Abstract
Neuropsychiatric disorders (e.g., autism, schizophrenia) are partially characterized by social cognitive deficits, including impairments in the ability to perceive others’ emotional states, which is an aspect of social cognition known as theory of mind (ToM). There is also evidence that inflammation may be implicated in the etiology of these disorders, but experimental data linking inflammation to deficits in social cognition is sparse. Thus, we examined whether exposure to an experimental inflammatory challenge led to changes in ToM. One hundred and fifteen (n=115) healthy participants were randomly assigned to receive either endotoxin, which is an inflammatory challenge, or placebo. Participants completed a social cognition task, the Reading the Mind in the Eyes (RME) test, at baseline and at the peak of inflammatory response for the endotoxin group. The RME test, a validated measure of ToM, evaluates how accurately participants can identify the emotional state of another person by looking only at their eyes. We found that endotoxin (vs. placebo) led to decreases in performance on the RME test from baseline to the peak of inflammatory response, indicating that acute inflammation can lead to decreases in the ability to accurately and reliably comprehend emotional information from others. Given that deficits in ToM are implicated in neuropsychiatric disorders, including those which may have an inflammatory basis, these results may have implications for understanding the links between inflammation, social cognition, and neuropsychiatric disorders.
Keywords: inflammation, social cognition, endotoxin, theory of mind, cytokines, Mind in the Eyes
1. Introduction
One of the most important aspects of human social interaction is the ability to infer others’ mental states and emotions. Indeed, theory of mind (ToM)—defined as the capacity to attribute thoughts, desires, intentions, and beliefs to others (Premack & Woodruff, 1978)—is an aspect of social cognition which is essential for dealing with complex social relationships. This capacity was likely developed in order to allow primates, including humans, to adapt to the complexities that accompany living in organized social groups (Brüne, 2001; Brüne & Brüne-Cohrs, 2006). Impairments or deficits in ToM are implicated in an array of neuropsychiatric disorders, including autism spectrum disorders and schizophrenia (Baron-Cohen et al., 1985; Brüne & Brüne-Cohrs, 2006; Couture et al., 2010; Frith & Corcoran, 1996; Sprong et al., 2007). In fact, deficits in ToM have been hypothesized to underlie the core behavioral symptoms of autism (Baron-Cohen et al., 1985), and social cognitive abilities such as ToM are more strongly related to functional outcomes (e.g., work functioning, living independently) than non-social cognition in individuals with schizophrenia (Fett et al., 2011).
Recent research has also suggested that inflammation, including proinflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α, may play a role in the pathophysiology of autism and schizophrenia (Fineberg & Ellman, 2013; Meyer et al., 2011; Meyer et al., 2009; Michel et al., 2012; Onore et al., 2012; Patterson, 2009). It has been hypothesized that prenatal exposure to infection, including the subsequent maternal proinflammatory response, can alter the development of the fetal brain (Fineberg & Ellman, 2013; Meyer et al., 2011; Meyer et al., 2009; Michel et al., 2012; Onore et al., 2012; Patterson, 2009). Indeed, elevated levels of maternal inflammatory markers during pregnancy are associated with increased risk for neuropsychiatric disorders such as schizophrenia and autism spectrum disorders (Brown et al., 2004; Brown et al., 2013; Buka et al., 2001; Canetta et al., 2014), and animal work has shown that offspring of mothers who were exposed to an inflammatory challenge during pregnancy show long-lasting changes in inflammation persisting through adulthood (Romero et al., 2008). Furthermore, individuals with autism spectrum disorders exhibit elevated levels of proinflammatory cytokines (e.g., IL-6) compared to healthy controls (Ashwood et al., 2011a; Masi et al., 2014; Ricci et al., 2013; Suzuki et al., 2011), and proinflammatory cytokines are also associated with more impaired behavioral outcomes in individuals with autism (Ashwood et al., 2011a; Ashwood et al., 2011b; Enstrom et al., 2010; Onore et al., 2012). Similarly, a recent meta-analysis of cytokine alterations in schizophrenia found that inflammatory cytokines were elevated during acute exacerbations of the illness (Miller et al., 2011), suggesting that acute changes in proinflammatory cytokines may contribute to the pathogenesis and clinical course of schizophrenia. Thus, disruptions in inflammatory processes may play a critical role in the pathophysiology of schizophrenia and autism.
While prior work has found that proinflammatory cytokines may contribute to the etiology of these disorders characterized by ToM deficits, the literature demonstrating a causal relationship between inflammation and deficits in social cognition, particularly ToM, is sparse. Only one prior study, to our knowledge, has examined the link between inflammation and social cognition, specifically examining the impact of endotoxin, an experimental inflammatory challenge, on the Reading the Mind in the Eyes test, a ToM task in which participants are asked to infer the mental or emotional state of another person by looking at only their eyes (Kullmann et al., 2013). Experimentally-induced inflammation in this study did not impact social cognitive performance as assessed by the ToM task. However, the study had a small sample (n=18) and subjects were administered a very low dose of endotoxin (0.4 ng/kg). Prior work has shown that the effect of endotoxin on physiological responses and neurobehavioral functions in humans is dose-dependent (Grigoleit et al., 2011), suggesting that a higher dose of endotoxin may be necessary to induce effects on social cognition.
