Abstract
Objective
To determine whether dry needling of an active myofascial trigger point (MTrP) reduces pain and alters the status of the trigger point to either a non-spontaneously tender nodule or its resolution.
Design
A prospective, non-randomized, controlled interventional clinical study
Setting
University campus
Participants
Fifty-six subjects with neck or shoulder girdle pain > 3 months duration and active MTrPs were recruited from a campus-wide, volunteer sample. Fifty-two completed the study (23 male/33 female) with mean age of 35.8 years.
Interventions
Three weekly dry needling treatments of a single active MTrP
Main Outcome Measures
Primary Outcomes: Baseline and post treatment evaluations of pain using the verbal analogue scale, the Brief Pain Inventory and the status of the MTrP as determined by digital palpation. Trigger points were rated: active (spontaneously painful), latent (requiring palpation to reproduce the characteristic pain) and resolved (no palpable nodule).
Secondary Outcomes: Profile of Mood States, Oswestry Disability Index, Short Form 36, Cervical Range of Motion.
Results
Primary outcomes: 41 subjects had a change in trigger point status from active to latent or resolved; and 11 had no change (p < .001). Reduction in all pain scores was significant (p<.001).
Secondary outcomes: significant improvement in post-treatment cervical rotational asymmetry in subjects with unilateral/bilateral MTrPs (p=.001, p=21, respectively); in pain pressure threshold in subjects with unilateral/bilateral MTrPs, (p=.006, p=.012), respectively; improvement in the SF-36 mental health and physical functioning subscales (p=.019, p=.03) respectively; decrease in the Oswestry disability scale (p=.003).
Conclusions
Dry needling reduces pain and changes MTrP status. Change in trigger point status is associated with a statistically and clinically significant reduction in pain. Reduction in pain is associated with improved mood, function and level of disability.
Keywords: Myofascial pain, trigger point, dry needling
Introduction
Myofascial pain syndrome (MPS) is a common and significant clinical problem, accounting for 15% of general medical visits.1 MPS negatively impacts function and participation in life activities.2, 3
MPS has generated controversy in part because there has been disagreement about diagnostic criteria. The syndrome has had many names including fibrositis, myofasciitis, and myogelosis4, 5 reflecting lack of agreement about etiology, pathophysiology and the primary tissue involved. MPS has been confused with other pain syndromes such as fibromyalgia and neuropathic pain and while confusion remains, there is general acceptance of the term MPS and its diagnostic components.6, 7, 8
There is active debate about whether the MTrP is a necessary condition for the diagnosis of MPS and whether it should be the target for pain relief. This paper explored this relationship in part because there seems to be agreement that the MTrP is an objective finding associated with MPS that is reliably identified and useful in assessing pain.9,10, 11, 12
In this study, we used Travell and Simons' definition of MPS: a regional pain syndrome in which there is a palpable, discreet nodule within a taut band of skeletal muscle that is spontaneously painful.9, 10 This is referred to as an active trigger point (a-MTrP), defined as a spontaneously painful nodule. A latent myofascial trigger point, (l-MTrP), is a trigger point that is not spontaneously painful and requires palpation or motion/activity to induce pain.
Dry needling is a non-pharmacological treatment for MPS commonly used for reducing pain associated with a-MTrPs.13, 14 It is frequently performed by a clinician using a 32 gauge acupuncture needle inserted into the palpably painful nodule using a superficial (10-20 mm) or deep (25-40mm) needling technique. Elicitation of one or more local twitch responses is a goal of dry needling and often benefits those with pain secondary to MTrPs.3
The effectiveness of dry needling has been difficult to demonstrate due to the lack of objective measures of pain. Currently, assessment of people with MPS relies upon patient self-reports of pain. Patient reported outcomes (PROs) are reliable measures, but their sensitivity to change, the variety of ways of expressing pain by individual patients, and correlations with physical findings, and other objective measures has made validation difficult.
Our research team used the status of the MTrP as the treatment target and an outcome measure in order to assess the changes that resulted from treatment; and determine whether change in its status correlated with change in post-treatment level of pain.
This paper presents the results of a prospective, interventional clinical study designed to assess whether dry needling of an a-MTrP alters patient reported pain and contemporaneously alters the status of the trigger point. We selected a technique widely used in clinical practice shown to be effective in reducing MPS, but whose effect on the MTrP is not known.3, 13, 14 We also measured the impact of dry needling on self-reports of mood, and function.
To our knowledge, this is the first study to investigate the association between dry needling, and its effect on pain reduction and MTrP status.
Methods
The study was approved by the Chesapeake Institutional Review Board. Subjects were recruited by posting flyers around a university community. No remuneration was offered to participants. All provided consent.
Study entry required that participants were adults (age 18-65 years), experienced pain without provocation for at least 3 months in the neck/shoulder girdle region and a palpable MTrP in one or both of the specific locations of the upper trapezius. The spontaneous pain had to be in the area of the prescribed MTrP locations and its palpation had to exacerbate pain. Radiation to head, neck or face on palpation was acceptable, but not required for inclusion. All evaluations and treatments were performed by 2 experienced clinicians, each with more than 20 years of treatment experience. Patients selected which day of the week was preferable for treatment and followup, thereby were assigned to the physician who treated on a specific day of the week. That is, physician #1 treated on Fridays and physician #2 on Thursdays. Occasionally, patients were seen on the alternative day if scheduling required a change.
