Abstract
We previously determined that burst size necrosis is the chief mode of mononuclear cell death in the lungs of mice with tuberculosis. The present study explored the link between infection-induced necrosis of mononuclear phagocytes and neutrophil accumulation in the lungs of mice challenged with one of four Mycobacterium tuberculosis strains of increasing virulence (RvΔphoPR mutant, H37Ra, H37Rv and Erdman). At all time points studied, Erdman produced the highest bacterial load and the highest proportion and number of M. tuberculosis-infected neutrophils. These parameters, and the proportion of TUNEL-positive cells, tracked with virulence across all strains tested. Differences in neutrophil infection were not reflected by levels of chemoattractant cytokines in bronchoalveolar lavage fluid, while interferon-γ (reported to suppress neutrophil trafficking to the lung in tuberculosis) was highest in Erdman-infected mice. Treating Erdman-infected mice with ethambutol decreased the proportion of mononuclear phagocytes with high bacterial burden and the ratio of infected neutrophils to infected mononuclear cells in a dose-dependent manner. We propose that faster replicating M. tuberculosis strains cause more necrosis which in turn promotes neutrophil recruitment. Neutrophils infected with M. tuberculosis constitute a biomarker for poorly controlled bacterial replication, infection-induced mononuclear cell death, and increased severity of immune pathology in tuberculosis.
Keywords: tuberculosis, neutrophils, macrophages, cell death
1. Introduction
We previously reported that necrotic cell death of mononuclear phagocytes (MPs) infected with Mycobacterium tuberculosis Erdman is associated with high intracellular bacterial burden and is the predominant form of cell death in tuberculosis (TB) disease in vivo [1]. Data from in vivo and in vitro experiments support the concept of burst size cytolysis in MPs harboring 20–40 bacilli [1–3]. This necrotic cell death mode relies on mycobacterial genes regulated by the PhoPR 2-component system but it is independent of Esx-1 [2]. Neutrophils comprised roughly half of all M. tuberculosis-infected lung leukocytes 3 weeks after aerosol challenge with Erdman. Bacterial burden increases logarithmically at this early stage of TB disease, prior to the full expression of adaptive immunity in the lung [1]. At later time points, when total lung bacillary load is held to a plateau level by host immunity, the proportion of MPs permissive for M. tuberculosis replication (and therefore susceptible to burst size necrosis) falls by ~90%. In the same interval post-infection (p.i.) the proportion of neutrophils amongst all M. tuberculosis-infected phagocytes falls to ~10%. Mice lacking interferon gamma (IFN-γ) fail to restrict Erdman replication and have a rising proportion of infected neutrophils until death. These results suggest a model where neutrophils are recruited to TB lesions in response to signals associated with burst size necrosis of MPs.
In the present study, we investigated the relation between M. tuberculosis replication and neutrophil recruitment to the lung. Wild-type C57BL/6 mice were challenged with one of four M. tuberculosis strains differing in virulence: Erdman, H37Rv, H37Ra, or an H37Rv mutant lacking the phoPR coding region (RvΔphoPR). This approach was necessary because there are no known inhibitors of burst size necrosis or any mouse strains resistant to this mode of infection-induced cell death. Results confirmed that bacterial replication during the innate phase of TB defense correlated with the total number of lung leukocytes harboring acid-fast bacilli (AFB), the proportion of M. tuberculosis-infected (AFB+) leukocytes that were neutrophils and the distributions of AFB burden per cell in MPs and neutrophils. The data from this study support a model where the rate of M. tuberculosis replication and consequent burst size necrosis of MPs promotes neutrophil recruitment to TB lesions despite potentially counter-regulatory cytokine signals.
2. Materials and methods
2.1. Mice
C57BL/6 mice (Jackson Laboratory) were housed in a pathogen-free environment at Animal Medicine facility at The University of Massachusetts Medical School (UMMS). Experiments were conducted in accordance with National Institutes of Health guidelines regarding laboratory animals and followed protocols approved by the Institutional Animal Care and Use Committee and the Institutional Biosafety Committee at UMMS.
2.2. Mycobacteria and infection
M. tuberculosis Erdman, H37Rv, H37Ra (provided by H. Remold, Brigham and Women’s Hospital) and RvΔphoPR mutant (provided by K. Papavinasasundaram, UMMS) [2] were used in this study. Aerosol infections delivered ~100 colony forming units (CFU) of Erdman, H37Rv or H37Ra and ~400 CFU of RvΔphoPR (Glas-Col Inhalation Exposure System).
2.3. CFU Assay
Lung homogenates were serially diluted with PBS-Tween (0.05%) and plated in duplicate on Middlebrook 7H11 agar plates supplemented with Middlebrook OADC Enrichment. Plates were incubated at 37°C and CFU were counted 2, 3 and 4 weeks after plating.
2.4. Cell preparation
Whole lung leukocytes were isolated from infected mice as previously described [4]. Single lung cell suspensions were used for flow cytometry and cytospin preparation for AFB staining.
2.5. Enumeration of intracellular bacteria
Intracellular AFB were enumerated from isolated lung leukocytes as described Repasy et al. [1].
2.6. Flow cytometry
Cell suspensions were incubated in CD16/CD32 mAb (BD Biosciences) to block Fc binding. The following reagent and conjugated antibodies (eBioscience) were used: CD11b-PerCP-Cyanine5.5 (M1/70), CD45-APC (30-F11), Ly-6G-Alexa Fluor 700 (RB6-8C5); Live/Dead Fixable Dead Cell Stain Kit (Invitrogen). Data acquisition by BD LSRII flow cytometer and analysis with FlowJo software (TreeStar). Excluding dead cells and lymphocytes by forward and side scatter, neutrophils were gated on CD45+ cells and defined as Ly-6Ghi CD11bhi cells.
2.7. Histology, TUNEL assay and immunostaining
Lungs of infected mice were inflated with 10% buffered formalin and fixed for 24 h. Paraffin embedded sections were stained with hematoxylin and eosin (H&E) or terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (In Situ Cell Death Detection Kit, Roche). For immunostaining, primary Ab against myeloperoxidase (MPO; LS Bio) and secondary Ab Alexa 555 (Invitrogen) were used, along with In Situ Cell Death Detection Kit with fluorescein-dUTP (Roche) to detect TUNEL+ cells. Lung sections were analyzed using Nikon Eclipse E400 Microscope equipped with a Nikon DS-Ri1 camera using NIS-Elements Microscope Imaging Software or Spot Advance Software.