Given that inflammation is thought to contribute to disorders marked by social cognitive deficits, but that little is known about the effect of inflammation on social cognition, we aimed to examine the effect of a relatively higher dose of endotoxin (0.8 ng/kg), previously shown to alter socioemotional responding, on social cognitive performance in a large sample (n=115) of healthy adults. We hypothesized that endotoxin, compared to placebo, would lead to decreases in performance on a ToM task.
2. Material and methods
2.1 Participants and procedures
One hundred and fifteen healthy participants (69 female; mean age: 24.2 ± 6.6) completed the study. Participants were recruited from UCLA and the greater Los Angeles community using flyers around the UCLA campus, advertisements in campus and local newspapers, and online postings (i.e., Craigslist, ClinicalTrials.gov NCT01671150). Interested participants were screened for eligibility using a two-step process, consisting of a structured telephone interview and an in-person screening session. During the phone interview, prospective participants were screened for and excluded if they had any of the following conditions: claustrophobia, left-handedness, or metal in their body (relevant for a neuroimaging portion, reported separately); chronic physical or mental health problems; history of allergies, autoimmune, or other severe chronic diseases; current use of prescription medications; or recent nightshift work or time zone shifts greater than three hours. If participants were still eligible, they completed an additional interview at the UCLA Cousins Center for Psychoneuroimmunology to ensure eligibility. During the in-person session, participants completed the Structured Clinical Interview for DSM-IV Disorders (SCID; First et al., 2012) and were asked about their medical and medication history. In addition, height, weight, and vital signs were assessed. Urine samples were collected to examine drug use (marijuana, opiates, cocaine, amphetamines, methamphetamines), and blood samples were collected to screen for abnormalities (e.g., complete blood cell count, chemistry panel, liver function tests), as well as pregnancy for females. Participants’ eligibility was determined by the study physician (M.R.I.). After the in-person screening session, any participant who: 1) had body mass index (BMI) greater than 30, 2) reported physical health problems or medication use, 3) evidenced an Axis I psychiatric disorder based on SCID assessment, 4) showed evidence of drug use from a positive urine test, 5) had a positive pregnancy test, or 6) showed abnormalities on the screening laboratory tests were ineligible for the study. Participants who met study eligibility criteria were then scheduled for the study.
The study was conducted between March 2011 and August 2013 (when the intended sample size of n=115 was reached, which was based on effect sizes from prior research (Eisenberger et al., 2010) regarding the primary aims of the study (reported in Moieni et al., in press)) at the UCLA Clinical and Translational Research Center (CTRC) using a randomized, double-blind, placebo-controlled design. Upon arrival to the CTRC, a nurse, who was blind to condition, inserted a catheter with a heparin lock into the dominant forearm (right) for hourly blood draws and one into the non-dominant forearm (left) for a continuous saline flush and for drug administration. Ninety minutes after arrival to the CTRC, each participant was randomly assigned to receive either low-dose endotoxin (0.8 ng/kg of body weight) or placebo (same volume of 0.9% saline), which was administered by the nurse as an intravenous bolus. The endotoxin was derived from Escherichia coli (E. coli group O:113: BB-IND 12948 to M.R.I.) and was provided by the National Institutes of Health Clinical Center as a reference endotoxin for studies of experimental inflammation in humans (A. F. Suffredini et al., 1999); previous research has demonstrated the safety of this reference endotoxin across many different samples (Andreasen et al., 2008; A. Suffredini et al., 1999). The random allocation sequence was generated by a consultant who was not involved in running participants and was kept by the UCLA Pharmacy to ensure proper drug preparation for each participant. Randomization was done using a computerized uniform random number generator; males and females were randomized separately in permuted blocks of 4. Participants were enrolled in the study by a study coordinator (I.J.). The two conditions were not different in appearance or method of administration. However, it is possible that participants in the endotoxin condition who felt very symptomatic may have suspected that they were in the active condition. For this reason, participants who were out of range on self reports of physical sickness symptoms in the endotoxin condition were removed from all analyses (described below).