Inter-observer reliability for the two treating physicians was tested using 14 treatment-naïve volunteers with and without pain. Each provided informed consent for evaluation. Two sites were examined independently by each of the two examiners and scored as active, latent or non-painful nodule/normal. Inter-rater reliability was assessed using a kappa statistic. The kappa statistic for site 2 is 0.74 (p=.003) and for site 3, the kappa statistic is 0.87 (p<.001). Entry exclusions included: chronic fatigue syndrome, fibromyalgia, chronic Lyme disease, cervical radiculopathy, head/neck/shoulder girdle surgeries, new medication or change within 6 weeks and current use of acupuncture.
All study subjects received 3 successive dry needling sessions weekly. Post-treatment evaluations were performed at 3 weeks. Treatment technique was standardized as follows: 4 predetermined examination areas were palpated and point(s) were identified.2 They were: 2cm medial to the acromioclavicular joint on L and R sides and two additional sites in the upper trapezius as it turns cephalad lateral to the spinous process of C7. Trigger points reported to be spontaneously painful were a-MTrP; those not spontaneously painful, but painful upon palpation were designated l-MTrPs. Only one a-MTrP was selected for treatment. If there was more than one a-MTrP, we selected the most symptomatic site for dry needling. Hence, there may have been untreated a-MTrPs.
Some subjects had a-MTrPs on only one side, which we defined as “unilateral”. Some subjects had at least one a-MTrP on each side, which we defined as “bilateral”. We defined “responders” as patients whose status changed from a-MTrP to l-MTrP, or a-MTrP to an asymptomatic palpable nodule or no nodule palpable. “Non-responders” were those whose a-MTrP remained active (spontaneously painful). This status was determined by a treating physician (not always the one who performed the dry needling treatment) who palpated and assessed whether the findings were consistent with a-MTrP, l-MTrP, non-painful nodules or no palpable nodule. The selected a-MTrP was prepared by wiping the area with an alcohol pad and a 32-gauge needle with its plastic guide tube in place was placed over the a-MTrP (Figure 1). A tapping motion was used to advance the needle. Occasionally, needle movement was performed around the nodule following a 4- points-of-compass technique with rotation along its long axis in an effort to elicit a small muscle twitch. This was achieved in approximately 70% of subjects on the first, 66% on the second and 50% on the third treatment. Change in VAS was not statistically correlated with eliciting the twitch response.
Figure 1. Demonstration of Needle Insertion into Myofascial Trigger Point.
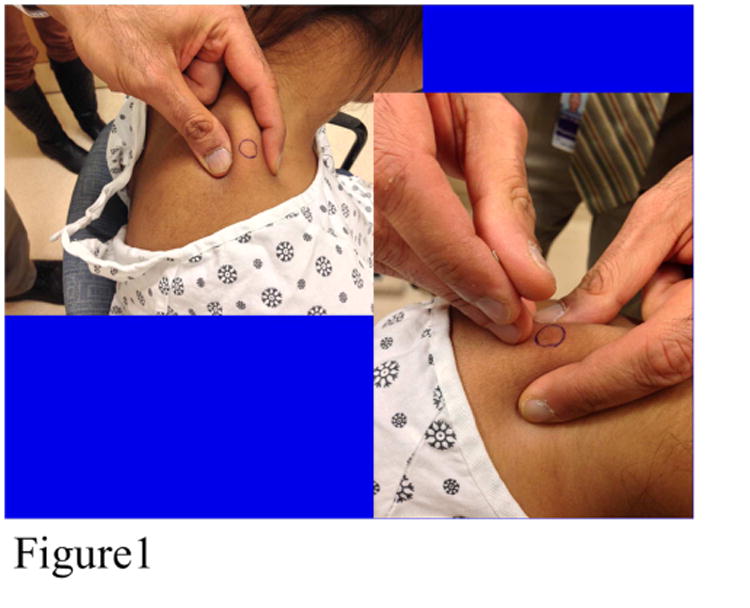
All evaluations were performed at baseline and after the third treatment at 3 weeks. Primary outcomes were measures of pain reduction and change in trigger point status from a-MTrP to either l-MTrP or no palpable nodule. A verbal analogue scale was used for pain assessment. It was scored 0-10 (0 being no pain and 10 being unbearable pain). The question was asked as follows: “Are you having pain now? Please rate it on a scale from 0-10. Do you have pain on the right side of your neck? Please rate this 0-10. Do you have pain on the left side of your neck? Please rate this 0-10”. Palpation was performed on 4 standard sites. Nodules were either active (spontaneously painful), latent (required overpressure to elicit pain) or not palpable (and no pain associated with palpation).
