy for stress exposure, predicts adult health outcomes (Cohen, Doyle, & Baum, 2006; Marmot, Ryff, Bumpass, Shipley, & Marks, 1997) and support further studies of mediating mechanisms, such as inflammation and stress physiology (Cohen et al., 2012; McEwen, 2012). Significantly, it is not yet clear if stress exposure has reliable effects on children's somatic health, i.e., if there are childhood-onset effects on health associated with stress exposure. On the one hand, analyses of large-scale studies such as the National Health Interview Survey (Chen, Martin, & Matthews, 2006) indicate that parental reports of children's general health problems are more common in low social class strata. Somewhat stronger evidence derives from studies associating specific measures of stress with measured health biomarkers, including documented somatic illness (Caserta et al., 2008; Caserta, Wyman, Wang, Moynihan, & O'Connor, 2011; Chen, Cohen, & Miller, 2010; Evans & Kim, 2007; Liang, Jemerin, Tschann, Wara, & Boyce, 1997). Nonetheless, this area of study is at a nascent stage, as illustrated by the limited and inconsistent evidence so far reported, and the diversity in research methods employed (Broyles et al., 2012; Mills, Scott, Wray, Cohen-Woods, & Baune, 2013; O'Connor, Moynihan, & Caserta, 2013; Slopen, Koenen, & Kubzansky, 2012). Further research in this area is needed to clarify the nature of the effects, establish mechanisms of effect, and consider the applications to prevention and intervention.
In the current study we measure child stress exposure based on observed parent-child relationship quality for three reasons. First, a wealth of research indicates that parent-child relationship quality is among the most reliable predictors of behavioral health and mediates effects of distal risks, such as economic stress and family transitions (Burchinal, Vernon-Feagans, & Cox, 2008; Conger, Ge, Elder, Lorenz, & Simons, 1994; Hetherington, Bridges, & Insabella, 1998; Simons, Lin, Gordon, Conger, & Lorenz, 1999). Second, if parent-child relationship quality did reliably predict childhood-onset health problems, then existing parenting and family interventions (Sanders, Markie-Dadds, Tully, & Bor, 2000; Scott, Briskman, & O'Connor, 2014; Webster-Stratton, Hollinsworth, & Kolpacoff, 1989) might provide one strategy for promoting children's overall health. Third, experimental animal studies suggest that the caregiving environment may regulate multiple biological systems in the offspring, including aspects of immunology (Coe, Lubach, Schneider, Dierschke, & Ershler, 1992; Hofer, 1994; M. Laudenslager, Capitanio, & Reite, 1985; Levine, Franklin, & Gonzalez, 1984); these findings warrant concerted translational research.
Our primary health outcome focus is immune system function, and more specifically antibody response to immunization. Our study design capitalized on the natural experiment of immunization to examine parent-child relationship quality as a predictor of antibody response to the quadrivalent meningococcal polysaccharide-protein conjugate vaccine (MCV4). The meningococcal conjugate vaccine is now routinely administered at approximately 11 years of age in the US. We assessed parent-child relationship quality using observer reports of conflict and warmth/support in a moderately stressful problem-solving interaction as a predictor of subsequent antibody response following immunization.
Predicting antibody response to vaccine is an established experimental paradigm in animal (M. L. Laudenslager et al., 1988) and adult psychoneuroimmunology research (Glaser, Sheridan, Malarkey, MacCallum, & Kiecolt-Glaser, 2000). For example, Glaser and colleagues (Glaser et al., 2000) showed that a high-stress group, caregivers of spouses with dementia, showed a more rapid 6-month decline in antibody concentrations to pneumococcal polysaccharide vaccine compared with those not currently caring for a spouse with dementia. Studies of adults have also demonstrated that several psychological factors, such as stress and negative affect, may alter immune response to other immunizations, such as hepatitis B vaccine (Marsland, Cohen, Rabin, & Manuck, 2001). Evidence that a history of stress exposure may compromise antibody response in children and adolescents is much more limited, and is so far based on quite diverse measures of risk exposure (Boyce et al., 1995; O'Connor, Winter, et al., 2013). We provide one of the first longitudinal studies to assess if quality of the parent-child relationship, as a key marker of child stress, predicts subsequent antibody response to vaccine at 4 weeks, 3 months, and 6 months post-vaccination.
Factors that may predict antibody response that may also be associated with parent-child relationship quality were also investigated. One of these was cortisol reactivity, which was assessed in response to the parent-child problem-solving stress paradigm. Cortisol, a steroid hormone that is the product of the hypothalamic-pituitary-adrenal (HPA) axis, plays a major role in stress regulation and metabolism. The relevance for the current study is that cortisol has well-known dynamic influences on multiple aspects of immune function (Vedhara et al., 1999), including in pediatric samples (Boyce et al., 1995). Additionally, cortisol reactivity has been incorporated in numerous developmental studies of child stress exposure and is perhaps the most frequently assessed biomarker in studies of parent-child relationship quality in young children (Hertsgaard, Gunnar, Erickson, & Nachmias, 1995; Spangler & Grossmann, 1993) and adolescents (Kobak, Zajac, & Levine, 2009; Spies, Margolin, Susman, & Gordis, 2011); furthermore, it is considered in many health composites (Matthews & Gallo, 2011), such as allostatic load (Evans & Kim, 2012; Rogosch, Dackis, & Cicchetti, 2011). We investigate the degree to which associations between parent-child relationship quality and antibody production may be mediated by alterations in cortisol production associated with parent-child relationship quality. Second, we examine BMI given the sizable research linking BMI to antibody production in both adults (Sheridan et al., 2012) and children (Halsey et al., 1999), and the links between parent-child and family relationship quality and child BMI (Anderson, Gooze, Lemeshow, & Whitaker, 2012; Evans, Fuller-Rowell, & Doan, 2012; Parks et al., 2012; Stenhammar et al., 2010; Wu, Dixon, Dalton, Tudiver, & Liu, 2011). That is, like cortisol, BMI may be an important mediator of an association between parent-child relationship quality and antibody response; we test this hypothesis in the current study. Finally, we begin to consider specific clinical applications of antibody response by assessing a further index of immune function, physical illness. Illness was determined by active surveillance for an acute illness via biweekly phone calls and illness visits, an approach with methodological advantages over parental reports of general health. In a sample of 5-10 year-olds, Caserta (Caserta et al., 2008) found that more frequent physical illness in younger children was predicted from parental psychiatric symptoms, an indirect index of caregiving stress. We expand this work by tracking illnesses over a 12-month period as predicted from observer reports of interaction quality of parents and children to the problem-solving stress paradigm.
In summary, the current study contributes to existing research by assessing observations of parent-child interactions, widely considered a measurement “gold standard” in developmental and clinical research, and their impact on vaccine response and other markers of children's physical health. Prior research in adults suggested that stress exposure, indexed by conflicted/negative parent-child relationship quality, would be associated with weaker vaccine responses, although no directly relevant pediatric data are available.