Throughout the study, vital signs and blood draws (to assess cytokine levels) were collected at baseline and then approximately every hour for the next six hours. Participants also completed hourly measures of sickness symptoms and socioemotional responses (reported in Moieni et al., in press). The social cognition task, a pre-specified secondary behavioral outcome, was completed at baseline and approximately two hours post-injection (1 hour and 40 minutes post injection), the peak inflammatory response for the endotoxin group (please see Figure 1 and Moieni et al., in press, for further details on timing). Participants were discharged from the CTRC following the last blood draw upon approval from the study’s physician; approval was granted once self-reported symptoms returned to baseline levels. The Data Safety Monitoring Board met periodically during the course of the study in order to evaluate participant safety by comparing reports from the nurses, the study coordinator (I.J.), and participants, but data were not formally analyzed during or for these meetings. Of the 115 participants that completed the study, five participants experienced unexpected, but not harmful, symptoms (e.g., diarrhea, nausea) that prevented them from completing all components of the study protocol (i.e., the aforementioned neuroimaging component). An additional participant lost consciousness prior to drug administration upon insertion of the intravenous catheter; this participant did not complete the study due to safety concerns.
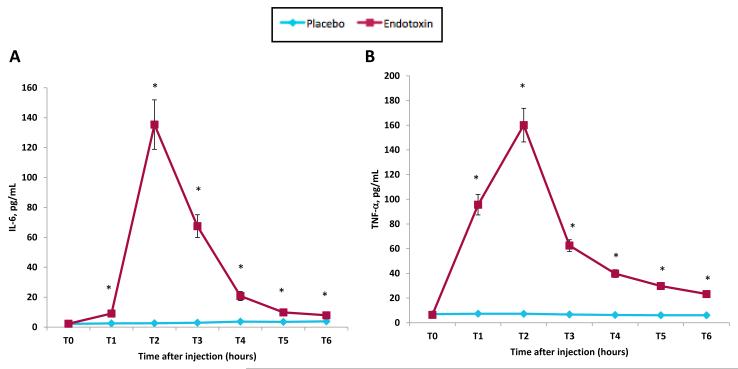
Figure 1.
Changes over time in: plasma levels of (A) IL-6 and (B) TNF-α (raw values that have not been log-transformed are shown). Time points with asterisks indicate significant time (baseline vs. each subsequent time point) by condition interactions. Error bars depict the standard error of the mean. Because participants completed a neuroimaging session (reported separately) starting at exactly 2 hours post-injection, T2 was assessed prior to this scanning session at 1 hour 40 minutes post-injection and T3 was assessed immediately after the scan at 3 hours and 30 minutes post-injection; T4-T6 were assessed hourly after T3.
At the end of the study, participants were thanked, debriefed, and paid for their participation ($200 for the day of the study and $20 for the prior screening session for a total of $220). All subjects provided written consent before participating, as obtained by the study physician (M.R.I). Prior to providing consent, the consent form was reviewed with all subjects, and they were encouraged to discuss any questions or concerns they had with the study physician. Further details about methods are available in the report detailing the pre-specified primary outcomes of this study (i.e., depressed mood, social disconnection; Moieni et al., in press). All procedures were approved by the UCLA Human Subjects Protection Committee.
2.2 Behavioral assessments
2.2.1 Self-reported sickness symptoms
Physical sickness symptoms (headaches, muscle pain, shivering, nausea, breathing difficulties, and fatigue) were assessed from T0-T6. Participants rated the extent to which they felt each symptom on a scale from 0 (no symptoms) to 4 (very severe symptoms). Participants who reported anything other than “no symptoms” (i.e., reporting anything other than “0”) for 3 or more physical sickness symptoms at baseline were removed from all analyses (2 participants were removed using these criteria). Additionally, in order to reduce the likelihood that any between-group differences in social or affective responses were due to severe sickness symptoms, any participant who reported “very severe” (i.e., reporting “4”) sickness symptoms for 3 or more symptoms across all time points were removed (2 participants were removed using these criteria). For the rest of the participants, scores from each of these items were then dichotomized for each time point: any reports of 3 (“severe”) or greater for any of the symptoms were scored as a physical symptom response, zero otherwise.
2.2.2 Feelings of confusion
Because some prior work has found that inflammation can lead to decreases in performance on non-social cognitive tasks (Reichenberg et al., 2001) and increases in mental confusion (Brydon et al., 2008), we measured feelings of confusion from T0-T6 using a short-form version of the Profile of Mood States (POMS; Baker et al., 2002; McNair et al., 1971) in order to account for potential changes in confusion, which may reflect changes in global cognition. Participants rated the extent to which they felt “confused” and “bewildered” on a scale from 0 (not at all) to 4 (extremely). Measures of confusion were calculated by averaging scores from these two items at each timepoint.
2.2.3 Depressed mood
As previously reported (Moieni et al., in press), we measured depressed mood from T0-T6 using the POMS. Participants rated the extent to which they felt: “unhappy,” “sad,” blue,” “hopeless,” “discouraged,” “miserable,” “helpless,” and “worthless” on a scale from 0 (not at all) to 4 (extremely). Measures of depressed mood were calculated by averaging scores from each of these items at each timepoint. Although results from depressed mood analyses were reported on previously (Moieni et al., in press), they are evaluated here as covariates to ensure that any effects of endotoxin on social cognitive performance were not primarily driven by changes in depressed mood.