Secondary outcomes included range of motion (ROM) which was determined in 3 planes of movement (flexion/extension, side bending and rotation) using the CROM® (Deluxe Cervical Range of Motion Instrument, Model #12-1156 (Fabrication Enterprises, White Plains, NY). A ratio of measures of ROM over the normal range was determined for the left and right sides. The asymmetry was evaluated at baseline and at end of treatment (3 weeks). Two additional measures of pain included: a measure of pain pressure threshold (PPT) and the Brief Pain Inventory (BPI).15 PPT was obtained at four sites, following a standard procedure for assessing relative comparisons among the anatomical sites using a pressure algometer (Commander Algometer, Tech Medical, Salt Lake City, Utah http://www.jtechmedical.com/Commander/commander-algometer). (Figure 2) Subjects were instructed to identify the moment at which symptoms underwent a qualitative shift from pressure to pain during algometer compression. The reading at that time was determined to be the PPT score. A high score, that which requires more pressure to be applied to produce pain, was associated with improved pain symptoms.
Figure 2.
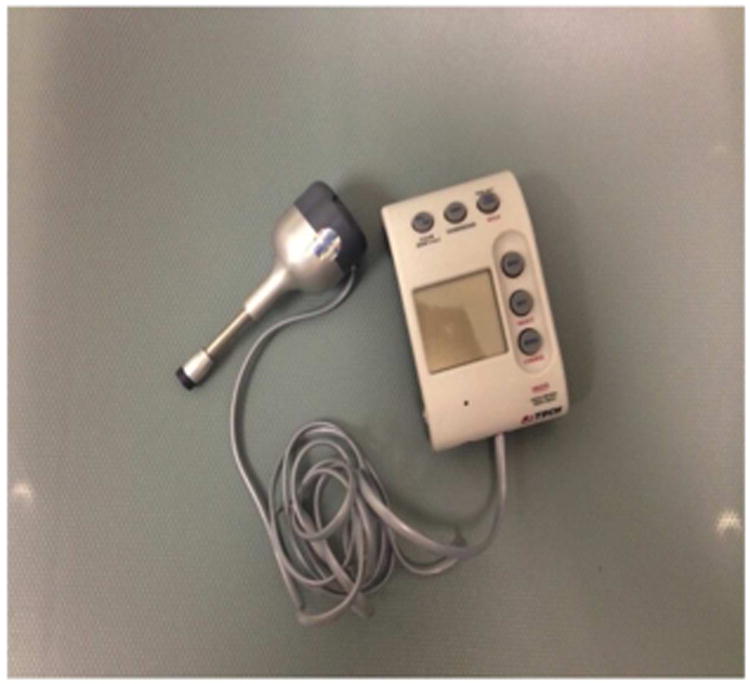
Algometer® Used for Measuring Pain Pressure Threshold, Tech Medical, Salt Lake City, UT.
Additional measures included Oswestry Disability Scale, a measure of disability secondary to the spine and adjacent musculoskeletal system. Subjects were instructed to reply with reference to the neck and upper thoracic area in terms of limitations. 16 Short Form 36 (SF36), a health status questionnaire,17 a short version of the Profile of Mood States,18 a symptom checklist of mood that included anxiety, depressive symptoms, sadness, et al. Subjects with high scores on Oswestry, POMS and VAS were considered more symptomatic/more disabled. A high score on SF36 was considered better health status.
Sample size was determined to be 90 subjects with an assumption that 5% of patients would spontaneously improve the status of the MTrP without dry needling. We wished to detect an increase of 10% for responders post-treatment. We conducted a conditional power analysis after 56 patients were accrued and determined the study to be substantially over-powered and our hypothesized percent of responders was underestimated.19
STATXACT20 was used to conduct an exact binomial test that the percent of responders exceeded 5% at the 0.05 (two-sided)_alpha level. Paired t-tests compared pain and variables of interest before and after treatment. These variables included both objective and self-reported outcomes. Analysis of covariance was used to detect changes in outcome measures for responders vs. non-responders.
Change from baseline in VAS, BPI, and PPT scores was analyzed, adjusted for baseline value, age, gender, group (unilateral/bilateral), and exercise status, by response to treatment, using regression analysis. For all models, studentized residual plots were inspected. For VAS scores and BPI scores, the residuals appeared homoscedastic with no outliers. For PPT scores, one subject was considered as an outlier. A Q-Q plot of residuals exhibited no indication of non-normality.
Each model was adjusted for gender, age, and exercise status and none was significant in any of the models. Regression diagnostics were graphically depicted, including checks for outliers and heteroscedasticity, and Q-Q plots to verify the normal error assumption. There were no outliers and no transformations were deemed necessary. All regression analyses were conducted using SAS software, version 9.3 (SAS Institute, Cary, NC).
Results
Fifty-two subjects were included in the study. Fifty-six were eligible and underwent study baseline procedures. Two had not completed three weekly dry needling sessions and dropped out for unknown reasons. One started new treatments after the first study treatment and one did not have complete follow-up data for analysis. Table 1 presents the distribution of the descriptive variables and a summary of treatments that subjects had selected for their pain prior to study entry.