Material and methods
Sample and procedure
Children 9-10 years of age and a primary caregiver (in 89% the biological mother) were recruited by an invitation letter from three pediatric practices serving diverse populations in a medium-sized US city; the intent was to oversample families at high psychosocial risk. Interested families were subsequently screened by phone for eligibility. Children were eligible if they were free of chronic illness affecting immune function and were able to complete the research measures in English. Given the study's focus on vaccine responses, the assessment protocol was devised according to the standard US immunization schedule. Specifically, children were recruited 12 months prior to the immunization target age of 11 years in order to obtain pre-vaccination or baseline data. Baseline demographic data for the current study were collected 12 months pre-vaccination; observations of parent-child interactions were collected 6 months prior to vaccination; blood samples to assess the antibody response were collected just prior to vaccination and at approximately 4 weeks, 3 months, and 6 months following vaccination. Vaccine was administered by the child's primary care provider and was not part of this protocol. Of the 188 families who started the study, observational data on parent-child interactions were collected on 165 families; a smaller number of children were included in analyses of antibody response (see vaccine results below). All research visits took place in a hospital clinical research center. For each visit a research nurse collected blood from the child from venipuncture; this typically occurred early in the course of the visit after an acclamation period to the clinic and low stress or physical activity (completing questionnaires). Families were compensated for each visit. The study was approved by the local institutional review board; parents provided written informed consent and children provided written assent to participate.
Measures
Observed parent-child relationship quality
We employed an observational assessment paradigm that has been used and validated in numerous studies of diverse samples of families (Conger et al., 1992; Hagan, Hollier, O'Connor, & Eisenberg, 1992; Joseph, O'Connor, Briskman, Maughan, & Scott, 2014; Kim, Hetherington, & Reiss, 1999; Simons et al., 1999). Following a 5-minute warm-up planning task, the parent and child were observed in a 10-minute moderately stressful “hot topic” problem-solving interaction in which they were asked to discuss and resolve two specific topics about which they disagreed (one nominated from each partner from a standard list, e.g., curfew, homework, chores). The study nurse was not in the room during the videotaped interaction. Parent and child behaviors were subsequently coded from videotape using an established global coding system (Hagan et al., 1992; Hetherington, 1999) by trained research assistants who were blind to all study data. The 5-point Likert scales were: Warmth/Support; Assertiveness; Involvement; Communication; Anger/Rejection; and Coercion. Three coders with at least a bachelor's degree in psychology were trained in the coding system by an experienced coder until they reached acceptable reliability on each scale. Twenty-five percent of the videotapes were coded by multiple coders for reliability; reliability was based on the intraclass correlation (ICC). Consistent with prior research, the main interaction variables identified a clear 2-factor solution, indexing a Positive/Warmth dimension defined by warmth/support (ICC=.84), communication (ICC=.90), assertiveness (ICC=.62), and involvement (ICC=.87). Internal consistency for the Positive/Warmth scale, based on the alpha, was .91. The Negative/Conflict dimension was defined by anger/rejection (ICC = .90) and coercion (ICC= .87); the internal consistency for this dimension was .93. Because of a technical problem, behavioral codes for the problem-solving interaction were not available for one family.
Meningococcal serogroup specific antibody responses.
We assessed antibody responses to the quadrivalent meningococcal conjugate vaccine (Menactra, Sanofi Pasteur) administered at age 11 years, approximately 6 months following the observational assessment. Because of our interest in assessing peak antibody response (evident at 3-6 weeks) and decay in antibody response, measurement of antibody concentrations were planned for prior to and approximately 4 weeks, 3 months, and 6 months following routine vaccination; cases in which pre-vaccination samples were not obtained or initial antibody concentration was not obtained within a defined window were excluded (see below). Antibody responses were determined by ELISA as previously described (Gheesling et al., 1994; Sikkema et al., 2000), with modifications as per (Sikkema et al., 2000). Briefly, 96-well medium-binding EIA plates (COSTAR #3591, Fisher, Suwanee, GA) were coated with meningococcal polysaccharide serogroups A, C, W-135 or Y with methylated human serum albumin (mHSA) (National Institute for Biological Standards and Control, Hertfordshire, UK) in pyrogen free phosphate buffered saline (PBS). Final concentrations of the polysaccharide and mHSA were optimized for the plate lot based on box titrations. Eleven serial dilutions of anti-meningococcal human reference serum CDC 1992 (National Institute for Biological Standards and Control, Hertfordshire, UK) starting at 0.36ug/ml IgG antibodies for each serogroup were used to create a standard curve on each plate. Four dilutions of subject serum sample starting at 1:50 or 1:100 were added to the plates, washed and incubated at room temperature for 2 hours with 100ul per well goat anti-human IgG- alkaline phosphatase conjugate (Thermo Pierce -Fisher, Suwanee, GA). Plates were again washed, the reactions were developed with p-nitrophenyl phosphate substrate in 1X diethanolamine buffer (Thermo Pierce -Fisher, Suwanee, GA) and read on a SeptraMAX 340 (Molecular Devices, Sunnyvale, CA). Concentrations of IgG for each serogroup in each sample were extrapolated from the 5 parameter regression of reference serum CDC 1992. An average of at least 2 dilutions of the sample was accepted as the final result. Any sample with a coefficient of variance above 15% was not used in the calculation. Competition experiments were performed to confirm the specificity of each assay as well as to determine the limit of detection of meningococcal IgG which was 0.15 ug/ml for all serogroups.
Pre-vaccination antibody concentrations to the four serogroups indicated prior exposure to Neisseria meningitides or cross-reacting commensal organisms via colonization, as would normally be expected. Therefore, the pre-vaccination antibody concentrations were subtracted from the 4-week post-vaccination level to define initial response; additionally, time since dose was residualized from each post-vaccine value because of its influence on vaccine response. The four polysaccharide capsule-specific serotypes included in MCV4 (A, C, W-135, and Y) are considered independent antibody responses and so are analyzed separately.
Baseline cortisol and cortisol reactivity to interaction stress
Cortisol was included as a correlate of parent-child relationship quality and as a possible mediator of the association between parent-child relationship quality and antibody response. Baseline saliva samples were collected upon arrival at the research center and then immediately prior to and 15- and 30-minutes after the end of the stress paradigm interaction. Saliva was collected using the passive drool method; following collection, samples were spun and frozen at −20°C until assayed. All samples were assayed using commercially available High Sensitivity Salivary Cortisol Enzyme Immunoassay kits (Salimetrics, State College, PA) and run in duplicate; average intra- and inter-assay coefficients of variation are 4.13% and 8.89%. Cortisol values were log transformed for analyses. The majority of visits occurred in mid-late afternoon (average time at initial cortisol value, 1:48pm, SD 2:48), but there was variation due to schedule demands; time of visit was included as a covariate in all cortisol analyses. Excluding cases whose pre-stress cortisol sample was obtained in the morning (<10% before 10am) did not substantively influence the findings, and so we include all cases and include time as a covariate.
BMI
Body mass index, measured concurrently with the stress paradigm interaction assessment, was calculated using the standard formula, weight (kg)/height (m)2.
Physical illness
Occurrence and frequency of physical illness were based on active surveillance over a 12-month period following the observed stress paradigm interaction visit; a 12-month period of surveillance was instituted to adjust for seasonality of illness because families were enrolled in the study throughout the calendar year. Families were contacted by phone every two weeks to inquire about the health of the child and remind parents to contact the team for illnesses; parents were provided a digital thermometer to determine fever, defined as a temperature >38°C. When an illness occurred the family was encouraged to bring the child for a study visit or to be evaluated by their primary care provider. Information on illnesses was obtained either from the parent, the primary care provider, or from a study visit where the child was evaluated by a Pediatric Infectious Diseases physician (MTC). Signs, symptoms, and physical exam findings were recorded at illness visits. Diagnostic studies were performed as clinically indicated. We focus in this report on any acute illness as assessed from phone surveillance, whether or not it was subsequently confirmed by physician assessment or positive laboratory finding.