2.8. Cytokine measurement
Lungs of infected mice were flushed three times with lavage fluid (0.2% BSA 0.2mM EGTA in PBS) then filter-sterilized. Preparation of cell-free lung lysates were performed as described [4]. Bronchoalveolar lavage (BAL) fluid and supernatant were stored at −80°C until used. BAL fluid and lung lysates were individually assayed for IFN-γ, IL-1α, IL-1β, IL-17, JE (CCL2), KC, MIP-2, (R&D Systems) and S100A8/A9 (CusaBio) by ELISA following the manufacturers’ protocols.
2.9. Ethambutol treatment
Four days p.i. Erdman-infected mice were treated with ethambutol (EMB; Sigma-Aldrich) ad libitum in drinking water at concentrations of 67, 200 and 600 μg/ml. Water bottles containing EMB were replaced weekly and treatment continued for 11 days until 2 week p.i. time point.
2.10. Statistical analysis
For Figure 1D, the data followed an approximate Poisson distribution; therefore we compared the median values by strain at each time point and bin using a Poisson regression model, and expressed the differences as ratios to a reference group and tested their statistical significance. For all other data, statistical analysis was performed using SigmaPlot v11.0 (Systat Software, Inc.). One-way ANOVA with Tukey or Holm-Sidak post-test was used for comparison with more than two groups and Student’s t-test was used when comparing two groups. A P value <0.05 was considered statistically significant.
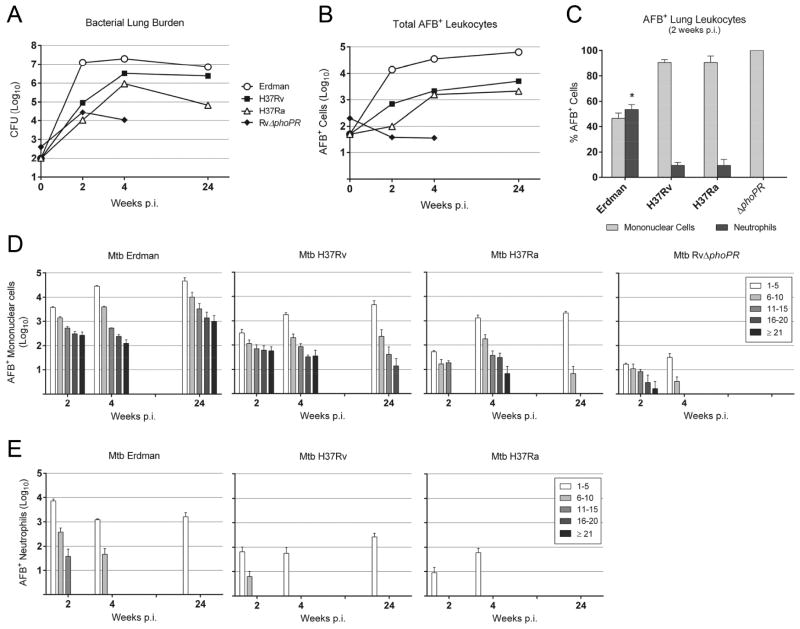
Fig. 1.
Growth and distribution of M. tuberculosis strains in murine hosts. (A) Total lung CFU of M. tuberculosis Erdman, H37Rv, H37Ra at 2, 4 and 24 weeks after aerosol infection (RvΔphoPR at 2 and 4 weeks only). The values are presented as mean log10 CFU (n=4). Mice infected with Erdman had significantly higher CFU than the other mycobacterial strains, P<0.001 for all strains at 2 and 4 weeks p.i. and P<0.01 for H37Rv at 24 weeks p.i. Total lung CFU of H37Rv was significantly higher than H37Ra or RvΔphoPR, P<0.001 at 2 and 4 weeks p.i. and P<0.001at 24 weeks p.i. (B) Whole lung leukocytes were isolated and cytospin preparations were made for acid-fast staining. The total number of M. tuberculosis-infected (AFB+) lung leukocytes was calculated by multiplying the percent of enumerated AFB+ cells by the total number of leukocytes in each sample. The data represent the mean log10 AFB+ cells (n=3). Erdman-infected mice had the greatest number of AFB+ leukocytes, P<0.001 for 2 and 4 weeks p.i. and P<0.01 for 24 weeks p.i. H37Rv-infected mice had significantly more AFB+ leukocytes than mice infected with H37Ra or RvΔphoPR at 2 weeks p.i., P<0.01. At 4 weeks p.i., both H37Rv and H37Ra-infected mice had significantly more AFB+ leukocytes than RvΔphoPR-infected mice, P<0.001. (C) The distribution of infection between neutrophils and mononuclear phagocytes was determined by counting AFB+ leukocytes from lungs harvested at 2 weeks p.i. Results are expressed as mean % AFB+ cells ± SD (n=3). * P<0.001. Intracellular AFB loads of M. tuberculosis Erdman, H37Rv, H37Ra and RvΔphoPR in mononuclear phagocytes (D) and neutrophils (E) at indicated time points were enumerated by microscopy. The number of AFB per cell was counted at the indicated time points with counts grouped into five bins of 1–5, 6–10, 11–15, 16–20, or ≥21 AFB. Data are presented as log10 AFB+ cells in each bin ± SD. Statistically significant differences (P<0.05) are not shown for sake of clarity. All strains, bins and time points were compared using a Poisson regression model. Among significant differences, Erdman-infected mice yielded more AFB+ mononuclear phagocytes and more AFB+ neutrophils for all bins at all time points compared to the other M. tuberculosis strains.
3. Results
3.1. Lung bacterial burden and leukocyte infection reflect M. tuberculosis virulence
To investigate the influence of virulence on the distribution of intracellular M. tuberculosis infection among lung leukocyte populations, wild-type C57BL/6 mice were challenged with one of four M. tuberculosis strains. The panel of bacteria included two commonly used virulent laboratory strains (Erdman and H37Rv) and two strains of reduced virulence (H37Ra and RvΔphoPR). Total lung burden was measured at 2, 4 and 24 weeks p.i. by counting CFU (Fig. 1A). All four strains exhibited logarithmic growth during the innate phase of host defense in the first 2 weeks p.i. Erdman had the highest replication rate in this interval, achieving 2 log10 greater mean CFU than H37Rv which had the second highest number. Of the attenuated strains, mean CFU for RvΔphoPR was ~0.5 log10 lower than H37Rv while H37Ra was a further ~0.5 log10 lower than RvΔphoPR. Mean lung CFU for Erdman, H37Rv and H37Ra increased by 4 weeks p.i., while RvΔphoPR dropped by nearly 0.5 log10 and was at least 2 log10 lower than the other strains. At 24 weeks p.i., Erdman and H37Rv had declined slightly while H37Ra dropped more than 1 log10 from its 4 week p.i. high point. M. tuberculosis growth reflected the hierarchy of bacterial virulence. Results confirmed the faster doubling time for Erdman compared to H37Rv noted by North and Izzo in CB-17 mice infected intravenously with 2×105 bacilli [5].