2.2.4 Feelings of social disconnection
As previously reported (Moieni et al., in press), feelings of social disconnection were also assessed from T0-T6. Participants rated items such as: “I feel lonely,” “I feel disconnected from others,” and “I feel isolated from others” on a scale from 1 (not at all) to 5 (very much so). The questionnaire included 12 items and was created using items from a social disconnection scale (Eisenberger et al., 2010), a loneliness scale (Joiner et al., 2002) and adding two additional items: “I feel lonely” and “I feel liked” (Russell, 1996), and scores were averaged at each timepoint to create a measure of self-reported social disconnection. Similar to the depressed mood analyses, results from the social disconnection analyses were reported on previously (Moieni et al., in press); they are evaluated here as covariates to ensure that effects of endotoxin on social cognitive performance were not primarily driven by changes in social disconnection.
2.2.5 Social cognition task
Social cognition performance was assessed at baseline (T0) and approximately two hours post-endotoxin/placebo administration (T2), the peak of response for the endotoxin group, using the Reading the Mind in the Eyes (RME) test, which assesses ToM capabilities (Baron-Cohen et al., 2001). For this task, participants were shown a series of pictures that each showed a set of eyes displaying a subtle affective expression and were asked to choose the word which “best describes what the person in the picture is thinking or feeling,” using a selection of four possible word choices. Examples of expressions shown include eyes that expressed feeling upset, thoughtful, confident, suspicious, regretful, or playful. We divided the 36-item RME test into two sets of 18 facial expressions such that participants were shown one set at baseline and the other set at T2; the order of the two sets was counterbalanced across participants. The number of correct responses was totaled and a percentage of correct answers was calculated for each time-point; all results for the social cognition task are reported in terms of percentage of correct responses.
2.3 Plasma levels of cytokines
Whole blood samples were collected in pre-chilled EDTA tubes. After collection, the samples were centrifuged at 4°C, plasma was harvested into multiple aliquots, and then stored in a −70°C freezer until the completion of the study.
Using a Bio-Plex 200 (Luminex) Instrument, Bio-Plex software v4.1, and a 5-parameter logistic curve fit, plasma levels of IL-6 and TNF-α were quantified by means of high sensitivity bead-based multiplex immunoassays (Performance High Sensitivity Human Cytokine, R& D Systems, Minneapolis, MN). This R&D Systems multiplex assay has been shown to have excellent intra- and inter-assay reproducibility for these two analytes in a recent temporal stability study of circulating cytokine levels (Epstein et al., 2013) and very strong correlations (r > .94) across a wide range of concentrations with high sensitivity ELISA kits from the same manufacturer (Breen et al., 2014). All multiplex assays were performed on plasma samples diluted 2-fold according to the manufacturer’s protocol, and all calculated concentrations generated by the BioPlex Manager software were included in data analyses. Due to the strength of the parent study design (Eisenberger et al., 2010; Eisenberger et al., 2009), which utilized repeated measures of cytokine values over seven time points for each subject, each time point was evaluated in a single determination. Every subject demonstrated the expected profile of change of cytokine concentrations over time, based on these previous studies (Eisenberger et al., 2010; Eisenberger et al., 2009).
Paired samples from each subject (baseline and all subsequent time points) were assayed on the same 96-well plate; multiplex assays were chosen for the analyses because of the large dynamic range necessary to evaluate both low physiologic (baseline) and very high (post-endotoxin) cytokine concentrations in the same assay. The ranges of detection for IL-6 and TNF- were 0.2-3800 pg/mL and 0.8-3100 pg/mL, respectively, and no samples exceeded the upper limit of detection for either analyte. The mean intra-assay CV% of the standards was < 8% for IL-6 and TNF- ; the inter-assay CV% of an internal laboratory quality control sample was < 13% for both analytes.
2.4 Statistical analyses
To assess between-group differences in the effect of endotoxin vs. placebo on cytokines and social cognitive performance, we used a standard statistical software program (SPSS 22.0) to conduct repeated-measures analyses of variance (ANOVA). These analyses tested time (e.g., baseline vs. T2) by condition (endotoxin vs. placebo) interactions. Effect sizes were calculated for changes in social cognitive performance. Follow-up analyses were conducted using paired t-tests to examine differences between baseline and T2 scores within each group (e.g., changes in social cognitive performance within the endotoxin group from baseline to T2).