Socio-demographic risks
Socio-economic status was measured by objective indicators, namely family income and education; in addition, a child subjective measure of social class, based on the McArthur Scale of Subjective Social Status (MSSS) (Adler, 2000; Goodman et al., 2001), was included. Children were asked to place themselves on the ladder following standard instructions with 10 representing the highest level and one the lowest in relation to “your school” (assessments were also made in relation to “American society” but all significant associations were limited to the “your school” reference, and so only this measure is retained in analyses). We also consider as possible covariates child age and sex, race/ethnicity, family size, sleep quality and total sleep time from a 7-day sleep diary (completed around the time of the stress paradigm observation visit), and pubertal status based on child self-reported Tanner stage.
Data analysis
Descriptive data are followed by correlations to examine which of the physical health variables was associated with observed parent-child relationship quality. For antibody responses to vaccination, a repeated measures analysis of variance was employed; the outcomes, antibody concentrations at 4-weeks, 3-months, and 6-months post-vaccination, were analyzed as continuous variables and were ln transformed for analyses. Consistent with prior psychological studies of vaccine response, we examine variation in vaccine response as an index of immune function (e.g., rather than vaccine effectiveness; although see below). Parent-child interaction variables are included as between-subjects predictors. Consistent with research on vaccine responses, we analyze complete data and do not substitute for missing data. In addition, samples for antibody analysis were excluded where we did not obtain blood samples pre-vaccination or in a defined window for the initial maximum antibody response at 4 weeks post-vaccination (see details below). Counts of illnesses, cortisol and BMI are treated as continuous variables. Cortisol measures included a) the cortisol level recorded at the beginning of the visit and b) reactivity to the stress paradigm interaction calculated as a slope derived from the pre-stress, and 15 and 30 minutes post-stress measures. A further set of analyses examine the possibility that the prediction of antibody responses from parent-child relationship quality was mediated by cortisol or BMI. According to the logic of mediation analyses (MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002), the prediction of antibody responses from parent-child relationship quality could be mediated by a third variable (cortisol reactivity, BMI) on the condition that the mediator variable is significantly associated with both parent-child relationship quality and antibody response, and that statistically controlling for the mediator reduces the association between parent-child relationship quality and antibody response.
Results
Baseline demographic data on the families from whom we obtained observational data on parent-child interactions are provided in Table 1; findings indicate that the sample included a high proportion of minority families and families at elevated psychosocial risk (Table 1). Preliminary analyses also indicated modest correlations between child and parent behavior within affective dimension: for Warmth/Positivity, r(164)=.41, p<.001; for and Conflict/Negativity, r(164)=.37, p<.001; correlations between dimension within person were of a similar but slightly higher magnitude: for child behavior, r(164)= −.47, p<.001; for parent behavior, r(164)= −.59,p<.001. Modest to weak associations were found between parent and child behavior in the interaction and measures of demographic and psychosocial factors (Table 2); child-parent conflict tended to be elevated in families with higher levels of socio-economic disadvantage. Additionally, minority status was associated with lower levels of child Warmth/Positivity (means [SD]: non-minority, 3.20 [.78]; minority, 2.83 [.86]; F(1,162)=7.67, p<.01), lower levels of parent Warmth/Positivity (means [SD]: non-minority, 4.15 [.60]; minority, 3.65 [.84]; F(1,162)=17.26, p<.01), and higher levels of parent Conflict/Negativity (means [SD]: non-minority, 1.37 [.62]; minority, 2.30 [1.22]; F(1,162)=31.44, p<.01).
Table 1.
Characteristics of the cohort with observational data at baseline assessment.
| Mean (SD)/N (%) | |
|---|---|
| Child factors | |
| Age (years) | 9.56 (.50) |
| Sex (female) | 84 (51%) |
| Body Mass Index | 19.86 (5.22) |
| Race/Ethnicity | |
| African-American | 75 (46%) |
| White | 64 (39%) |
| Other + mixed | 26 (16%) |
| Hispanic | 8 (5%) |
| MSSS | 4.32 (1.59) |
| Family Factors (mother/primary caregiver) | |
| Education | |
| < HS degree | 19 (12%) |
| HS degree | 30 (18%) |
| < University degree | 58 (35%) |
| University degree or greater | 58 (35%) |
| Low Income (<$15,000/year) | 60 (37%) |
| Live-in partner | 92 (57%) |
Note: MSSS = McArthur Scale of Subjective Social Status (“your school”). HS = high school. Self-declared Hispanic designation is provided independent of race categories. n=165.
Table 2.
Associations between parent-child observed behaviors and concurrent socio-economic and psychosocial risks.
| Child behavior | Parent Behavior | |||
|---|---|---|---|---|
| Negative/Conflict | Warmth/Positive | Negative/Conflict | Warmth/Positive | |
| Maternal education | −.12 | .21** | −.37** | .35** |
| Income | −.03 | .19* | −.35*** | .28*** |
| Parent has partner | −.07 | .19* | −.29** | .33** |
| MSSS | .01 | .06 | .05 | .03 |
| Pubertal status | ||||
| Boys | .12 | .06 | .04 | −.02 |
| Girls1 | .11 | −.03 | .20 | −.15 |
Note: MSSS = McArthur Scale of Subjective Social Status with respect to “your school.”
p <.05
p<.01.
girls pubertal development score is based on breast development.
Antibody responses
Of the children with observational data, we excluded 8 children who received meningococcal vaccine from a different manufacturer and an additional 31 children from whom we did not obtain a pre-vaccination blood draw. Of the remaining 125 children, there was variation in the timing of the first blood draw (i.e., 4-week) post-vaccination because of cancelled and re-scheduled visits. Antibody concentration obtained long before or after the target of 28 days post-vaccination would mis-estimate maximum response. We therefore excluded on an a priori basis and blind to results and all identifying information those children whose initial 28 day post-vaccination blood sample was not obtained within 18-38 days of vaccination, i.e., 10 days on either side of the 4-week target window (see below and footnote1); antibody concentration at 3 and 6 months post-vaccination would be less sensitive to time since vaccination and so no inclusion criteria were made for these later assessments. These conditions resulted in a maximum sample of 90 children for analyses of antibody concentrations. Analyses indicated that the above exclusions were not reliably associated with key study variables; for example, children from whom we did not obtain pre-vaccination blood were not significantly different from those children on whom we did obtain a pre-vaccination blood sample on relationship quality measures, health outcomes or covariates listed.
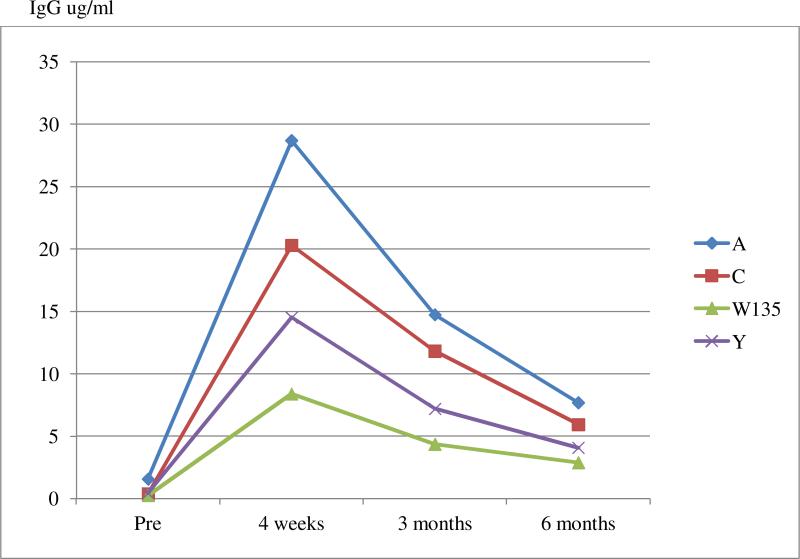
Antibody concentrations (Appendix I) displayed the expected maximum response at the 4-week post-vaccination assessment followed by a leveling off to 6-months post-vaccination; response data concur with prior studies and licensure data on effectiveness. For example, for serogroup C, 90% of the children showed a 4-fold increase in pre-vaccination antibody concentration at the 4-week assessment, a convention used in prior studies (Bertolini, Costa, van der Heijden, Sato, & Marques, 2012; Borrow et al., 2001).