We investigated the correlation between total lung bacterial burden with infection of phagocytic cells by examining Zhiel-Neelsen-stained lung leukocytes and counting AFB+ cells (Fig. 1B). The total number of AFB+ leukocytes increased logarithmically in the first 2 weeks p.i. for all strains except RvΔphoPR. Between 2 weeks and 4 weeks p.i., total AFB+ cells from mice infected with Erdman, H37Rv and H37Ra continued rising logarithmically. In mice infected with RvΔphoPR, total AFB+ lung leukocytes were confined to a plateau level nearly 3 log10 lower than the Erdman group. Between 4 weeks and 24 weeks p.i., there was a trend for increased total AFB+ cells in mice infected with Erdman, H37Rv and H37Ra but rising at a much slower rate than in the first 4 weeks p.i. The small increase of AFB+ leukocytes for these strains occurred despite a decline in total lung bacterial burden that was modest for Erdman and H37Rv but >1 log10 for H37Ra. Comparing the distribution of AFB+ leukocytes at 2 weeks p.i., the relative proportion of infected neutrophils among all infected phagocytes was much greater in the Erdman group than the other groups (Fig. 1C). No AFB+ neutrophils were identified from mice infected with RvΔphoPR.
3.2. Phagocyte bacterial burden reflects M. tuberculosis replication rate and host immunity
To evaluate the distribution of M. tuberculosis burden on a per cell basis over the course of TB disease, AFB were counted in individual MPs and neutrophils (Fig. 1D, E). The distribution of bacillary loads within MPs at 2 weeks p.i. largely matched the prediction for a burst size model with the majority of infected cells containing 1–5 bacilli and fewer than 10% containing ≥21 AFB (Supplementary Fig. 1). An exception was H37Ra where no MPs containing >15 bacilli were identified at 2 weeks p.i. By 4 weeks p.i., the proportion of MPs with high AFB loads was reduced by a factor of 10 or more (Fig. 1D, Supplementary Fig. 1) consistent with immune-mediated enhancement of antimicrobial activity that is effective in most but not all M. tuberculosis-infected MPs and with the greatest impact on the less virulent strains. By 24 weeks p.i., Erdman was the only strain where MPs harboring ≥21 AFB could be detected. In H37Rv-infected mice, MPs had ≤20 AFB while MPs from H37Ra-infected mice contained ≤10 AFB. Overall, the capacity for persistent bacterial replication under immune pressure was greatest for Erdman, less so for H37Rv and nil for H37Ra.
The maximum neutrophil AFB load in Erdman-infected mice was 11–15 at 2 weeks p.i., falling to 6–10 at 4 weeks and 1–5 at 24 weeks (Fig. 1E). At 2 weeks p.i., mice challenged with H37Rv or H37Ra had a lower proportion and total number of AFB+ neutrophils than the Erdman group. The distribution of bacterial loads in neutrophils was limited to ≤10 AFB for H37Rv at 2 weeks p.i. falling to ≤5 by 4 weeks p.i. Neutrophils from H37Ra-infected mice contained ≤5 AFB at 2 weeks and 4 weeks p.i. No AFB+ neutrophils were identified at any time point in lung leukocytes from mice infected with RvΔphoPR, and by 24 weeks p.i. none were seen in H3Ra-infected mice.
3.3. Progressive lung pathology correlates with M. tuberculosis virulence
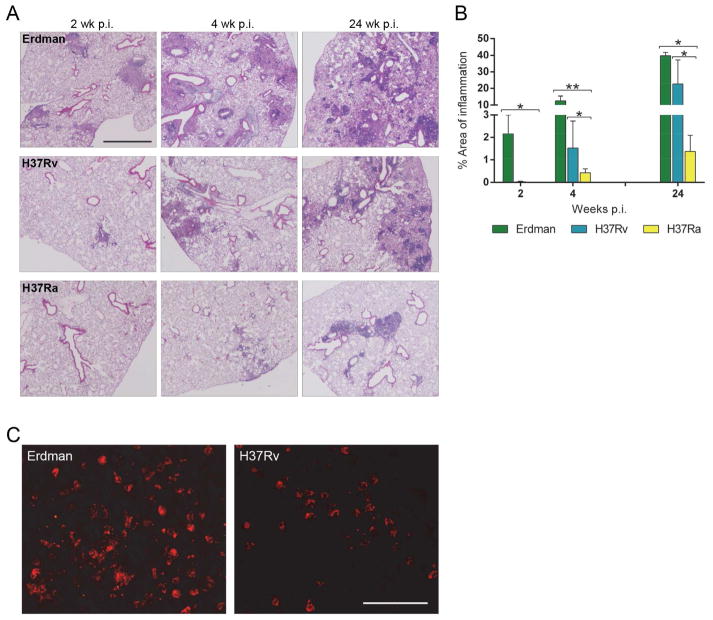
Pulmonary immune pathology was compared in mice infected with Erdman, H37Rv or H37Ra (Fig. 2A). Inflammatory lesions from each group were quantified by morphometry (Fig. 2B). At 2 weeks p.i., an average of 35 lesions per mouse was identified in the Erdman-challenged group occupying ~2% of total lung area. In contrast, only one lung lesion per mouse was visible in the H37Rv-infected group (0.03% of total lung area) and no lesions were visible in lungs from mice in H37Ra group. Inflammation in Erdman group occupied 12.5% of total lung area by 4 weeks p.i. with values of 1.5% and 0.4% with H37Rv or H37Ra group, respectively. At the 24 week p.i. time point, lung architecture was extensively damaged in the Erdman group with 40% of lung area involved. Inflammation progressed to involve 22.6% of lung area in H37Rv-infected mice and 1.3% of lung area in the H37Ra group. Following the expression of adaptive immunity (4 and 24 weeks p.i.) total AFB+ leukocytes were nearly equal in H37Rv and H37Ra group (Fig. 1B), yet the area of inflammation was much lower in H37Ra-infected lungs (Fig. 2B). Inflammatory lung infiltrates in the H37Ra group were nearly devoid of neutrophils, whereas neutrophils were common in the lesions of mice infected with Erdman and, to a lesser extent, H37Rv (Fig. 2C). We examined 4-week p.i. lung sections immunostained for MPO to detect neutrophils, finding no signal from H37Ra-infected mice (data not shown) whereas MPO+ neutrophils were present in both Erdman and H37Rv groups.
Fig. 2.