To examine differences in self-reported sickness symptoms, a dichotomous outcome, we used a non-parametric Fisher’s Exact Test to examine differences in sickness symptoms by condition (endotoxin vs. placebo) at each time point. Because the cytokine values were not normally distributed at any time point, values were log-transformed, and due to known effects of BMI on cytokines, we controlled for BMI in all cytokine analyses. Additionally, subjects whose scores were over three standard deviations on the dependent variables of interest were removed from the respective analyses in order to improve the robustness of the results to replication, improve accuracy, and reduce errors (Osborne & Overbay, 2004). All analyses were pre-specified and based on a priori hypotheses.
3. Results
3.1 Characteristics of the sample
Participants who were outliers (±3 SD) on the Beck Depression Inventory (BDI; Beck et al., 1988) were removed from all analyses (2 participants were removed using these criteria). Additionally, as mentioned above, four participants were removed for being out of range on sickness symptoms. Of the remaining 109 participants (65 female; mean age: 24.1 ± 6.3), 51 were randomized into the placebo condition and 58 were randomized into the endotoxin condition. For further demographic information about this sample, please see the previous report (Moieni et al., in press)
3.2 Physiological responses to endotoxin
As reported previously (Moieni et al., in press), endotoxin (vs. placebo) led to significant increases in proinflammatory cytokines IL-6 and TNF-α from baseline to one hour post-injection through six hours-post injection (Figure 1; T1-T6; all p’s <.01). Endotoxin (vs. placebo) participants also reported significantly greater sickness symptoms at T2 (Fisher’s Exact p < .001), but not at any other time points (Fisher’s Exact p’s > .2). Consistent with previous work (e.g., Eisenberger et al., 2010), the peak response (as indexed by IL-6, TNF-α, and self-reported sickness symptoms) occurred at 2 hours post-injection (T2) in the endotoxin group. These results are described in further detail in a separate report (Moieni et al., in press).
3.3 Social cognitive performance
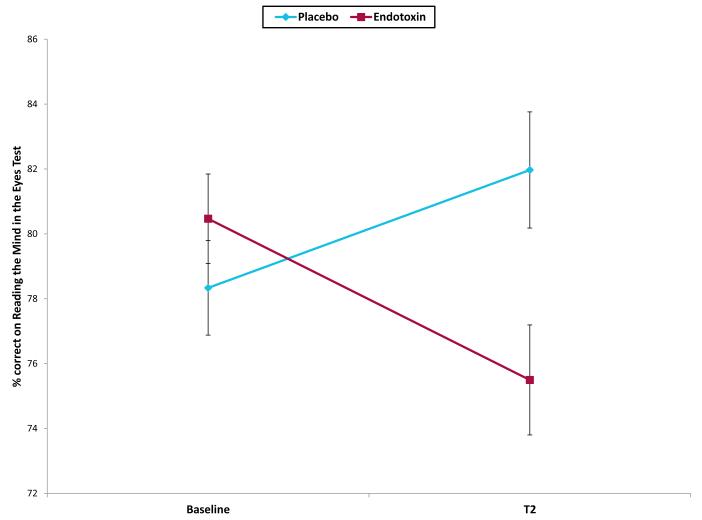
The two groups did not perform differently from each other on the RME test at baseline (placebo mean 78.3 ± 1.42; endotoxin mean 80.5 ±1.41; p = .3). However, there was a significant condition by time interaction, such that endotoxin (vs. placebo) led to significantly greater decreases in social cognitive performance, as measured by performance on the RME test from baseline to T2 (Figure 2; condition*time: F(1,106)=12.21, p < .01; ηp2= .10). Follow-up paired sample t-tests revealed that the endotoxin group showed a significant decrease in performance on the RME test from baseline to T2 (t(56)=−2.85, p < .01), whereas the placebo group showed a significant increase in performance on the RME test from baseline to T2 (t(50)=2.10, p<.05), which could be due a practice effect. Interestingly, these results remained significant even after controlling for self-reported sickness symptoms at T2 (F(1,104)=8.97, p < .01; η 2p=.08), suggesting that decreases in performance on the RME test were not solely due to endotoxin-induced sickness symptoms. There were no sex differences in these effects (F(1,104)=.03, p > .8).
Figure 2.
Performance on the Reading the Mind in the Eyes (RME) test at baseline and T2 (peak of inflammatory response for the endotoxin group). Error bars depict the standard error of the mean.
Furthermore, inflammation did not lead to changes in feelings of mental confusion from baseline to T2 (F(1,104)=.58, p > .4), and decreases in RME performance remained after controlling for self-reported feelings of mental confusion at T2 (F(1,104)=12.29, p < .01; η 2p=.11), suggesting that the decreases in social cognitive performance were not reflective of global changes in cognition or confusion.