Table 1. Descriptive Variables.
| Characteristics | Active Subjects | |
|---|---|---|
| N | % | |
| Gender | ||
| Male | 23 | 41.1 |
| Female | 33 | 58.9 |
| Age | ||
| Mean | 35.8 yr (20-62) | |
| Pain Distribution | ||
| Bilateral | 42 | 75 |
| Unilateral (Right/Left) | 9/5 | 16.1/8.9 |
| Pain Duration | ||
| <3years | 21 | 37.5 |
| >3 years | 35 | 62.5 |
| Use of Medication | ||
| Analgesics | 37 | 66 |
| Mood | 11 | 19.6 |
| Sleep | 1 | 1.8 |
| Opioids/Narcotics | 0 | 0 |
| Supplements/Vitamins | 30 | 53.6 |
| Use of Non-Pharmacologica Treatment | ||
| Exercise | 43 | 76.6 |
| Physical Modalities (heat, cold, electrical stimulation) | 32 | 57 |
| Massage | 17 | 30 |
| Chiropractic | 8 | 14 |
Table 2 presents the frequencies for the primary outcome in bilateral and unilateral groups, respectively. There were 41 responders and 11 non-responders (p < .001). A conditional power analysis was conducted. Under the current trend of the data, under the hypothetical trend of the data, and under the null hypothesis, the conditional power was 1, meaning that there was no positive probability of a non-significant result using the full sample size.
Table 2. Primary outcome for Treated Subjects with Bilateral and Unilateral Active Trigger Points.
| Bilateral Active Trigger Points | Unilateral Active Trigger Points | ||||
|---|---|---|---|---|---|
| Baseline | Follow-up | Count | Baseline | Follow-up | Count |
| Active | Active | 7 | Active | Active | 4 |
| Active | Latent | 12 | Active | Latent | 14 |
| Active | Normal | 6 | Active | Normal | 9 |
Table 3 presents baseline and follow-up characteristics for physical findings, pain and self-reports. We measured a significant improvement in rotational asymmetry in both the unilateral and bilateral groups (p=.001, p=.021, respectively). ROM extension and flexion had not improved. There was a significant change in side bending ROM in the unilateral group only (p=.001) and a significant improvement in PPT at the treated site in both groups (p=.006, p=.012, respectively). The baseline and follow-up characteristics for pain measurements and self-reports showed a significant reduction in BPI scores (p<.001). There was a significant reduction in VAS on the treated side in both unilateral and bilateral groups (p<.001); and on the untreated side only in the bilateral group (p<.001). There was a significant increase in the SF-36 pain subscale score (p=.002) and a decrease in the POMS tension and mood scores (p=.012, p=.013, respectively).
Table 3. Baseline and Follow-up Characteristics: Physical Findings, Pain and Self Reported Outcomes (Mean ± SD).
| Characteristic | n | Baseline | Follow-up | p-value |
|---|---|---|---|---|
| Physical Findings | ||||
| Cervical ROM Extension (degrees) | 51 | 73.8 ± 12.8 | 74.3 ± 12.0 | .741 |
| Cervical ROM Flexion (degrees) | 51 | 55.2 ± 11.0 | 57.1 ± 8.3 | .192 |
| Rotation Asymmetry Unilateral (degrees) | 27 | 8.1 ± 6.3 | 3.1 ± 5.4 | .001 |
| Rotation Asymmetry Bilateral (degrees) | 24 | 5.4 ± 4.4 | 2.4 ± 3.2 | .021 |
| Side Bending Unilateral (degrees) | 27 | 5.6 ± 3.8 | 2.7 ± 2.9 | .001 |
| Side Bending Bilateral (degrees) | 24 | 5.5 ± 6.4 | 3.1 ± 3.2 | .109 |
| PPT Treated Site Unilateral (pounds) | 27 | 7.6 ± 3.3 | 9.4 ± 3.7 | .006 |
| PPT Treated Site Bilateral (pounds) | 24 | 6.7 ± 3.0 | 8.4 ± 3.1 | .012 |
| Pain (scores) | ||||
| BPI | 49 | 3.4 ± 1.6 | 2.3 ± 1.9 | <.001 |
| VAS Treated Side Unilateral | 27 | 3.5 ± 2.4 | 0.9 ± 1.3 | <.001 |
| VAS Treated Side Bilateral | 25 | 3.0 ± 1.4 | 0.9 ± 1.2 | <.001 |
| VAS Untreated Side Unilateral | 27 | 1.0 ± 1.9 | 0.4 ± 1.1 | .203 |
| VAS Untreated Side Bilateral | 25 | 2.6 ± 1.2 | 0.9 ± 1.2 | <.001 |
| SF36 Pain | 50 | 62.5 ± 18.4 | 69.3 ± 16.5 | .002 |
| Self Reported Outcomes | ||||
| POMS Confusion | 49 | 0.28 ± 0.39 | 0.23 ± 0.35 | .418 |
| POMS Depression | 49 | 0.11 ± 0.23 | 0.07 ± 0.18 | .151 |
| POMS Fatigue | 49 | 0.77 ± 0.81 | 0.54 ± 0.69 | .056 |
| POMS Tension | 49 | 0.47 ± 0.50 | 0.28 ± 0.33 | .012 |
| POMS Mood | 49 | 0.29 ± 1.91 | -0.38 ± 1.79 | .013 |
| POMS Vigor | 49 | 1.49 ± 0.94 | 1.58 ± 0.93 | .261 |
| POMS Anger | 49 | 0.15 ± 0.35 | 0.08 ± 0.27 | .12 |
| SF36 General Health | 50 | 76.9 ± 19.1 | 76.8 ± 18.6 | .913 |
| SF36 Mental Health | 50 | 75.9 ± 11.8 | 79.1 ± 11.4 | .017 |
| SF36 Physical Functioning | 50 | 88.5 ± 14.3 | 91.4 ± 11.3 | .03 |
| SF36 Emotional | 50 | 83.4 ± 21.5 | 88.8 ± 16.3 | .051 |
| SF36 Physical Role | 50 | 85.1 ± 17.0 | 86.9 ± 16.7 | .471 |
| SF36 Social Functioning | 50 | 87.8 ± 16.9 | 89.7 ± 15.9 | .253 |
| SF36 Vitality | 50 | 58.7 ± 17.0 | 60.7 ± 16.9 | .258 |
| Oswestry Disability Score | 50 | 10.8±6.0 | 8.5 ± 7.1 | .004 |
Abbreviations: BPI, brief pain inventory; PPT, pressure pain threshold; POMS, profile of mood states; ROM, range of motion; SF-36, short form 36; VAS, verbal analogue scale.