Histopathology of M. tuberculosis-infected lungs. Wild-type mice were challenged with ~100 CFU of M. tuberculosis Erdman, H37Rv or H37Ra via aerosol route. Infected lungs were harvested at indicated time points and processed for H&E staining. (A) Representative photomicrographs at 2, 4 and 24 weeks p.i. (scale bar = 1000 μm) (B) Cross-sectional areas of all lung tissue sections were examined by light microscopy and measured for total lung area and areas of inflammation. Calculation for the percent of area of inflammation was derived from ((cumulative summation of inflamed areas)/(total lung area surveyed) × 100). Values are mean ± SD (n=3). * P<0.05, ** P<0.001. (C) Representative images of MPO+ neutrophils in red fluorescence from lung sections of Erdman- and H37Rv-infected mice at 4 weeks p.i. (scale bar = 50 μm).
3.4. Increased cell death in lung lesions of M. tuberculosis Erdman-infected mice
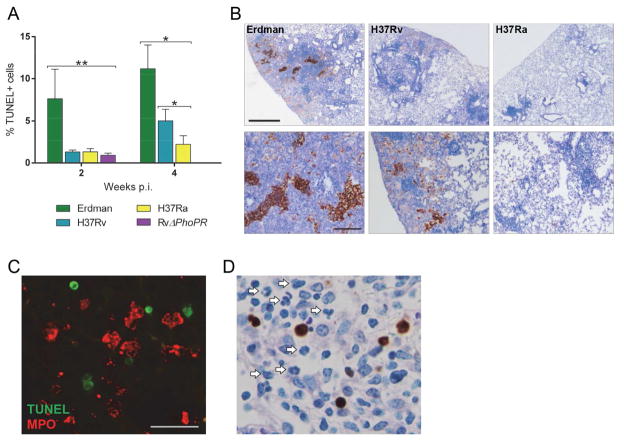
To compare the extent of cell death at the tissue level in infected mice, lungs were harvested for TUNEL assay. Lung sections from 2 and 4 weeks p.i. were examined to identify five fields of inflammatory infiltrates having the most abundant TUNEL+ cells in each section. In the selected fields, all TUNEL+ and TUNEL− cells were counted (Fig. 3A). At 2 weeks p.i., Erdman yielded an average of 7.6% TUNEL+ cells, nearly 6 fold higher than the other strains. At this time point, the proportion of TUNEL+ cells was comparable in mice infected with H37Rv, H37Ra or RvΔphoPR. At 4 weeks p.i., lesions from Erdman group contained a mean of 11.1% TUNEL+ cells, while 5.0% and 2.3% of cells were TUNEL+ in H37Rv and H37Ra groups, respectively, and no TUNEL+ cells were identified in RvΔphoPR-infected mice. At 24 weeks p.i., extensive lung tissue damage made it unfeasible to tally individual TUNEL+ cells, particularly in Erdman-infected mice (Fig. 3B). Areas of TUNEL staining were confined to necrotic lesions with densely packed leukocytes having both nuclear and cytoplasmic staining and mostly indistinguishable cellular borders.
Fig. 3.
Cell death in lung lesions correlates with M. tuberculosis virulence. (A) Mice were infected with M. tuberculosis Erdman, H37Rv, H37Ra or RvΔphoPR by aerosol. Lung sections were processed for colorimetric TUNEL assay and TUNEL+ cells were enumerated by examining all cross-sectional areas of lung tissue sections and selecting 5 fields with the most TUNEL+ signals. For each field, all TUNEL+ and TUNEL− cells were counted and all 5 fields were tallied. Percent TUNEL+ cells was calculated as ((total TUNEL+ cells)/(total cells) ×100). Values are mean ± SD (n=3). * P<0.01, ** P<0.001. (B) Representative images of highest TUNEL signals from lung tissue sections at 24 weeks p.i. (upper panels, scale bar = 500 μm; lower panels, scale bar = 200 μm). (C) Representative immunofluorescent image of a lung section from an Erdman-infected mouse at 4 weeks p.i. TUNEL+ cells are identified by green fluorescence and MPO+ neutrophils are identified by red fluorescence (scale bar = 25 μm). (D) Colorimetric TUNEL assay on a representative lung section from an Erdman-infected mouse 4 weeks p.i. revealing neutrophils (arrows) in the vicinity of TUNEL+ cells (brown).
If neutrophils are recruited to the site of TB disease by signals released from MPs undergoing infection-induced necrosis, then they should be found in proximity to dying cells. This is evident from neutrophil engulfment of M. tuberculosis and was further tested by combined MPO immunostaining and TUNEL assay of lung sections from 4 weeks p.i. (Fig. 3C). Neutrophils accumulated in lesions where TUNEL+ cells were also present and virtually all TUNEL+ cells were MPO−, suggesting that the dead cells were predominantly MPs [6,7]. This impression was also supported by light microscopy of slides processed for colorimetric TUNEL assay (Fig. 3D). Although we were unable to count individual TUNEL+ cells at 24 weeks p.i., we did observe clusters of neutrophils positioned in areas of abundant TUNEL signals (Supplementary Fig. 2).
3.5. Neutrophil infection in the lungs correlates with bacterial virulence
The dynamics of neutrophil recruitment and intracellular infection were strikingly different in mice infected with an M. tuberculosis strain of high (Erdman) vs. intermediate (H37Rv) virulence. As a proportion of all CD45+ lung leukocytes, neutrophils peaked at 4 weeks p.i. (58% and 37% for Erdman or H37Rv group, respectively) and then fell to <3% at 24 weeks for both groups (Fig. 4A). Between 2 weeks and 4 weeks p.i., total lung neutrophils increased 4.5 fold in mice infected with Erdman and 3.5 fold with H37Rv but dropped more than 1 log10 by the 24 week time point (Fig. 4B). At each time point, total lung neutrophils recovered from either group were not significantly different but the total number and proportion of AFB+ neutrophils was significantly higher in the Erdman group compared to H37Rv (Fig. 4B). At 2 weeks p.i., AFB were detected in 3.84% of lung neutrophils from the Erdman group but in only 0.06% of neutrophils from H37Rv group. These distributions fell to 0.15% and 0.01%, respectively, at 4weeks p.i. and then rebounded to 6.65% for Erdman and 3.22% for H37Rv at 24 weeks.
Fig. 4.