Endotoxin led to increases in depressed mood (F(1,101)=8.03, p < .01) and feelings of social disconnection (F(1,107)=15.89, p < .001) from baseline to T2 (see Moieni et al., in press for details). However, decreases in RME performance remained after controlling for depressed mood at T2 (F(1,104)=10.70, p < .01; η 2p=.09) and feelings of social disconnection at T2 (F(1,105)=10.38, p < .01; η 2p=.09), suggesting that the observed changes in social cognition were not solely due to other observed socioemotional changes.
Finally, within the endotoxin group, changes on the RME test from baseline to T2 did not correlate with changes in IL-6 or TNF-α (p’s > .9).
4. Discussion
The findings from this study extend the literature by demonstrating, for the first time, that experimental inflammation can lead to deficits in social cognition. More specifically, endotoxin-induced inflammation led to significant decreases in performance on a ToM task, suggesting that inflammation can impair the ability to reliably perceive others’ emotional states, and these effects were not simply due to greater sickness symptoms or mental confusion. Decreases in this capability may ultimately cause deficits in the ability to successfully sustain the complex social interactions crucial to maintaining social relationships. Although a prior study found no effects of endotoxin-induced inflammation on performance on the RME test, this could have been due to a lower dose of endotoxin and/or a smaller sample size (Kullmann et al., 2013). Furthermore, the prior study involved participants completing the RME task in an MRI scanner, which is also distinct from the present study. Given that animal work has shown that stressful environmental contexts can impact the behavioral effects of endotoxin (Willette et al., 2007), and other work has shown that stress can impair social cognitive processes (Merz et al., 2010), it is also possible that the context of the scanning environment led to differences in subjects’ performance across these two studies.
The findings from the present study suggest that a consequence of heightened inflammation may be declines in the ability to correctly infer the mental and emotional states of others. This is important given that proinflammatory cytokines are thought to play a role in the pathophysiology of neuropsychiatric disorders such as autism and schizophrenia (Fineberg & Ellman, 2013; Meyer et al., 2011; Meyer et al., 2009; Michel et al., 2012; Onore et al., 2012; Patterson, 2009), which often include impairments in ToM (Baron-Cohen et al., 1985; Brüne & Brüne-Cohrs, 2006; Couture et al., 2010; Frith & Corcoran, 1996; Sprong et al., 2007). Thus, this experimental work showing that heightened proinflammatory activity can lead to decreases in ToM suggests that proinflammatory activity that may be contributing to the social cognitive deficits observed in these disorders.
Interestingly, changes in performance on the RME test were not correlated with changes in cytokines within the endotoxin group. This suggests that either decreases in ToM do not have a dose-dependent relationship with increases in cytokines or that the RME test is not sensitive enough to map onto incremental changes in cytokines. Future research should investigate additional ToM tasks in order to investigate whether changes in levels of cytokines correlate with changes in ToM performance on other tasks.
Furthermore, a limitation of this study is that participants’ response times in answering the RME items were not recorded. This information could provide clues as to the level of effort participants put into inferring the correct emotional states of others on the task (e.g., gauging whether participants were “giving up” immediately or answering incorrectly after much thoughtful deliberation). Future studies incorporating this information about timing may provide a more comprehensive picture of inflammation-induced changes in social cognition. However, in the present study, we attempted to overcome this limitation by statistically controlling for physical sickness symptoms, aiming to reduce effects primarily due to participants “giving up” immediately due to increasing sickness symptoms. Indeed, the endotoxin-induced decreases in ToM performance remained after controlling for sickness symptoms, indicating that it is unlikely that participants in the endotoxin group were impaired on the RME test because of increasing sickness symptoms.
Given that depressed mood and feelings of social disconnection increased in response to endotoxin in this sample (Moieni et al., in press), we also explored whether controlling for these socioemotional changes accounted for changes in RME performance. Decreases in RME performance remained after controlling for depressive symptoms and feelings of social disconnection, suggesting that the observed changes in social cognition were not solely due to other affective or socioemotional changes. However, we still recognize that these results should be interpreted with caution. While we found that changes in RME performance were not due to factors such as sickness symptoms, depressed mood, or feelings of social disconnection, there may still be unmeasured motivational factors which could account for the observed changes. As such, future research should attempt to parse out whether these decreases in RME performance are truly due to inflammatory-induced changes in ToM or a compromised motivational state.
Another limitation of this study is that we did not include a task assessing non-social cognition. However, by reporting and statistically controlling for feelings of mental confusion, we tried to account for potential changes in non-social cognition. We found that inflammation did not lead to changes in feelings of mental confusion and that the decreases in social cognitive performance remained after controlling for feelings of confusion. These results suggest that changes in mental confusion, which may reflect changes in non-social cognition, were not responsible for the observed decreases in social cognitive performance.