For serogroup C, a repeated measures analysis of variance indicated a significant between-subjects effect for parent Negative/Conflict behavior, which predicted lower antibody concentrations (F(1,76) = 4.39, p<.05); in parallel analyses, a similar but non-significant between-subjects effect was found for child Negative/Conflict behavior (F(1,76) = 2.56, p=.11). Additionally, a greater antibody response to serogroup C was associated with parent and child positive behavior, although neither variable was statistically significant in a repeated measures analysis of variance (for parent Positive/Warmth behavior, F(1,76) = 2.42, p=.12; for child Positive/Warmth behavior (F(1,76) = 3.41, p=.07). Additional analyses indicated that child reports of subjective social class also predicted lower concentrations; no other covariates (i.e., of those listed above) were significant predictors.
The final prediction model included child reports of subjective social class, maternal education (as a control for objective social class), and observer reports of parent Negative/Conflict behavior (Table 3). There was a significant within-subjects effect of time, which captured the expected decrease in antibody concentrations from 4-weeks to 3- and 6-months post-vaccination. Additionally, significant effects were obtained for child subjective social class and parent Negative/Conflict behavior; these effects were consistent across the three post-vaccination assessments, i.e., there were no interactions by time. For parent Negative/Conflict behavior, the consistent negative effect on antibody concentration is illustrated by follow-up correlation analyses, which indicated that parent Negative/Conflict behavior was consistently associated with lower antibody concentration at each post-vaccination assessment: 4 weeks, r = −.23, p<.05; 3 months, r = −.20, p<.05; 6 months r = −.19, p=.09. Further follow-up analyses indicated that parent Negative/Conflict behavior was a robust predictor of lower antibody concentrations in the child: including parent positive behavior or child negative or positive behavior had minimal effect on and did not mediate the effect of parent Negative/Conflict behavior.
Table 3.
Between- and within-subjects effects from repeated measures analysis of variance predicting antibody response.
| Effects | F | (df) | p |
|---|---|---|---|
| Between-subjects | (1,74) | ||
| MSSS | 4.41 | .04 | |
| Parent education | .79 | .38 | |
| Parent Negative/Conflict | 5.54 | .02 | |
| Within-Subjects | (2,73) | ||
| Time | 3.56 | .03 | |
| Time X MSSS | .06 | .94 | |
| Time X Parent education | .68 | .51 | |
| Time X Parent Negative/Conflict | .61 | .55 |
Note: MSSS = McArthur Scale of Subjective Social Status with respect to “your school.” Results are for serogroup C.
Further analyses indicated that the prediction model was particular to serogroup C; there was no reliable evidence that observed parent or child behavior or child subjective social class predicted variation in response to serogroup A, W-135, or Y1.
Cortisol baseline and reactivity
Overall levels of salivary cortisol showed a significant mean decrease from pre-interaction (raw mean and SD: 1.92 nmol/l SD 1.52) to 15-minutes (1.62 nmol/l SD 1.21) and 30-minutes (1.48 nmol/l SD 1.04) following the interaction; a repeated measures ANOVA on the ln-transformed data indicated a significant linear decrease, F(2,158) = 22.00, p<.01). Nonetheless, there was sizable variation in slope, with approximately one-quarter showing an increase in cortisol from pre-observational task levels. Baseline cortisol level was moderately negatively associated with reactivity or slope (r(163) = −.18, p<.05); additionally, time of initial cortisol sampling was negatively associated with baseline value (r(158) = −.39, p<.01) and slope (r(158) = −.17, p<.05). None of the covariates listed above was significantly (p<.05) associated with cortisol baseline or cortisol reactivity.
As shown in Table 4, child cortisol reactivity during the interaction was significantly associated with child Negative/Conflict behavior (r(163) = .19, p<.05) and child Positive/Warmth behavior (r(163) = −.18, p<.05). A regression analysis indicated that child Negative/Conflict behavior significantly predicted an increase (or reduced decrease) in cortisol (B= .05 [SD .02]; beta =.16, p<.05) independently of baseline value (B= −.15 [SD .05]; beta =−.24, p<.01) and time of collection (B= −.03 [SD .01]; beta =−.26, p<.01), which were also significant predictors. Follow-up analyses indicated that the effect of child Negative/Conflict behavior on cortisol reactivity was trivially changed when parent Negative/Conflict was included in the model (B= .04, beta =.13) or when child Positive/Warmth behavior was included in the model (B= .04, beta =.12), neither of which was an independent predictor of cortisol reactivity. Cortisol reactivity was not significantly associated with serogroup C antibody response, however, and so the possibility that cortisol reactivity mediated the association between parent-child relationship quality and antibody response was rejected.
Table 4.
Bivariate correlations between observer ratings of interaction quality and health outcomes.
| Physical Illness | Cortisol | BMI | ||
|---|---|---|---|---|
| Initial value | Slope | |||
| Child behavior | ||||
| Negative/Conflict | .04 | −.11 | .19* | .17* |
| Warmth/Support | .03 | .04 | −.18* | −.10 |
| Parent Behavior | ||||
| Negative/Conflict | −.12 | −.06 | .13 | .18* |
| Warmth/Support | .11 | −.04 | −.05 | −.16* |
Note: BMI = body mass index; Any illness= number of illness in the subsequent 12-month period.
p<.05.
BMI
Child BMI was significantly associated with child and parent Negative/Conflict behavior and parent Positive/Warmth behavior in the expected direction (Table 4). Several covariates were significantly associated with child BMI: parental education (r(161) = −.17, p<.05); child age (r(161) = .18, p<.05); and pubertal development (for girls, r(7) = .37, p<.01; for boys, r(76) = .39, p<.01). Child BMI was also higher among minority youth (means [SD]: for minority youth, 21.57 [6.59]; non-minority youth 19.29 [5.08]; F(1,159) = 5.32, p<.05). A regression model was subsequently conducted to examine parent-child relationship quality as a predictor of BMI after accounting for covariates; models were run separately for girls and boys to account for pubertal effects. For girls, none of the interaction variables was significantly associated with BMI after accounting for significant covariates cited above (the prediction from child Negative/Conflict behavior remained marginally significant, B=1.31, SE .71, p=.068). There was no evidence that interaction quality was associated with BMI in boys after accounting for developmental and socio-economic confounds. The lack of a robust association between parent-child interaction quality and child BMI also means that BMI did not mediate the association between parent-child relationship quality and antibody response.
Illnesses
40 children (24%) had at least 1 illness over the 12-month period following the interaction assessment visit (range 1-9). Observer ratings of parent-child relationship quality did not predict illness (Table 4), measured as a continuous variable or dichotomized as any illness.