Neutrophil accumulation in the lungs of mice with TB. Mice were infected with M. tuberculosis Erdman or H37Rv by aerosol. At 2, 4 and 24 weeks p.i. isolated lung leukocytes were analyzed by flow cytometry. Neutrophils were defined as Ly-6Ghi CD11bhi cells. For AFB+ neutrophils, lung leukocytes were harvested and cytospin slides were processed for Ziehl-Neelsen staining. AFB+ neutrophils were identified based on nuclear morphology and acid-fast bacilli. (A) The proportion CD45+ Ly-6Ghi CD11bhi neutrophils among all CD45+ lung leukocytes was higher in Erdman-infected mice (light bars) at 2 and 4 weeks p.i. compared to H37Rv-infected mice (dark bars). (B) Total lung neutrophil counts and AFB+ neutrophil counts in mice infected with M. tuberculosis Erdman (light bars) or H37Rv (dark bars). AFB+ neutrophil counts in both groups (hatched bars). Total neutrophil counts in Erdman-infected and H37Rv-infected mice were not significantly different at any time point but the number of AFB+ neutrophils was significantly greater in Erdman-infected mice at 2 and 4 weeks p.i. P<0.001. Data are presented as the mean ± SD (n=3) * P<0.01.
3.6. Cytokine profile of tuberculous lung
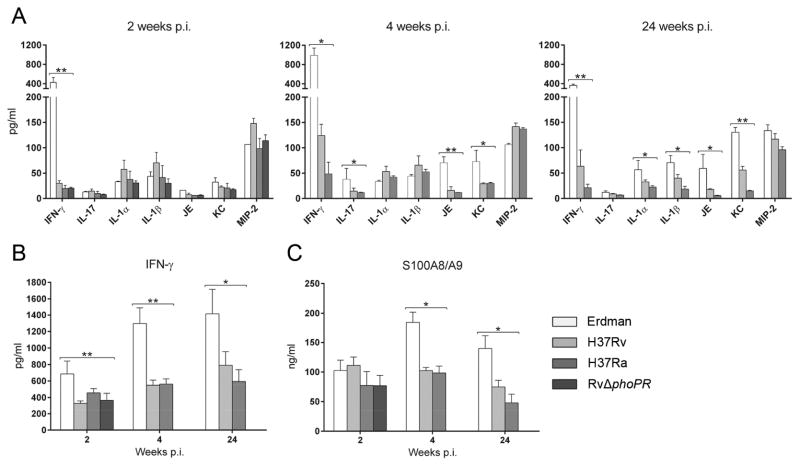
To test if neutrophil accumulation in TB lesions correlated with typical recruiting signals, we measured eight pro-inflammatory cytokines in BAL fluid from mice infected with one of the four M. tuberculosis strains. (Fig. 5A). At 2 weeks p.i., the levels of IL-1α, IL-1β, IL-17, JE, KC and MIP-2 were comparable in all groups, including RvΔphoPR-infected mice. At 4 and 24 weeks p.i., the levels of IL-17, JE and KC in Erdman-infected mice were two to three times higher than in H37Rv-infected mice. The Erdman group also had higher levels of IL-1α and IL-1β at 24 weeks. In contrast to the other cytokines measured, the level of IFN-γ in BAL fluid from Erdman-infected mice at 2 weeks p.i. was 14 times higher than in mice infected with H37Rv or the attenuated M. tuberculosis strains. At the two later time points there was a rise and then a fall in IFN-γ levels for all M. tuberculosis strains but it remained substantially higher in the Erdman group. Consistent with the BAL fluid results, the level of IFN-γ was also higher in whole lung lysate of mice infected with Erdman compared to H37Rv (Fig. 5B).
Fig. 5.
Lung cytokine levels during TB disease. The indicated cytokines were measured by ELISA of BAL fluid (A, C) and cell-free lung homogenates (B) from mice infected with indicated M. tuberculosis strains. Data are presented as the mean concentration ± SD (n=3 or 4). *P<0.05, ** P<0.001.
Damage-associated molecular pattern molecules (DAMPs) have been identified as signals for neutrophil recruitment in necrotic cell death [8,9]. DAMPs may call neutrophils to TB lesions in the period of logarithmic increase in lung bacterial load coinciding with the peak of burst size necrosis. Recently, Gopal et al. [10] linked calgranulins S100A8 and S100A9 (recognized DAMPs) to neutrophil accumulation and damaging lung pathology in TB. We measured the S100A8/A9 protein complex in BAL fluid from mice infected with Erdman, H37Rv, H37Ra or RvΔphoPR (Fig. 5C). The levels were comparable at 2 weeks p.i. in mice infected with any of these four M. tuberculosis strains but at 4 and 24 weeks, the level in BAL fluid of Erdman group was significantly higher than mice from other groups. The high levels of S100A8/A9 at the later time points in Erdman-infected mice may have been both a cause and effect of greater neutrophil accumulation in that group but there was no indication that these DAMPs were a determining factor accounting for the predominance of AFB+ neutrophils in the Erdman group at 2 weeks p.i.
3.7. Mycobacterial replication and neutrophil recruitment in TB
It is conceivable that a property of M. tuberculosis Erdman other than its high replication rate and resultant burst size macrophage necrosis was responsible for the high rate of neutrophil infection in pulmonary TB lesions. To address that question, mice were challenged with Erdman then treated with the bacteriostatic drug EMB starting 4 days p.i. Mice were sacrificed on day 14 p.i. for determination of lung CFU, total AFB+ leukocytes and the distribution of AFB load per phagocyte in MPs and in neutrophils. The 2 week time point was selected as the period of peak bacterial replication before the full expression of adaptive immunity. Compared to untreated mice, there was a dose dependent decrease in lung CFU with EMB treatment of ~0.5 log10 at 67 μg/ml and a further ~0.5 log10 at 200 μg/ml (Fig. 6A). Lung CFU in mice treated with EMB at 600 μg/ml was ~4 log10 lower than untreated controls. The total number of AFB+ leukocytes mirrored the CFU counts (Fig. 6B). Treatment with EMB had a significant, dose dependent impact on the distribution of intracellular AFB loads in MPs, consistent with inhibition of bacterial replication (Fig. 6C). In untreated control mice, the total number of MPs containing 1–5 AFB was 1 log10 greater than the number of cells with ≥21 AFB. This spread increased to 1.7 log10 and 2.3 log10 in mice treated with EMB at 67 μg/ml and 200 μg/ml, respectively. No MPs containing >6 AFB were identified in mice given EMB at 600 μg/ml. There was a disproportionate reduction of AFB+ neutrophils compared to AFB+ MPs in mice treated with EMB (Fig. 6D). The ratio of AFB+ neutrophils to AFB+ MPs was 0.36 in control mice, falling to 0.19 and 0.17 in mice treated with 67 μg/ml and 200 μg/ml, respectively. No AFB+ neutrophils were identified in mice treated with 600 μg/ml EMB.
Fig. 6.