In sum, these findings indicate that heightened inflammation can lead to decreases in ToM capabilities. To our knowledge, this study provides the first evidence that experimentally-induced inflammation can lead to decreases in the ability to accurately and reliably comprehend emotional information from others, which is a critical aspect of social interaction that is altered in neuropsychiatric disorders such as schizophrenia and autism. Thus, proinflammatory cytokines, which are hypothesized to be linked to schizophrenia and autism, may drive social deficits and contribute to impairment in social cognition found in individuals with schizophrenia and autism. Future research is needed to develop a more comprehensive understanding of inflammation-induced changes in social cognition and the consequences of such changes in both healthy populations and in individuals with neuropsychiatric disorders (e.g., schizophrenia, autism).
Supplementary Material
Highlights.
First study to show experimental inflammation leads to deficits in social cognition
Acute inflammation led to decreases in performance on theory of mind task
Results have implications for understanding disorders like autism and schizophrenia
Acknowledgements
We would like to thank the staff and support of the UCLA Clinical and Translational Research Center, as well as Anthony Suffredini, M.D. at the National Institutes of Health, Warren Grant Magnuson Clinical Center, for providing the standard reference endotoxin, as well as Spencer Bujarski, M.A. for providing statistical consulting; neither Dr. Suffredini nor Mr. Bujarski were compensated. This research was funded by an R01 from NIMH to NIE (5R01MH091352). The authors also acknowledge the additional support provided by R01AG034588; R01AG026364; R01CA160245-01; R01CA119159; R01HL095799; R01DA032922-01; P30AG028748 to MRI; and UCLA CTSI UL1TR000124, and the Cousins Center for Psychoneuroimmunology. Additionally, the first author was supported by a pre-doctoral NRSA individual fellowship from NIA (1F31AG048668) and a pre-doctoral NRSA training fellowship from NIGMS (5T32GM084903). The aforementioned funders provided financial support for the study, but they were not involved in the conduct of the study in any other capacity (e.g., design, data collection, manuscript preparation, etc.).
Footnotes
Clinical Trials Registration. ClinicalTrials.gov NCT01671150
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen A, Krabbe K, Krogh-Madsen R, Taudorf S, Pedersen B, Moller K. Human endotoxemia as a model of systemic inflammation. Current Medicinal Chemistry. 2008;15(17):1697–1705. doi: 10.2174/092986708784872393. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain, Behavior, and Immunity. 2011a;25(1):40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Altered T cell responses in children with autism. Brain, Behavior, and Immunity. 2011b;25(5):840–849. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F, Denniston M, Zabora J, Polland A, Dudley WN. A POMS short form for cancer patients: psychometric and structural evaluation. Psycho-Oncology. 2002;11(4):273–281. doi: 10.1002/pon.564. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” test revised version: A study with normal adults, and adults with Asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry. 2001;42(2):241–251. [PubMed] [Google Scholar]
- Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8(1):77–100. [Google Scholar]
- Breen E, Perez C, Olmstead R, Eisenberger N, Irwin M. 135. Brain, Behavior, and Immunity. 2014;40:e39. [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, Susser ES. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. American Journal of Psychiatry. 2004;161(5):889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Brown AS, Sourander A, Hinkka-Yli-Salomäki S, McKeague I, Sundvall J, Surcel H. Elevated maternal C-reactive protein and autism in a national birth cohort. Molecular Psychiatry. 2013;19(2):259–264. doi: 10.1038/mp.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne M. Social cognition and psychopathology in an evolutionary perspective. Psychopathology. 2001;34(2):85–94. doi: 10.1159/000049286. [DOI] [PubMed] [Google Scholar]
- Brüne M, Brüne-Cohrs U. Theory of mind—evolution, ontogeny, brain mechanisms and psychopathology. Neuroscience and Biobehavioral Reviews. 2006;30(4):437–455. doi: 10.1016/j.neubiorev.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biological Psychiatry. 2008;63(11):1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH. Maternal cytokine levels during pregnancy and adult psychosis. Brain, Behavior, and Immunity. 2001;15(4):411–420. doi: 10.1006/brbi.2001.0644. [DOI] [PubMed] [Google Scholar]