These represented improvements. There was significant improvement in the scores of the SF-36 mental health and physical functioning subscales (p=.019, p=.03, respectively) and the Oswestry disability scale scores (p=.003)). The regression model was significant for VAS scores (model F = 32.37, p<.001, R2 = 0.81, n = 52). Baseline values for VAS were also significant (p < .001). Other adjustment variables were not significant. For BPI scores, the regression model was marginally significant (model F = 2.36, p = .047, R2 = 0.25, n = 49). For PPT, the regression model was not significant (model F = 2.13, p = .069, R2 = 0.22, n = 51). Only baseline PPT was significant in the model.
Table 4 presents the least squares means (standard errors) for change from baseline in VAS, BPI, and PPT among responders and non-responders from the adjusted regression models. The mean change from baseline in VAS score was -2.87±0.16 for responders and -1.00±0.30 for non-responders. The means were significantly different (p<.001). The mean change from baseline in BPI score was -1.32±0.22 for responders and 0.04±0.38 for non-responders. The means were significantly different (p=.002). Mean change from baseline in PPT was not statistically significantly different in responders and non-responders.
Table 4. Least Squares Means ± S.E. of Change from Baseline of VAS, BPI, and PPT, Adjusted for Baseline, Site, Gender, Age, and Exercise Status.
| VAS (score) | BPI (score) | PPT (pounds) | |
|---|---|---|---|
| Responders | -2.87±0.16 | -1.32±0.22 | 2.12±0.50 |
| Non-responders | -1.00±0.30 | 0.04±0.38 | 0.85±0.96 |
Abbreviation: VAS, verbal analogue scale; BPI, brief pain inventory; PPT, pain pressure threshold.
Discussion
Much has been written about MTrPs and their possible relationship to MPS.21-24 The contribution of the MTrP in the pathogenesis of MPS is an area of active investigation and has raised important questions about muscle and fascia in inciting and perpetuating soft-tissue pain.25-27 Debate continues about whether the MTrP is necessary for MPS diagnosis and whether it needs to be the target of treatment.
The pathogenesis of the MTrP is elusive and current explanations about its relationship to MPS remain incomplete. Trauma, muscle overload, and muscle overuse have been cited as etiologic agents with trauma being one of the leading contenders.24, 25 Tissue injuries result in the release of noxious substances that bind to, sensitize, and/or activate nociceptors. This leads to the transmission of signals that indicate tissue damage and inflammation, and may set up persistent pain states. 26 The relative contributions of the central and peripheral nervous systems in generating and perpetuating pain have not yet been fully understood, although there is preliminary evidence for pain dysregulation in MPS.28, 29, 30 Disrupted descending inhibition in individuals with chronic musculoskeletal pain may lead to a muscle pain complaint, irrespective of peripheral tissue damage. 30
To explore relationships between MTrPs and MPS we reasoned that if treatment directed at the MTrP was shown to improve myofascial pain3, 14, 31, 32, 33, 34 we could measure changes in pain and MTrP status at the same time. We elected to use a single MTrP, in a defined anatomical area and use experienced “calibrated” examiners to study it carefully. The examiners participated in a test of inter-rater reliability that demonstrated no statistically significant differences between their clinical assessments. This approach would provide an opportunity to assess pain related to the MTrP and allow for determining the relationship, if any, between pain reduction and MTrP status change. We used objective measures of the MTrP (palpation and size35, and correlated these with patient self-reports of pain, mood, health status and disability. One review article addressing the reliability of palpation suggested that it varies widely.34 However, none of these 9 studies used examiners who had demonstrated inter-rater reliability and performed evaluation and treatment on a single muscle.
Our major findings were that pain reduction, as measured using all 3 of the pain assessments, is significantly correlated with change in the MTrP status as determined by MTrP palpation from active to latent or normal (no palpable nodule) following dry needling. We noted that there was a clinically significant improvement in pain scores (a drop of ≥2) on the VAS.36 Treatment was correlated with significant, clinically relevant reduction in pain compared with baseline and improvement in mood and function. Needling was also positively correlated with a significant increase in cervical ROM attributable to the upper trapezius (i.e. side bending and rotation). There was a significant decrease in asymmetry between the left and right sides after treatment. We are aware of some concerns about the reliability of pain measures and therefore used 3 instruments. One of these, the PPT, is an instrumented measure. All showed significant reduction following treatment.