Ethambutol (EMB) treatment of mice with TB. Mice were infected with M. tuberculosis Erdman by aerosol and treated with ethambutol at 67, 200 or 600 μg/ml for 11 days, beginning on day 4 p.i. At 2 weeks p.i. infected lungs were harvested and plated for CFU (A) or enumerated for AFB+ cells from cytospin slides with Ziehl-Neelsen staining (B–D). (A) Compared to untreated mice, there was a dose-dependent reduction of lung CFU in EMB-treated mice. Data represent mean (n=4). * P<0.01, ** P<0.001. (B) Total AFB+ phagocytes also fell with increasing dosages of EMB. * P<0.01, ** P<0.001 compared to untreated mice. (C) EMB treatment resulted in a dose-dependent reduction of total AFB+ MPs and a progressively lower proportion of mononuclear cells with high AFB loads. (D) EMB treatment resulted in a dose-dependent reduction of total AFB+ neutrophils and a lower proportion of neutrophils with high AFB loads at each dose increment. Only untreated mice had neutrophil AFB loads of 11–15 per cell while mice treated with 600 μg/ml EMB had no detectable AFB+ neutrophils. (B–D) Data represent mean ± SD (n=3).
4. Discussion
We previously reported data supporting a model of burst size necrosis for M. tuberculosis-infected MPs in vitro and in vivo [1–3]. Following aerosol challenge, horizontal spread of M. tuberculosis Erdman, particularly into neutrophils, was curtailed by an effective adaptive immune response in wild-type C57BL/6 mice whereas infected neutrophils increased until death in IFN-γ−/− mice that were incapable of limiting bacterial replication in MPs [1]. The present study extended that investigation by challenging wild-type mice with four M. tuberculosis strains of graded virulence to study the effects mycobacterial replication on intracellular bacillary load, burst size necrosis and neutrophilic inflammation.
Whether classified on the basis of replication rate, total lung bacterial load or extent of immune pathology, Erdman was the most virulent of the four M. tuberculosis strains tested followed by H37Rv and then H37Ra. As expected, RvΔphoPR was the most attenuated strain. The steep growth curve of Erdman between inhalation of ~100 CFU and 2 weeks p.i. was matched by highest rate of horizontal transmission into neutrophils in that period. These results demonstrate a correlation between bacterial doubling time and horizontal spread of infection to recruited phagocytes during the innate phase of host immunity, and a correlation of bacterial replication rate with burst size necrosis and neutrophil accumulation at the site of infection.
Upon aerosol challenge, resident alveolar macrophages host the first round of M. tuberculosis replication. Only single bacilli can reach the alveolar airspaces after inhalation of infectious aerosols, so the initial infection of alveolar macrophages begins at an MOI of one bacillus per macrophage. Our data unexpectedly revealed that RvΔphoPR was capable of intracellular replication in vivo, exceeding 5 AFB per cell in 50% of infected MPs and reaching ≥21 AFB in 3% (Supplementary Fig. 1). The number of RvΔphoPR bacilli in the lung increased by 4–5 doublings from the delivered aerosol dose. Despite this replicative capacity there was no evidence of horizontal spread by RvΔphoPR into recruited leukocytes (Fig. 1B). That conclusion fits with the attenuated capacity for burst size macrophage necrosis in vitro that we reported for this strain [2] along with the complete absence of neutrophil infection in vivo identified in the current study.
The disparities between H37Rv and Erdman were striking, as were some similarities between H37Rv and H37Ra. The H37Rv isolate used in this study was fully virulent in terms of reaching a plateau lung burden of ~106 CFU that persisted for 24 weeks with progressively increasing pulmonary immune pathology. It is known that H37Rv can spontaneously lose expression of the cell wall lipid phthiocerol dimycocerosate (PDIM), resulting in its attenuation [11,12]. Although the H37Rv isolate used here retained PDIM (data not shown), it exhibited slower in vivo growth compared to Erdman in the first 4 weeks after aerosol challenge and substantially less horizontal spread into neutrophils. At 2 weeks p.i., the proportion of AFB+ neutrophils among all AFB+ phagocytes was 7 fold lower in mice infected with H37Rv than in Erdman-infected mice and the proportion of M. tuberculosis-infected neutrophils within the total lung neutrophil population was 65 fold lower in the H37Rv group.
The initial growth curve for H37Rv in the lung was steeper than H37Ra, and H37Rv maintained a high plateau burden at 24 weeks p.i. by which time H37Ra CFU declined more than 1 log10. Despite these differences, the total number of AFB+ leukocytes in the lungs of mice infected with H37Rv or H37Ra was surprisingly close at the 4 and 24 week time points (Fig. 1B). At 24 weeks, the maximum intracellular bacillary burden in MPs had shifted towards lower values for H37Ra (≤10 AFB) compared to H37Rv (≤20 AFB). Among factors contributing to these dynamics, we note that the survival of macrophages challenged with M. tuberculosis at MOI=1 in vitro is prolonged by IFN-γ activation, which prevents bacilli from growing to a burst size load [13]. We reported that IFN-γ exerts an opposite effect and accelerates necrosis of macrophages with a high intracellular bacillary load (MOI=25) [14]. This could explain the elimination of MPs containing >10 AFB at 24 weeks after infection with H37Ra which has limited or no replicative capacity at that late phase of TB disease and therefore does not continuously drive host cells to high AFB loads. With the expression of adaptive immunity, survival of MPs with a low intracellular H37Ra burden would be extended while the most heavily infected cells would be eliminated. The protective role of IFN-γ in TB disease is indisputable. However, its differential effect on regulating cell death of infected macrophages based on the bacterial load raises the question as to its possible contribution to the elevated levels of necrosis of infected MPs and inflammation as seen in the Erdman group.
Macrophage infection with M. tuberculosis has been linked to a diverse range of apoptotic and necrotic cell death modes, based mostly on evidence from in vitro experiments [15]. Macrophages may respond to attenuated mycobacterial strains, including H37Ra, by undergoing caspase-mediated apoptosis that might provide host-protective effects [16,17]. Comparable to our data, similar finding of fewer bacteria per infected phagocyte was also observed in mice challenged by aerosol with a pro-apoptotic (ΔnuoG) mutant of H37Rv [18]. The slower growth rate of H37Ra in the first 4 weeks p.i. along with the scarcity of cells harboring >15 AFB could reflect macrophage apoptosis and the delivery of bacilli to acidified vacuoles following efferocytosis by naïve macrophages [19]. Efferocytosis also provides a mechanism for spreading infection to naïve MP [17,20], which might explain the ability of H37Ra to invade nearly as many lung leukocytes as H37Rv in our study. In contrast to H37Ra and some other attenuated strains, virulent M. tuberculosis strains suppress macrophage apoptosis to preserve a protected replication niche which they ultimately escape by triggering burst size necrosis [1–3]. Under in vivo conditions it is likely that additional factors play a role in cytolysis but the majority of non-viable lung leukocytes in mice with TB contain >10 bacilli [1].