Discussion
A considerable body of empirical research suggests that the quality of parent-child relationships has a powerful influence on children's behavioral health, with evidence of a causal connection derived from intervention studies (Hutchings et al., 2007; Scott et al., 2010; Webster-Stratton, 1998). Findings from the current study suggest that the effects of parent-child relationship quality, and particularly the management of conflict in the relationship, may extend to markers of physical health in children. By far the most novel and significant finding is the prediction of vaccine responses from the quality of the parent-child interaction. Prior research in older adults found associations between self-reported psychosocial factors and elevated stress physiology and antibody responses to vaccine challenge (Glaser et al., 2000; Vedhara et al., 1999), and one cross-sectional study of college students found that lower antibody concentrations to meningococcal serogroup C at a median of 9-12 months following vaccination were associated with greater levels of anxiety/insomnia, social dysfunction, and perceived stress (Burns, Drayson, Ring, & Carroll, 2002). Vaccine responses have attracted attention in studies of child stress and development (Boyce et al., 1995; McDade, Beck, Kuzawa, & Adair, 2001), but the prediction of vaccine response from relationship quality is novel. The robustness of this prediction is suggested by the persistence of reduced antibody levels at the three post-vaccination assessments at 4-weeks, 3- and 6- months, and the similarity in effect across parent and child behavior and for both affective dimensions (although the prediction from parent Negative/Conflict was the most statistically reliable). Furthermore, the prediction from parent-child interaction quality was independent of socio-demographic controls, one of which, children's subjective sense of social class, was also a predictor.
Mechanisms underlying this association will require further study. It is natural to propose that some aspect of stress circuitry in the child induced by conflicted parent-child relationships may be responsible for the less robust antibody response. In the current study we considered two potential mediators, but neither mediated the association between parent-child relationship quality and antibody response. That is, parent-child relationship quality was associated with both child cortisol reactivity and BMI; however, neither was reliably associated with antibody response, and so unable to account for the association between parent-child relationship quality and antibody response. Other stress system candidates exist that might mediate the link between negativity and stress in the parent-child relationship and immune response, and require further study.
Each capsular polysaccharide contained in MCV4 is antigenically distinct. We found that parent-child conflict reliably predicted response to serogroup C. The prediction of variation in serogroup C is novel and may be clinically important as serogroup C accounts for a significant percentage of sporadic and epidemic meningococcal disease from middle childhood through early adulthood (Harrison, 2010). Importantly, however, although meningococcal disease is a significant public health concern (Cohn et al., 2010), we used this vaccination program as a natural experiment to assess immune response to family and caregiving stress rather than examine disease susceptibility per se.
It is unclear why the effect seemed particular to serogroup C. Consistent with previous clinical and licensure data, there was generally greater response to serogroup A than serogroups C, W-135 or Y (Appendix I), but there was sizable between-individual variation in all serogroups. In the case of serogroup C, 90% of the children showed the standard 4-fold increase in antibody concentration at 4-weeks post-vaccination, indicating a typical response (Bertolini et al., 2012; Borrow et al., 2001). Previous studies have consistently demonstrated MCV4 vaccine hyporesponsiveness to serogroup C in subjects previously vaccinated with a meningococcal pure polysaccharide vaccine (MPSV), which was not evident with other serogroups, and suggests that serogroup C may be more sensitive to immune system perturbations (Khalil et al., 2014). Another possibility is that associations were not obtained for other serogroups because a substantial antibody response (for serogroup A) or a more modest response (serogroups Y and W135) to vaccination meant that there was less variation in which to detect a signal associated with parent-child relationship stress. A perhaps parallel situation was recently reported in the case of predicting hepatitis B antibody concentration in infants from maternal prenatal anxiety (O'Connor, Winter, et al., 2013).
As a further investigation of the link between parent-child relationship quality and immune function, we examined the likelihood of acute symptomatic illness over the subsequent 12-month period using an in-depth active surveillance methodology. In contrast to results of a study of younger children (Caserta et al., 2008), we did not find a significant association between caregiving stress and acute illness. This apparent age-related difference may reflect developmental changes in stress and health as well as age-based differences in rates of illness and types of exposure.
The association between parent-child relationship quality and health measures was not limited to vaccine response. Consistent with previous studies of infants (Spangler & Grossmann, 1993) and adolescents (Kobak et al., 2009), parent-child relationship quality was associated with cortisol reactivity. The effects were most robust for child negative/conflicted behavior; that is, it was behaving toward the parent in an angry, coercive manner that predicted cortisol reactivity. However, as noted, this effect did not help explain the weaker vaccine response. Consistent with previous research (Brotman et al., 2012; Gerards, Sleddens, Dagnelie, de Vries, & Kremers, 2011), parent-child interaction quality predicted child BMI, another potential key index of health risk in young people. Importantly, however, the association between parent-child relationship quality and BMI was reduced to non-significance after accounting for confounds; the implication is that BMI did not mediate the association between parent-child conflict and antibody response to vaccination.
Several limitations of the study deserve attention. First, inevitably, the health biomarkers included here were based on different time scales (e.g., cortisol was measured concurrently with the observational assessment whereas immune response was assessed over a 6-month period and illnesses over a full year). It is possible that the lack of a mediated association between parent-child relationship quality and antibody response may simply be a result of the different time-scale of the cortisol and vaccine responses measures. Second, our findings on vaccine response were specific to serogroup C; as noted, it is not clear why parent-child relationship quality or child perceptions of social class did not also predict variation in the other antigenically distinct serogroups, although studies showing stress and psychosocial prediction to multiple serogroups is rare. Third, the sampling strategy targeted comparatively high-risk children and families; results obtained here may not generalize to low-risk samples. Set against these limitations are several strengths of the study, including a detailed observational methodology, longitudinal design with serial blood draws, multiple measures of health, and a large ambulatory sample that over-sampled for high-risk youth.
Further research is needed to determine the extent of the scientific and clinical implications of these findings. One important avenue is greater integration of markers of physical health in mental and behavioral health settings (O'Connor et al., 2014; Ridout et al., 2014). Our findings add to a growing literature suggesting that established markers of stress and resilience for children, such as the quality of family relationships and emotional regulation within these relationships, have implications beyond mental health and deserve broader public health attention. In the context of future intervention research, the current findings have two particular advantages: a) in contrast to some previous studies that assess child health in relation to broad and impractical intervention targets such as social class, the current study identified a specific target for intervention, parent-child relationships; and b) evidence-based interventions to improve parent-child relationship quality have already been developed and may be applicable for child somatic health.
Highlights.
Animal and adult studies suggest that stress exposure history may be associated with weakened immune response; the application to pediatric samples is unclear.
The current study capitalized on the natural experiment provided by the universal administration of the meningococcal conjugate vaccine to children at age 11 years to examine if stress, indexed by quality of parent-child relationships, predicted weaker vaccine response.
Observer reports of more negative, hostile parent-child relationship quality, rated blind to all other study data, was associated with weaker vaccine response in the child at 4-weeks, and 3- and 6-months post vaccination.
The association held after accounting for covariates, and was not accounted for by measures of stress physiology, indexed by cortisol reactivity.
Acknowledgements
We are extremely grateful to all the families who took part in this study, pediatric practices who donated time and resources (Elmwood Pediatric Group, Panorama Pediatric Group, Pediatric Practice at the Golisano Children's Hospital) and to Linda Anderson, Nancy Nix, Ken Schnabel, Arthur Watts, Shouling Zhang for their assistance with the project. The project was funded by NIH HD038938 and in part by grants MH097293and General Clinical Research Grant 5 MO1 RR00044 from the National Center for Research Resources, NIH. The study funder had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Appendix
Appendix I.