The mean baseline measurement of pain for subjects with unilateral MTrP, was VAS 3.5 (± SD 2.4) which is considered moderate pain.37 The group with bilateral MTrPs had VAS score of 3.0 (±1.4), mild pain. Some clinicians may not wish to treat MTrP and myofascial pain if the level is mild. The decision to treat often depends upon several factors including frequency and persistence, intrusion into daily activities and peak pain levels. The measure at baseline was determined at a moment in time and the entry criterion was reportable pain, the clinical severity did not determine whether the research subject was to receive dry needling. After criteria were met, our primary outcome was a change in pain score, and the change was significant. Appropriate measurement is critical to assure the validity and reliability of this clinical study. Pain evaluations are not objective assessments and consensus about which are best to use for this study group has not been reached. This study employed standard, systematically applied and frequently used evaluations to assess people with MPS. We used the BPI and algometry to assess the level and nature of pain. While both the BPI and VAS measured pain intensity at the time of administration, the BPI also measured the impact of pain on daily functions, pain relief, pain quality, and the patient's perception of the cause of the pain. The statistical analysis showed that VAS and BPI adjusted means scores were significantly different after treatment and PPT scores were not. In the regression model, VAS was significant, BPI was marginally significant and PPT was not. We support the use of the VAS for assessment of treatment of MPS. PPT may be useful and has been shown to be reliable in evaluating MPS, but lacks sensitivity.38 In this study, we have defined a positive response to treatment as a statistically significant decrease in pain from baseline and improvement in MTrP status. The change was also clinically significant, that is a decrease in 2 points on the VAS.
We recommend a careful, systematic and comprehensive approach to the evaluation of patients with MPS. This approach should include objective measures of cervical spine ROM, trigger point palpation and self-reports of pain, fatigue, mood, disability and health status, which have been shown to be sensitive to change and provide important information about the impact of MPS on issues of importance to patients.
One review examined the level of evidence for dry needling in MPS.14 They identify the data as Level 1a, because the reports were randomized, placebo controlled trials. The outcomes were self-reports and did not include objective measures and did not link response to trigger point status.
To the best of our knowledge, this is the first report to demonstrate that there is a significant, contemporaneous change in the level of both pain and the status of the MTrP following dry needling. Dry needling is likely to provide pain reduction and resolution of the a-MTrP. We report that dry needling has a significant effect in reducing pain as measured by VAS, BPI and PPT; and in decreasing disability as measured by the Oswestry Disability Index in people with MPS and a-MTrPs. A randomized, placebo controlled, blinded trial is the gold standard and required to definitively demonstrate effectiveness. Our group is planning to do this. There are some limitations associated with this study. MPS has long been considered a local or regional pain syndrome, implying that the inciting factors for pain are local rather than resulting from central sensitization.39, 40 This study did not address this question. The results of this study do not answer questions about pathogenesis, etiology and relative contributions of various regulatory mechanisms for developing or resolving MPS or MTrPs. However, the data advance our understanding of this complex syndrome by linking improvement in symptoms with objective measures of the MTrP and establish a relationship between the MTrP and MPS. Subjects for this study were recruited on a university campus, and possibly represent an atypical cross-section of people with MPS. Nonetheless, computer-based activity is used by most of our subjects, and has been reported to be a significant risk factor for developing MPS.41
Lastly, this study was not a randomized, placebo controlled, blinded clinical trial, hence it cannot prove effectiveness. The treating clinicians also evaluated the subjects, creating potential bias despite their being experienced and standardizing their technique.42, 43 The two treating physicians evaluated and treated the subjects based on scheduling convenience, and any bias introduced as a result cannot be ruled out. Not all subjects responded with a twitch to the dry needling. Some believe this is an important part of the therapeutic effect.3, 42 The elicitation of the twitch response did not distinguish the responders from the non-responders in this study. This study was a carefully conducted, systematic prospective study using valid instruments designed to measure soft tissue pain and disability to which objective measures were also applied. This permitted us to develop a properly sized and designed clinical effectiveness trial for dry needling using self-reported outcomes and objective measures.
Conclusion
A three week course of dry needling had a significant effect on pain reduction in MPS. Pain reduction was significantly related to change in trigger point status from active (spontaneously painful) to latent or resolution. Importantly, pain reduction was significantly correlated with improvement in cervical spine side bending and rotation, in patient self-reports of improved physical and emotional well-being and mood; and reduction in disability.
Acknowledgments
Funding for this study was received from the National Institute of Arthritis, Musculoskeletal and Skin Diseases of the National Institutes of Health: 1R01AR057348
Abbreviations
- BPI
Brief Pain Inventory
- cm
Centimeter
- IL
Interleukin
- MPS
Myofascial Pain Syndrome
- MTrP
Myofascial Trigger Point
- a-MTrP
Active myofascial trigger point
- 1-MTrp
Latent myofascial trigger point
- PPT
Pain Pressure Threshold
- PRO
Patient Reported Outcomes
- POMS
Profile of Mood States
- ROM
Range of Motion
- SF-36
Short Form-36
- VAS
Verbal Analogue Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jay Shah, Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD 20892.