Neutrophils are increasingly recognized as major hosts for intracellular infection by M. tuberculosis in mice and in humans [1,21,22]. They play a complex role in TB pathogenesis with host protective effects in the initiation of adaptive immunity [23,24] but promoting damage when present in excess at later stages of disease [25–28]. The determinants of neutrophil recruitment to sites of TB disease therefore assume importance as potential drug targets for host-directed therapies to limit tissue damage in TB. In the present study, Erdman exhibited disproportionately higher spread into neutrophils despite no significant difference in total lung neutrophil numbers compared to H37Rv. The presence of AFB+ neutrophils unambiguously places those cells at the site of TB disease where they must, at least initially, engulf bacilli released from necrotic MPs.
Neutrophils are recruited to sites of necrotic cell death in vivo, swarming to sites of sterile or infectious injury [29]. In contrast, apoptotic cells release lactoferrin which acts as a “stay away” signal specifically inhibiting neutrophil but not MP migration in response to “find me” signals, thereby facilitating the non-inflammatory clearance of apoptotic cells [30]. In our experiments, AFB+ neutrophils correlated with M. tuberculosis virulence and with time points after aerosol challenge when bacteria were replicating at high rates and consequently triggering high rates of MP necrosis. Infection with Erdman resulted in the highest number and proportion of AFB+ neutrophils among total neutrophils and among total AFB+ lung leukocytes despite being the strongest inducer of IFN-γ, which was shown by Nandi et al. [31] to limit neutrophil recruitment and retention in the lungs of M. tuberculosis-infected mice. Neutrophil recruitment in Erdman-infected mice was not explained by the levels of several typical chemoattractant factors in BAL fluid, suggesting that some other signals may override the regulatory influences. A range of neutrophil chemoattractant factors link DAMPs to neutrophil recruitment in TB or other settings, including S100A8/A9 proteins, leukotriene B4, formyl peptide receptor-2 ligands, HMGB1, purine metabolites, MIP-2 and ENA-78 among others [16,32–34]. This redundancy complicates the identification of dominant pathways. In the present study we were unable to associate neutrophil accumulation in Erdman-infected mice with levels of MIP-2, S100A8/A9 or HMGB1 (data not shown) in BAL fluid. We speculate that the higher level of S100A8/A9 at later time points in Erdman-infected mice was an effect rather than a cause of neutrophil accumulation.
In summary, the data presented here and in our published studies [1–3] are consistent with a model of TB pathogenesis where bacterial replication in MPs reaches threshold lethal burden enabling bacilli to escape and spread to recruited phagocytes, including neutrophils responding to signals associated with necrotic cell death. The persistently high proportion of neutrophils in TB lesions at the 24 week time point when areas of TUNEL-positivity were greatest (Fig. 3B) yet M. tuberculosis replication even for Erdman was relatively low compared to 2 weeks p.i. (Fig. 1A) suggests that a feed-forward mechanism might perpetuate inflammation in more severe TB lesions. Neutrophils have short half-lives in vivo, although there is contrasting evidence that their lifespan might be prolonged in TB by inhibition of apoptosis [18] or curtailed by M. tuberculosis-induced NETosis [1]. The high rate of burst size necrosis caused by fast-replicating virulent M .tuberculosis strains in early TB might drive neutrophil accumulation in lesions in excess of the capacity to clear their corpses. This in turn would result in sustained release of signals from dying neutrophils even as IFN-γ restricts bacterial replication to a limited number of permissive MPs. Such dynamics could be a factor in the equilibrium between stable, expanding and contracting TB lesions revealed by positron emission tomography [35]. Targeting pathways of neutrophil trafficking to sites of infection-induced necrosis might shift this equilibrium towards stability and contraction, thus limiting the tissue injury that is responsible for most morbidity and mortality in TB.
Supplementary Material
Supplementary Fig. 1. Proportion of bacillary loads in mononuclear phagocytes. Wild-type mice were challenged with M. tuberculosis Erdman, H37Rv, H37Ra or RvΔphoPR by aerosol. Lung leukocytes were harvested 2, 4 and 24 weeks p.i.(only 2 and 4 weeks for RvΔphoPR-infected mice) and cytospin slides were processed for Ziehl-Neelsen staining. AFB per mononuclear phagocyte were counted on cytospin samples and stratified into indicated bins. Results are expressed as mean % AFB+ mononuclear cells within each bin ± SD.
Supplementary Fig. 2. Neutrophil clusters in lung TB lesions. Lung sections from mice infected with M. tuberculosis Erdman or H37Rvwere processed for H&E staining or colorimetric TUNEL assay. At 4 and 24 weeks p.i. clusters of neutrophils were observed predominantly in the vicinity of macrophages (H&E staining) and TUNEL signals (brown staining). Scale bar = 25 μm.
Acknowledgments
This work was supported by grant HL081149 (to H.K.) from the National Heart Lung and Blood Institute.