Antibody Titer Mean Through 6 Months: 4 Serotypes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Post hoc analyses were also carried out using more restrictive (e.g., target 28 days +/− 1 week, i.e., 21-35 days) and less restrictive (e.g., target 28 days +/− 2 weeks) inclusion criterion for days since initial vaccine response. Findings on serogroup C were consistently significant across more and less restrictive brackets around the target 28 day assessment. For serogroup W-135, associations between child and parent negative behavior were significantly associated with lower antibody concentration using a more restrictive time bracket of 21-35 days but not across multiple brackets, and so we do not regard this as a robust effect.
Conflict of interest: The authors declare no financial or other conflicts of interest.
References
- Adler NE. The MacArthur Scale of Subjective Social Status. 2000 http://www.macses.ucsf.edu/Research/Psychosocial/notebook/subjective.html.
- Anderson SE, Gooze RA, Lemeshow S, Whitaker RC. Quality of early maternal-child relationship and risk of adolescent obesity. Pediatrics. 2012;129(1):132–140. doi: 10.1542/peds.2011-0972. doi: 10.1542/peds.2011-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini DV, Costa LS, van der Heijden IM, Sato HK, Marques HH. Immunogenicity of a meningococcal serogroup C conjugate vaccine in HIV-infected children, adolescents, and young adults. Vaccine. 2012;30(37):5482–5486. doi: 10.1016/j.vaccine.2012.06.069. doi: 10.1016/j.vaccine.2012.06.069. [DOI] [PubMed] [Google Scholar]
- Borrow R, Southern J, Andrews N, Peake N, Rahim R, Acuna M, Kaczmarski E. Comparison of antibody kinetics following meningococcal serogroup C conjugate vaccine between healthy adults previously vaccinated with meningococcal A/C polysaccharide vaccine and vaccine-naive controls. Vaccine. 2001;19(23-24):3043–3050. doi: 10.1016/s0264-410x(01)00050-0. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Adams S, Tschann JM, Cohen F, Wara D, Gunnar MR. Adrenocortical and behavioral predictors of immune responses to starting school. Pediatr Res. 1995;38(6):1009–1017. doi: 10.1203/00006450-199512000-00030. [DOI] [PubMed] [Google Scholar]
- Brotman LM, Dawson-McClure S, Huang KY, Theise R, Kamboukos D, Wang J, Ogedegbe G. Early childhood family intervention and long-term obesity prevention among high-risk minority youth. Pediatrics. 2012;129(3):e621–628. doi: 10.1542/peds.2011-1568. doi: 10.1542/peds.2011-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles ST, Staiano AE, Drazba KT, Gupta AK, Sothern M, Katzmarzyk PT. Elevated C-reactive protein in children from risky neighborhoods: evidence for a stress pathway linking neighborhoods and inflammation in children. PLoS One. 2012;7(9):e45419. doi: 10.1371/journal.pone.0045419. doi: 10.1371/journal.pone.0045419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchinal M, Vernon-Feagans L, Cox M. Cumulative Social Risk, Parenting, and Infant Development in Rural Low-Income Communities. Parenting, science and practice. 2008;8(1):41–69. doi: 10.1080/15295190701830672. doi: 10.1080/15295190701830672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns VE, Drayson M, Ring C, Carroll D. Perceived stress and psychological well-being are associated with antibody status after meningitis C conjugate vaccination. Psychosomatic medicine. 2002;64(6):963–970. doi: 10.1097/01.psy.0000038936.67401.28. [DOI] [PubMed] [Google Scholar]
- Caserta MT, O'Connor TG, Wyman PA, Wang H, Moynihan J, Cross W, Jin X. The associations between psychosocial stress and the frequency of illness, and innate and adaptive immune function in children. Brain Behav Immun. 2008;22(6):933–940. doi: 10.1016/j.bbi.2008.01.007. doi: S0889-1591(08)00032-9 [pii]10.1016/j.bbi.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta MT, Wyman PA, Wang H, Moynihan J, O'Connor TG. Associations among depression, perceived self-efficacy, and immune function and health in preadolescent children. Development and Psychopathology. 2011;23(4):1139–1147. doi: 10.1017/S0954579411000526. doi: 10.1017/S0954579411000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Cohen S, Miller GE. How low socioeconomic status affects 2-year hormonal trajectories in children. Psychological Science. 2010;21(1):31–37. doi: 10.1177/0956797609355566. doi: 10.1177/0956797609355566. [DOI] [PubMed] [Google Scholar]
- Chen E, Martin AD, Matthews KA. Understanding health disparities: the role of race and socioeconomic status in children's health. Am J Public Health. 2006;96(4):702–708. doi: 10.2105/AJPH.2004.048124. doi: AJPH.2004.048124 [pii]10.2105/AJPH.2004.048124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Schneider ML, Dierschke DJ, Ershler WB. Early rearing conditions alter immune responses in the developing infant primate. Pediatrics. 1992;90(3 Pt 2):505–509. [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosom Med. 2006;68(3):414–420. doi: 10.1097/01.psy.0000221236.37158.b9. doi: 68/3/414 [pii]10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(16):5995–5999. doi: 10.1073/pnas.1118355109. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, Messonnier NE. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998-2007: implications for prevention of meningococcal disease. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50(2):184–191. doi: 10.1086/649209. doi: 10.1086/649209. [DOI] [PubMed] [Google Scholar]
- Conger RD, Conger KJ, Elder GH, Jr., Lorenz FO, Simons RL, Whitbeck LB. A family process model of economic hardship and adjustment of early adolescent boys. Child Dev. 1992;63(3):526–541. doi: 10.1111/j.1467-8624.1992.tb01644.x. [DOI] [PubMed] [Google Scholar]
- Conger RD, Ge X, Elder GH, Jr., Lorenz FO, Simons RL. Economic stress, coercive family process, and developmental problems of adolescents. Child Dev. 1994;65(2 Spec No):541–561. [PubMed] [Google Scholar]
- Cui M, Durtschi JA, Donnellan MB, Lorenz FO, Conger RD. Intergenerational transmission of relationship aggression: a prospective longitudinal study. Journal of family psychology : JFP : journal of the Division of Family Psychology of the American Psychological Association. 2010;24(6):688–697. doi: 10.1037/a0021675. doi: 10.1037/a0021675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidov M, Grusec JE. Untangling the links of parental responsiveness to distress and warmth to child outcomes. Child Development. 2006;77(1):44–58. doi: 10.1111/j.1467-8624.2006.00855.x. doi: 10.1111/j.1467-8624.2006.00855.x. [DOI] [PubMed] [Google Scholar]
- DeGarmo DS, Forgatch MS, Martinez CR., Jr. Parenting of divorced mothers as a link between social status and boys' academic outcomes: unpacking the effects of socioeconomic status. Child Dev. 1999;70(5):1231–1245. doi: 10.1111/1467-8624.00089. [DOI] [PubMed] [Google Scholar]
- Evans GW, Fuller-Rowell TE, Doan SN. Childhood cumulative risk and obesity: the mediating role of self-regulatory ability. Pediatrics. 2012;129(1):e68–73. doi: 10.1542/peds.2010-3647. doi: 10.1542/peds.2010-3647. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and health: cumulative risk exposure and stress dysregulation. Psychological Science. 2007;18(11):953–957. doi: 10.1111/j.1467-9280.2007.02008.x. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and young adults' allostatic load: the mediating role of childhood cumulative risk exposure. Psychological Science. 2012;23(9):979–983. doi: 10.1177/0956797612441218. doi: 10.1177/0956797612441218. [DOI] [PubMed] [Google Scholar]