- Canetta S, Sourander A, Surcel H-M, Hinkka-Yli-Salomäki S, Leiviskä J, Kellendonk C, Brown AS. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. American Journal of Psychiatry. 2014;171(9):960–968. doi: 10.1176/appi.ajp.2014.13121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture S, Penn D, Losh M, Adolphs R, Hurley R, Piven J. Comparison of social cognitive functioning in schizophrenia and high functioning autism: more convergence than divergence. Psychological Medicine. 2010;40(04):569–579. doi: 10.1017/S003329170999078X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, Behavior, and Immunity. 2010;24(4):558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47(3):881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom AM, Onore CE, Van de Water JA, Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain, Behavior, and Immunity. 2010;24(1):64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein MM, Breen EC, Magpantay L, Detels R, Lepone L, Penugonda S, Birmann BM. Temporal stability of serum concentrations of cytokines and soluble receptors measured across two years in low-risk HIV-seronegative men. Cancer Epidemiology Biomarkers & Prevention. 2013;22(11):2009–2015. doi: 10.1158/1055-9965.EPI-13-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett A-KJ, Viechtbauer W, Dominguez M.-d.-G., Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Fineberg AM, Ellman LM. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biological Psychiatry. 2013;73(10):951–966. doi: 10.1016/j.biopsych.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. American Psychiatric Pub; 2012. [Google Scholar]
- Frith CD, Corcoran R. Exploring ‘theory of mind’in people with schizophrenia. Psychological Medicine. 1996;26(03):521–530. doi: 10.1017/s0033291700035601. [DOI] [PubMed] [Google Scholar]
- Grigoleit J-S, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S, Schedlowski M. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS ONE. 2011;6(12):e28330. doi: 10.1371/journal.pone.0028330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner TE, Lewinsohn PM, Seeley JR. The core of loneliness: Lack of pleasurable engagement--more so than painful disconnection--predicts social impairment, depression onset, and recovery from depressive disorders among adolescents. Journal of Personality Assessment. 2002;79(3):472–491. doi: 10.1207/S15327752JPA7903_05. [DOI] [PubMed] [Google Scholar]
- Kullmann JS, Grigoleit J-S, Wolf OT, Engler H, Oberbeck R, Elsenbruch S, Gizewski ER. Experimental human endotoxemia enhances brain activity during social cognition. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst049. nst049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi A, Quintana D, Glozier N, Lloyd A, Hickie I, Guastella A. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Molecular Psychiatry. 2014 doi: 10.1038/mp.2014.59. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. Education and Industrial Testing Service; San Diego, CA: 1971. [Google Scholar]
- Merz CJ, Wolf OT, Hennig J. Stress impairs retrieval of socially relevant information. Behavioral Neuroscience. 2010;124(2):288. doi: 10.1037/a0018942. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatric Research. 2011;69:26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophrenia Bulletin. 2009;35(5):959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M, Schmidt MJ, Mirnics K. Immune system gene dysregulation in autism and schizophrenia. Developmental Neurobiology. 2012;72(10):1277–1287. doi: 10.1002/dneu.22044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biological Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: Implications for sex differences in depression [published online ahead of print 02/04/2015] Neuropsychopharmacology. doi: 10.1038/npp.2015.17. (in press) doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain, Behavior, and Immunity. 2012;26(3):383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne JW, Overbay A. The power of outliers (and why researchers should always check for them) Practical assessment, research & evaluation. 2004;9(6):1–12. [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behavioural Brain Research. 2009;204(2):313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behavioral and Brain Sciences. 1978;1(04):515–526. [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Archives of General Psychiatry. 2001;58(5):445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Ricci S, Businaro R, Ippoliti F, Vasco VL, Massoni F, Onofri E, Ricciardi MR. Altered cytokine and BDNF levels in Autism spectrum disorder. Neurotoxicity Research. 2013;24(4):491–501. doi: 10.1007/s12640-013-9393-4. [DOI] [PubMed] [Google Scholar]
- Romero E, Guaza C, Castellano B, Borrell J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Molecular Psychiatry. 2008;15(4):372–383. doi: 10.1038/mp.2008.44. [DOI] [PubMed] [Google Scholar]
- Russell DW. UCLA Loneliness Scale (Version 3): Reliability, validity, and factor structure. Journal of Personality Assessment. 1996;66(1):20–40. doi: 10.1207/s15327752jpa6601_2. [DOI] [PubMed] [Google Scholar]
- Sprong M, Schothorst P, Vos E, Hox J, Van Engeland H. Theory of mind in schizophrenia Meta-analysis. British Journal of Psychiatry. 2007;191(1):5–13. doi: 10.1192/bjp.bp.107.035899. [DOI] [PubMed] [Google Scholar]
- Suffredini A, O’Grady N, Brade H, Opal S, Vogel S, Morrison D. Pathophysiological responses to endotoxin in humans. Endotoxin in health and disease. 1999:817–830. [Google Scholar]
- Suffredini AF, Fantuzzi G, Badolato R, Oppenheim JJ, O’Grady NP. New insights into the biology of the acute phase response. Journal of Clinical Immunology. 1999;19(4):203–214. doi: 10.1023/a:1020563913045. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Matsuzaki H, Iwata K, Kameno Y, Shimmura C, Kawai S, Takagai S. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS ONE. 2011;6(5):e20470. doi: 10.1371/journal.pone.0020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette AA, Lubach GR, Coe CL. Environmental context differentially affects behavioral, leukocyte, cortisol, and interleukin-6 responses to low doses of endotoxin in the rhesus monkey. Brain, Behavior, and Immunity. 2007;21(6):807–815. doi: 10.1016/j.bbi.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.