William Rosenberger, Department of Statistics, George Mason University, Fairfax, VA 22030.
Kathryn Armstrong, Center for the Study of Chronic Illness and Disability, George Mason University, Fairfax, VA 22030.
Diego Turo, Department of Bioengineering, George Mason University, Fairfax, VA 22030.
Paul Otto, Department of Bioengineering, George Mason University, Fairfax, VA 22030.
Juliana Heimur, Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD 20892.
Nikki Thaker, Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD 20892.
Siddhartha Sikdar, Department of Bioengineering, George Mason University, Fairfax, VA 22030.
Bibliography
- 1.Skootsky SA, Jaeger B, Oye RK. Prevalence of myofascial pain in general internal medicine practice. West J Med. 1989;151(2):157–60. [PMC free article] [PubMed] [Google Scholar]
- 2.Gerber LH, Sikdar S, Armstrong K, et al. A systematic comparison between subjects with no pain and pain associated with active myofascial trigger points. PM R. 2013;5(11):931–8. doi: 10.1016/j.pmrj.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tekin L, Akarsu S, Durmuş O, Cakar E, Dinçer U, Kıralp MZ. The effect of dry needling in the treatment of myofascial pain syndrome: a randomized double-blinded placebo-controlled trial. Clin Rheumatol. 2013;32(3):309–15. doi: 10.1007/s10067-012-2112-3. [DOI] [PubMed] [Google Scholar]
- 4.Llewellyn LJ. A discussion of Fibrositis. Proc R Soc Med. 1913;6:27–35. doi: 10.1177/003591571300600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schade H. Untersuchungen in der Erkältungstrage: III. Uber den Rheumatismus, insbesondere den Muskelrheumatismus (myogelose) Müench Med Wochenschr. 1921;68:95–99. [Google Scholar]
- 6.Travell JG, Simons DG. Myofascial pain and dysfunction : the trigger point manual. Baltimore, MD: Williams & Wilkins; 1983. [Google Scholar]
- 7.Borg-Stein J, Simons DG. Focused review: myofascial pain. Arch Phys Med Rehabil. 2002;83(3 Suppl 1):S40–7. S48–9. doi: 10.1053/apmr.2002.32155. [DOI] [PubMed] [Google Scholar]
- 8.Bennett R. Myofascial pain syndromes and their evaluation. Best Pract Res Clin Rheumatol. 2007;21(3):427–45. doi: 10.1016/j.berh.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Simons DG. Review of enigmatic MTrPs as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol. 2004;14(1):95–107. doi: 10.1016/j.jelekin.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Simons DG, Travell JG, Simons LS. Travell & Simons' myofascial pain and dysfunction : the trigger point manual. 2nd. Baltimore, MD: Williams & Wilkins; 1999. [Google Scholar]
- 11.Mense S, Masi AT. Increased muscle tone as a cause of muscle pain. In: Mense S, Gerwin RD, editors. Muscle pain: understanding the mechanisms. Heidelberg: Springer; 2011. pp. 207–49. [Google Scholar]
- 12.Tough EA, White AR, Richards S, Campbell J. Variability of criteria used to diagnose myofascial trigger point pain syndrome—evidence from a review of the literature. Clin J Pain. 2007;23(3):278–86. doi: 10.1097/AJP.0b013e31802fda7c. [DOI] [PubMed] [Google Scholar]
- 13.Tough EA, White AR, Cummings TM, Richards SH, Campbell JL. Acupuncture and dry needling in the management of myofascial trigger point pain: a systematic review and meta-analysis of randomized controlled trials. Eur J Pain. 2009;13(1):3–10. doi: 10.1016/j.ejpain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Kietrys DM, Palombaro KM, Azzaretto E, et al. Effectiveness of dry needling for upper-quarter myofascial pain: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2013;43(9):620–34. doi: 10.2519/jospt.2013.4668. [DOI] [PubMed] [Google Scholar]
- 15.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to Assess Pain in Cancer and Other Diseases. Pain. 1983;17(2):197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 16.Fairbank JC, Couper J, Davies JB, O'Brien JP. The Oswestry Low Back Pain Disability Questionnaire. Physiotherapy. 1980;66(8):271–73. [PubMed] [Google Scholar]
- 17.Ware JE, Jr, Sherbourne CD. The Mos 36-Item Short-Form Health Survey (Sf-36). I. Conceptual Framework and Item Selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 18.Shacham S. A Shortened Version of the Profile of Mood States. J Pers Assess. 1983;47(3):305–06. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 19.Proschan MA, Lan KKG, Wittes JT, editors. Statistical Manual of Clinical Trials. New York, NY: Springer; 2007. [Google Scholar]
- 20.Cytel Statistical Software and Services. StatXact with Cytel Studio, Version 10. Cambridge, MA: 2014. [Google Scholar]