- Gerards SM, Sleddens EF, Dagnelie PC, de Vries NK, Kremers SP. Interventions addressing general parenting to prevent or treat childhood obesity. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2011;6(2-2):e28–45. doi: 10.3109/17477166.2011.575147. doi: 10.3109/17477166.2011.575147. [DOI] [PubMed] [Google Scholar]
- Gheesling LL, Carlone GM, Pais LB, Holder PF, Maslanka SE, Plikaytis BD, et al. Multicenter comparison of Neisseria meningitidis serogroup C anti-capsular polysaccharide antibody levels measured by a standardized enzyme-linked immunosorbent assay. Journal of clinical microbiology. 1994;32(6):1475–1482. doi: 10.1128/jcm.32.6.1475-1482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Sheridan J, Malarkey WB, MacCallum RC, Kiecolt-Glaser JK. Chronic stress modulates the immune response to a pneumococcal pneumonia vaccine. Psychosom Med. 2000;62(6):804–807. doi: 10.1097/00006842-200011000-00010. [DOI] [PubMed] [Google Scholar]
- Goodman E, Adler NE, Kawachi I, Frazier AL, Huang B, Colditz GA. Adolescents' perceptions of social status: development and evaluation of a new indicator. Pediatrics. 2001;108(2):E31. doi: 10.1542/peds.108.2.e31. [DOI] [PubMed] [Google Scholar]
- Hagan MS, Hollier EA, O'Connor TG, Eisenberg N. Parent–child relationships in nondivorced, divorced single-mother, and remarried families. In: Hetherington EM, Clingempeel G, editors. Coping with marital transitions: A family systems perspective. Vol. 57. Monographs of the Society for Research in Child Development; 1992. pp. 94–148. [Google Scholar]
- Halsey NA, Moulton LH, O'Donovan JC, Walcher JR, Thoms ML, Margolis HS, Krause DS. Hepatitis B vaccine administered to children and adolescents at yearly intervals. Pediatrics. 1999;103(6 Pt 1):1243–1247. doi: 10.1542/peds.103.6.1243. [DOI] [PubMed] [Google Scholar]
- Harrison LH. Epidemiological profile of meningococcal disease in the United States. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50(Suppl 2):S37–44. doi: 10.1086/648963. doi: 10.1086/648963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertsgaard L, Gunnar M, Erickson MF, Nachmias M. Adrenocortical responses to the strange situation in infants with disorganized/disoriented attachment relationships. Child Development. 1995;66(4):1100–1106. [PubMed] [Google Scholar]
- Hetherington EM. Family functioning and the adjustment of adolescent siblings in diverse types of families. Monogr Soc Res Child Dev. 1999;64(4):1–25. doi: 10.1111/1540-5834.00045. [DOI] [PubMed] [Google Scholar]
- Hetherington EM, Bridges M, Insabella GM. What matters? What does not? Five perspectives on the association between marital transitions and children's adjustment. Am Psychol. 1998;53(2):167–184. doi: 10.1037//0003-066x.53.2.167. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta paediatrica. 1994;397:9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Hutchings J, Gardner F, Bywater T, Daley D, Whitaker C, Jones K, Edwards RT. Parenting intervention in Sure Start services for children at risk of developing conduct disorder: pragmatic randomised controlled trial. BMJ. 2007;334(7595):678. doi: 10.1136/bmj.39126.620799.55. doi: bmj.39126.620799.55 [pii]10.1136/bmj.39126.620799.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph MA, O'Connor TG, Briskman JA, Maughan B, Scott S. The formation of secure new attachments by children who were maltreated: an observational study of adolescents in foster care. Development and Psychopathology. 2014;26(1):67–80. doi: 10.1017/S0954579413000540. doi: 10.1017/S0954579413000540. [DOI] [PubMed] [Google Scholar]
- Khalil M, Al-Mazrou Y, Findlow H, Chadha H, Bosch Castells V, Oster P, Borrow R. Meningococcal serogroup C serum and salivary antibody responses to meningococcal quadrivalent conjugate vaccine in Saudi Arabian adolescents previously vaccinated with bivalent and quadrivalent meningococcal polysaccharide vaccine. Vaccine. 2014;32(43):5715–5721. doi: 10.1016/j.vaccine.2014.08.026. doi: 10.1016/j.vaccine.2014.08.026. [DOI] [PubMed] [Google Scholar]
- Kim JE, Hetherington EM, Reiss D. Associations among family relationships, antisocial peers, and adolescents' externalizing behaviors: gender and family type differences. Child Dev. 1999;70(5):1209–1230. doi: 10.1111/1467-8624.00088. [DOI] [PubMed] [Google Scholar]
- Kobak R, Zajac K, Levine S. Cortisol and antisocial behavior in early adolescence: the role of gender in an economically disadvantaged sample. Development and Psychopathology. 2009;21(2):579–591. doi: 10.1017/S0954579409000315. doi: 10.1017/S0954579409000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudenslager M, Capitanio JP, Reite M. Possible effects of early separation experiences on subsequent immune function in adult macaque monkeys. The American journal of psychiatry. 1985;142(7):862–864. doi: 10.1176/ajp.142.7.862. [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Fleshner M, Hofstadter P, Held PE, Simons L, Maier SF. Suppression of specific antibody production by inescapable shock: stability under varying conditions. Brain Behav Immun. 1988;2(2):92–101. doi: 10.1016/0889-1591(88)90010-4. [DOI] [PubMed] [Google Scholar]
- Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to blood pressure in the CARDIA study. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2009;28(3):338–346. doi: 10.1037/a0013785. doi: 10.1037/a0013785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Franklin D, Gonzalez CA. Influence of social variables on the biobehavioral response to separation in rhesus monkey infants. Child Development. 1984;55(4):1386–1393. [PubMed] [Google Scholar]
- Liang SW, Jemerin JM, Tschann JM, Wara DW, Boyce WT. Life events, frontal electroencephalogram laterality, and functional immune status after acute psychological stressors in adolescents. Psychosomatic medicine. 1997;59(2):178–186. doi: 10.1097/00006842-199703000-00010. [DOI] [PubMed] [Google Scholar]
- Lubach GR, Coe CL, Ershler WB. Effects of early rearing environment on immune responses of infant rhesus monkeys. Brain Behav Immun. 1995;9(1):31–46. doi: 10.1006/brbi.1995.1004. doi: S0889-1591(85)71004-5 [pii]10.1006/brbi.1995.1004. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M, Ryff CD, Bumpass LL, Shipley M, Marks NF. Social inequalities in health: next questions and converging evidence. Social science & medicine. 1997;44(6):901–910. doi: 10.1016/s0277-9536(96)00194-3. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Cohen S, Rabin BS, Manuck SB. Associations between stress, trait negative affect, acute immune reactivity, and antibody response to hepatitis B injection in healthy young adults. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2001;20(1):4–11. [PubMed] [Google Scholar]
- Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annual Review of Psychology. 2011;62:501–530. doi: 10.1146/annurev.psych.031809.130711. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Beck MA, Kuzawa C, Adair LS. Prenatal undernutrition, postnatal environments, and antibody response to vaccination in adolescence. Am J Clin Nutr. 2001;74(4):543–548. doi: 10.1093/ajcn/74.4.543. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Brain on stress: how the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl 2):17180–17185. doi: 10.1073/pnas.1121254109. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NT, Scott JG, Wray NR, Cohen-Woods S, Baune BT. Research review: The role of cytokines in depression in adolescents: a systematic review. Journal of child psychology and psychiatry. 2013;54:816–835. doi: 10.1111/jcpp.12080. [DOI] [PubMed] [Google Scholar]