Footnotes
Conflict of interest
None to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Repasy T, Lee J, Marino S, Martinez N, Kirschner DE, Hendricks G, et al. Intracellular bacillary burden reflects a burst size for Mycobacterium tuberculosis in vivo. PLoS Pathog. 2013;9:e1003190. doi: 10.1371/journal.ppat.1003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee J, Repasy T, Papavinasasundaram K, Sassetti C, Kornfeld H. Mycobacterium tuberculosis induces an atypical cell death mode to escape from infected macrophages. PloS One. 2011;6:e18367. doi: 10.1371/journal.pone.0018367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J, Remold HG, Ieong MH, Kornfeld H. Macrophage apoptosis in response to high intracellular burden of Mycobacterium tuberculosis is mediated by a novel caspase-independent pathway. J Immunol. 2006;176:4267–74. doi: 10.4049/jimmunol.176.7.4267. [DOI] [PubMed] [Google Scholar]
- 4.Vallerskog T, Martens GW, Kornfeld H. Diabetic mice display a delayed adaptive imuune response to Mycobacterium tuberculosis. J Immunol. 2010;184:6275–6282. doi: 10.4049/jimmunol.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.North RJ, Izzo AA. Mycobacterial virulence. Virulent strains of Mycobacterium tuberculosis have faster in vivo doubling times and are better equipped to resist growth-inhibiting functions of macrophages in the presence and absence of specific immunity. J Exp Med. 1993;177:1723–33. doi: 10.1084/jem.177.6.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altmann C, Andres-Hernando A, McMahan RH, Ahuja N, He Z, Rivard CJ, et al. Macrophages mediate lung inflammation in a mouse model of ischemic kidney injury. Am J Physiol Renal Physiol. 2011;302:F421–F432. doi: 10.1152/ajprenal.00559.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbarshahi H, Menzel M, Bauden MP, Rosendahl A, Andersson R. Enrichment of murine CD68+CCR2+ and CD68+CD206+ lung macrophages in acute pancreatitis-associated acute lung injury. PLoS One. 2011;7:42654. doi: 10.1371/journal.pone.0042654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pittmna K, Kubes P. Damage-assoicated molecular patterns control neutrophil. J Innate Immun. 2013;5:315–323. doi: 10.1159/000347132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kono H, Rock K. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gopal R, Monin L, Torres D, Slight S, Mehra S, McKenna KC, et al. S100A8/A9 proteins mediate neutrophilic inflammation and lung pathology during tuberculosis. Am J Respir Crit Care Med. 2013;188:1137–46. doi: 10.1164/rccm.201304-0803OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox JS, Chen B, McNeil M, Jacobs WR. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature. 1999;402:79–83. doi: 10.1038/47042. [DOI] [PubMed] [Google Scholar]
- 12.Camacho LR, Ensergueix D, Perez E, Gicquel B, Guilhot C. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Molec Microbiol. 1999;34:257–67. doi: 10.1046/j.1365-2958.1999.01593.x. [DOI] [PubMed] [Google Scholar]
- 13.Park JS, Tamayo MH, Gonzalez-Juarrero M, Orme IM, Ordway DJ. Virulent clinical isolates of Mycobacterium tuberculosis grow rapidly and induce cellular necrosis but minimal apoptosis in murine macrophages. J Leukoc Biol. 2006;79:80–6. doi: 10.1189/jlb.0505250. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Kornfeld H. Interferon- regulates the death of M. tuberculosis-infected macrophages. J Cell Death. 2010;3:1–11. doi: 10.4137/jcd.s2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moraco AH, Kornfeld H. Cell death and autophagy in TB. Semin Immunol. 2014;26:497–511. doi: 10.1016/j.smim.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keane J, Balcewicz-Sablinska K, Remold HG, Chupp GL, Meek BB, Fenton MJ, Kornfeld H. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect Immun. 1997;65:298–304. doi: 10.1128/iai.65.1.298-304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin CJ, Peters KN, Behar SM. Macrophages clean up: efferocytosis and microbial control. Curr Opin Microbiol. 2014;17:17–23. doi: 10.1016/j.mib.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host Microbe. 2012;11:81–90. doi: 10.1016/j.chom.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin CJ, Booty MG, Rosebrock TR, Nunes-Alves C, Desjardins DM, Keren I, et al. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe. 2012;12:289–300. doi: 10.1016/j.chom.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis JM, Ramadkrishnan L. The role of the granuloma in expansion and dissemination of early tuberculosis infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179:2509–19. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- 22.Eum SY, Kong JH, Hong MS, Lee YJ, Kim JH, Hwang SH, et al. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137:122–8. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blomgran R, Ernst JD. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J Immunol. 2011;186:7110–9. doi: 10.4049/jimmunol.1100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang DD, Lin Y, Moreno JR, Randall TD, Khader SA. Profiling early lung immune responses in the mouse model of tuberculosis. PLoS One. 2011;6:e16161. doi: 10.1371/journal.pone.0016161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller C, Hoffmann R, Lang R, Brandau S, Hermann C, Ehlers S. Genetically determined susceptibility to tuberculosis in mice causally involves accelerated and enhanced recruitment of granulocytes. Infect Immun. 2006;74:4295–309. doi: 10.1128/IAI.00057-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martens GW, Arikan MC, Lee J, Ren F, Vallerskog T, Kornfeld H. Hypercholesterolemia impairs immunity to tuberculosis. Infect Immun. 2008;76:3464–72. doi: 10.1128/IAI.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan H, Yan BS, Rojas M, Shebzukhov YV, Zhou H, Kobzik L, et al. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2005;434:767–72. doi: 10.1038/nature03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marzo E, Vilaplana C, Tapia G, Diaz J, Garcia V, Cardona PJ. Damaging role of neutrophilic infiltration in a mouse model of progressive tuberculosis. Tuberculosis. 2014;94:55–64. doi: 10.1016/j.tube.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, Germain RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–5. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bournazou I, Pound JD, Duffin R, Bournazos S, Melville LA, Brown SB, et al. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J Clin Invest. 2009;119:20–32. doi: 10.1172/JCI36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208:2251–62. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amaral EP, Ribeiro SC, Lanes VR, Almeida FM, de Andrade MR, Bomfim CC, et al. Pulmonary infection with hypervirulent Mycobacteria reveals a crucial role for the P2X7 receptor in aggressive forms of tuberculosis. PLoS Pathog. 2014;10:e1004188. doi: 10.1371/journal.ppat.1004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nouailles G, Dorhoi A, Koch M, Zerrahn J, Weiner J, Fae KC, et al. CXCL5-secreting pulmonary epithelial cells drive destructive neutrophilic inflammation in tuberculosis. J Clin Invest. 2014;124:1268–82. doi: 10.1172/JCI72030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berthelot F, Fattoum L, Casulli S, Gozlan J, Marechal V, Elbim C. The effect of HMGB1, a damage-associated molecular pattern molecule, on polymorphonuclear neutrophil migration depends on its concentration. J Innate Immun. 2012;4:41–58. doi: 10.1159/000328798. [DOI] [PubMed] [Google Scholar]
- 35.Lin PL, Coleman T, Carney JP, Lopresti BJ, Tomko J, Fillmore D, et al. Radiologic responses in cynomolgous macaques for assessing tuberculosis chemotherapy regimens. Antimicrob Agents Chemother. 2013;57:4237–44. doi: 10.1128/AAC.00277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Proportion of bacillary loads in mononuclear phagocytes. Wild-type mice were challenged with M. tuberculosis Erdman, H37Rv, H37Ra or RvΔphoPR by aerosol. Lung leukocytes were harvested 2, 4 and 24 weeks p.i.(only 2 and 4 weeks for RvΔphoPR-infected mice) and cytospin slides were processed for Ziehl-Neelsen staining. AFB per mononuclear phagocyte were counted on cytospin samples and stratified into indicated bins. Results are expressed as mean % AFB+ mononuclear cells within each bin ± SD.
Supplementary Fig. 2. Neutrophil clusters in lung TB lesions. Lung sections from mice infected with M. tuberculosis Erdman or H37Rvwere processed for H&E staining or colorimetric TUNEL assay. At 4 and 24 weeks p.i. clusters of neutrophils were observed predominantly in the vicinity of macrophages (H&E staining) and TUNEL signals (brown staining). Scale bar = 25 μm.