- 21.Fricton JR, Kroening R, Haley D, Siegert R. Myofascial Pain Syndrome of the Head and Neck: A Review of Clinical Characteristics of 164 Patients. Oral Surg Oral Med Oral Pathol. 1985;60(6):615–23. doi: 10.1016/0030-4220(85)90364-0. [DOI] [PubMed] [Google Scholar]
- 22.Bron C, Dommerholt JD. Etiology of Myofascial Trigger Points. Curr Pain Headache Rep. 2012;16(5):439–44. doi: 10.1007/s11916-012-0289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerwin RD, Dommerholt J, Shah JP. An Expansion of Simons' Integrated Hypothesis of Trigger Point Formation. Curr Pain Headache Rep. 2004;8(6):468–75. doi: 10.1007/s11916-004-0069-x. [DOI] [PubMed] [Google Scholar]
- 24.Stecco A, Gesi M, Stecco C, Stern R. Fascial components of the myofascial pain syndrome. Curr Pain Headache Rep. 2013;17(8):352. doi: 10.1007/s11916-013-0352-9. [DOI] [PubMed] [Google Scholar]
- 25.Mense S. The pathogenesis of muscle pain. Curr Pain Headache Rep. 2003;7(6):419–25. doi: 10.1007/s11916-003-0057-6. [DOI] [PubMed] [Google Scholar]
- 26.Mense S, Simons DG, Russell IJ. Muscle pain: understanding its nature, diagnosis and treatment. Baltimore, MD: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 27.Mense S. How do muscle lesions such as latent and active trigger points influence central nociceptive neurons? J Musculoskelet Pain. 2010;18(4):348–53. [Google Scholar]
- 28.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24(1):37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Niddam DM, Chan RC, Lee SH, Yeh TC, Hsieh JC. Central modulation of pain evoked from myofascial trigger point. Clin J Pain. 2007;23(5):440–8. doi: 10.1097/AJP.0b013e318058accb. [DOI] [PubMed] [Google Scholar]
- 30.Ge HY, Fernández-de-las-Peñas C, Yue SW. Myofascial trigger points: Spontaneous electrical activity and its consequences for pain induction and propagation. Chin Med. 2011;6:13. doi: 10.1186/1749-8546-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldry P. Superficial dry needling at myofascial trigger point sites. J Musculoskelet Pain. 1995;3:117–26. [Google Scholar]
- 32.Dommerholt J. Dry needling - peripheral and central considerations. J Man Manip Ther. 2011;19(4):223–37. doi: 10.1179/106698111X13129729552065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cummings TM, White AR. Needling therapies in the management of myofascial trigger point pain: a systematic review. Arch Phys Med Rehabil. 2001;82:986–92. doi: 10.1053/apmr.2001.24023. [DOI] [PubMed] [Google Scholar]
- 34.Lucas N, Macaskill P, Irwiq L, Moran R, Boqduk N. Reliability of physical examination for diagnosis of myofascial trigger points: a systematic review of the literature. Clin J Pain. 2009;25:80–9. doi: 10.1097/AJP.0b013e31817e13b6. [DOI] [PubMed] [Google Scholar]
- 35.Ballyns JJ, Shah JP, Hammond J, Gebreab T, Gerber LH, Sikdar S. Objective sonographic measures for characterizing myofascial trigger points associate with cervical pain. J Ultrasound Med. 2011;30:1331–40. doi: 10.7863/jum.2011.30.10.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Todd KH. Clinical versus statistical significance in the assessment of pain relief. Ann Emerg Med. 1996;27:439–41. doi: 10.1016/s0196-0644(96)70226-3. [DOI] [PubMed] [Google Scholar]
- 37.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: What is moderate pain in millimetres? Pain. 1997;72:95–7. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 38.Park G, Kim CW, Park SB, Kim MW, Jang SH. Reliability and Usefulness of Pain Pressure Threshold Measurement in Patients with Myofascial Pain. Ann Rehabil Med. 2011;35(3):412–17. doi: 10.5535/arm.2011.35.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Treaster D, Marras WS, Burr D, Sheedy JE, Hart D. Myofascial trigger point development from visual and postural stressors during computer work. J Electromyogr Kinesiol. 2006;16(2):115–24. doi: 10.1016/j.jelekin.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Fernández-de-las-Peñas C, Dommerholt J. Myofascial Trigger Points: Peripheral or Central Phenomenon? Curr Rheumatol Rep. 2014;16(1):395. doi: 10.1007/s11926-013-0395-2. [DOI] [PubMed] [Google Scholar]
- 41.Hoyle JA, Marras WS, Sheedy JE, Hart DE. Effects of postural and visual stressors on myofascial trigger point development and motor unit rotation during computer work. J Electromyogr Kinesiol. 2011;21(1):41–8. doi: 10.1016/j.jelekin.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Chu J, Schwartz I. The muscle twitch in myofascial pain relief: effects of acupuncture and other needling methods. Electromyogr Clin Neurophysiol. 2002;42:307–11. [PubMed] [Google Scholar]
- 43.Kalichman L, Vulfsons S. Dry needling in the management of musculoskeletal pain. J Am Board Fam Med. 2010;23:640–646. doi: 10.3122/jabfm.2010.05.090296. [DOI] [PubMed] [Google Scholar]