- O'Connor TG. Annotation: The 'effects' of parenting reconsidered: findings, challenges, and applications. J Child Psychol Psychiatry. 2002;43(5):555–572. doi: 10.1111/1469-7610.00046. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Moynihan JA, Caserta MT. Annual Research Review: The neuroinflammation hypothesis for stress and psychopathology in children - developmental psychoneuroimmunology. Journal of child psychology and psychiatry, and allied disciplines. 2013 doi: 10.1111/jcpp.12187. doi: 10.1111/jcpp.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor TG, Moynihan JA, Wyman PA, Carnahan J, Lofthus G, Quataert SA, Caserta MT. Depressive symptoms and immune response to meningococcal conjugate vaccine in early adolescence. Dev Psychopathol. 2014;26(4 Pt 2):1567–1576. doi: 10.1017/S0954579414001242. doi: 10.1017/S0954579414001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor TG, Winter MA, Hunn J, Carnahan J, Pressman EK, Glover V, Caserta MT. Prenatal maternal anxiety predicts reduced adaptive immunity in infants. Brain, behavior, and immunity. 2013;32:21–28. doi: 10.1016/j.bbi.2013.02.002. doi: 10.1016/j.bbi.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks EP, Kumanyika S, Moore RH, Stettler N, Wrotniak BH, Kazak A. Influence of stress in parents on child obesity and related behaviors. Pediatrics. 2012;130(5):e1096–1104. doi: 10.1542/peds.2012-0895. doi: 10.1542/peds.2012-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GR, Forgatch MS, Degarmo DS. Cascading effects following intervention. Dev Psychopathol. 2010;22(4):949–970. doi: 10.1017/S0954579410000568. doi: S0954579410000568 [pii]10.1017/S0954579410000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout KK, Parade SH, Seifer R, Price LH, Gelernter J, Feliz P, Tyrka AR. Interleukin 1B gene (IL1B) variation and internalizing symptoms in maltreated preschoolers. Dev Psychopathol. 2014;26(4 Pt 2):1277–1287. doi: 10.1017/S0954579414001023. doi: 10.1017/S0954579414001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosch FA, Dackis MN, Cicchetti D. Child maltreatment and allostatic load: consequences for physical and mental health in children from low-income families. Development and Psychopathology. 2011;23(4):1107–1124. doi: 10.1017/S0954579411000587. doi: 10.1017/S0954579411000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MR, Markie-Dadds C, Tully LA, Bor W. The triple P-positive parenting program: a comparison of enhanced, standard, and self-directed behavioral family intervention for parents of children with early onset conduct problems. Journal of consulting and clinical psychology. 2000;68(4):624–640. [PubMed] [Google Scholar]
- Scott S, Briskman J, O'Connor TG. Early Prevention of Antisocial Personality: Long-Term Follow-Up of Two Randomized Controlled Trials Comparing Indicated and Selective Approaches. The American journal of psychiatry. 2014 doi: 10.1176/appi.ajp.2014.13050697. doi: 10.1176/appi.ajp.2014.13050697. [DOI] [PubMed] [Google Scholar]
- Scott S, Sylva K, Doolan M, Price J, Jacobs B, Crook C, Landau S. Randomised controlled trial of parent groups for child antisocial behaviour targeting multiple risk factors: the SPOKES project. J Child Psychol Psychiatry. 2010;51(1):48–57. doi: 10.1111/j.1469-7610.2009.02127.x. doi: JCPP2127 [pii]10.1111/j.1469-7610.2009.02127.x. [DOI] [PubMed] [Google Scholar]
- Sheridan PA, Paich HA, Handy J, Karlsson EA, Hudgens MG, Sammon AB, Beck MA. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond) 2012;36(8):1072–1077. doi: 10.1038/ijo.2011.208. doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema DJ, Friedman KE, Corsaro B, Kimura A, Hildreth SW, Madore DV, Quataert SA. Relationship between serum bactericidal activity and serogroup-specific immunoglobulin G concentration for adults, toddlers, and infants immunized with Neisseria meningitidis serogroup C vaccines. Clinical and diagnostic laboratory immunology. 2000;7(5):764–768. doi: 10.1128/cdli.7.5.764-768.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lin KH, Gordon LC, Conger RD, Lorenz FO. Explaining the Higher Incidence of Adjustment Problems among Children of Divorce Compared with Those in Two-Parent Families. Journal of Marriage and the Family. 1999;61:1020–1033. [Google Scholar]
- Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: a systematic review. Brain, behavior, and immunity. 2012;26(2):239–250. doi: 10.1016/j.bbi.2011.11.003. doi: 10.1016/j.bbi.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Spangler G, Grossmann KE. Biobehavioral organization in securely and insecurely attached infants. Child Development. 1993;64(5):1439–1450. doi: 10.1111/j.1467-8624.1993.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Spies LA, Margolin G, Susman EJ, Gordis EB. Adolescents' cortisol reactivity and subjective distress in response to family conflict: the moderating role of internalizing symptoms. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2011;49(4):386–392. doi: 10.1016/j.jadohealth.2011.01.014. doi: 10.1016/j.jadohealth.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenhammar C, Olsson G, Bahmanyar S, Hulting AL, Wettergren B, Edlund B, Montgomery S. Family stress and BMI in young children. Acta paediatrica. 2010;99(8):1205–1212. doi: 10.1111/j.1651-2227.2010.01776.x. doi: 10.1111/j.1651-2227.2010.01776.x. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biological Psychiatry. 2006;60(8):819–824. doi: 10.1016/j.biopsych.2006.03.016. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Vedhara K, Cox NK, Wilcock GK, Perks P, Hunt M, Anderson S, Shanks NM. Chronic stress in elderly carers of dementia patients and antibody response to influenza vaccination. Lancet. 1999;353(9153):627–631. doi: 10.1016/S0140-6736(98)06098-X. doi: 10.1016/S0140-6736(98)06098-X. [DOI] [PubMed] [Google Scholar]
- Webster-Stratton C. Preventing conduct problems in Head Start children: strengthening parenting competencies. J Consult Clin Psychol. 1998;66(5):715–730. doi: 10.1037//0022-006x.66.5.715. [DOI] [PubMed] [Google Scholar]
- Webster-Stratton C, Hollinsworth T, Kolpacoff M. The long-term effectiveness and clinical significance of three cost-effective training programs for families with conduct-problem children. J Consult Clin Psychol. 1989;57(4):550–553. doi: 10.1037//0022-006x.57.4.550. [DOI] [PubMed] [Google Scholar]
- Webster-Stratton C, Reid MJ, Hammond M. Treating children with early-onset conduct problems: intervention outcomes for parent, child, and teacher training. J Clin Child Adolesc Psychol. 2004;33(1):105–124. doi: 10.1207/S15374424JCCP3301_11. doi: 10.1207/S15374424JCCP3301_11. [DOI] [PubMed] [Google Scholar]
- Wu T, Dixon WE, Jr., Dalton WT, 3rd, Tudiver F, Liu X. Joint effects of child temperament and maternal sensitivity on the development of childhood obesity. Maternal and child health journal. 2011;15(4):469–477. doi: 10.1007/s10995-010-0601-z. doi: 10.1007/s10995-010-0601-z. [DOI] [PubMed] [Google Scholar]