Abstract
Transfusion of stored red blood cells (RBCs) is associated with increased morbidity and mortality in trauma patients. Pro-oxidant, pro-inflammatory and nitric oxide (NO) scavenging properties of stored RBC are thought to underlie this association. In this study we determined the effects of RBC washing, nitrite and anti-heme therapy on stored RBC-dependent toxicity in the setting of trauma-induced hemorrhage. A murine (C57bl/6) model of trauma-hemorrhage and resuscitation with 1 or 3 units of RBC stored for 0–10d was used. Tested variables included whether washing RBC to remove lower MWt components that scavenge NO, NO-repletion therapy using nitrite or mitigation of free heme-toxicity by heme scavenging or preventing TLR4 activation. Stored RBC toxicity was determined by assessment of acute lung injury indices (airway edema and inflammation) and survival. Transfusion with 5d RBC increased acute lung injury indexed by BAL protein and neutrophil accumulation. Washing 5d RBC prior to transfusion did not decrease this injury, whereas nitrite therapy did. Transfusion with 10d RBC elicited a more severe injury resulting in ~90% lethality, compared to <15% with 5d RBC. Both washing and nitrite therapy significantly protected against 10d RBC-induced lethality, suggesting that washing may be protective when the injury stimulus is more severe. Finally, a spectral deconvolution assay was developed to simultaneously measure free heme and hemoglobin in stored RBC supernatants, which demonstrated significant increases of both in stored human and mouse RBC. Transfusion with free heme partially recapitulated the toxicity mediated by stored RBC. Furthermore, inhibition of TLR4 signaling, which is stimulated by heme, using TAK-242, or hemopexin-dependent sequestration of free heme significantly protected against both 5d and 10d mouse RBC-dependent toxicity. These data suggest that RBC washing, nitrite therapy and / or anti-heme and TLR4 strategies may prevent stored RBC toxicities.
Keywords: hemoglobin, hemin, nitric oxide, heme, acute lung injury, resuscitation, storage lesion
Graphical Abstract
Introduction
Transfusion with packed red blood cells (RBCs) is a front-line therapy for critically ill, anemic patients. However, recent studies have documented positive associations between the number of RBC units transfused, the age of the RBC unit being transfused and increased transfusion-related morbidity and mortality[1]. For example, our studies have shown increased incidence of acute lung injury, acute kidney injury, pneumonia, and mortality in bleeding trauma patients, a population that receives a significant portion of the stored blood in tertiary care centers [2–4]. Importantly, storage lesion toxicity is observed in diverse patient populations, suggesting common mechanisms related to gain of toxic functions by stored RBCs. This understanding has fueled numerous research efforts aimed at elucidating the mechanisms by which stored RBC may elicit injurious responses after transfusion[5]. Current thinking suggests these are related to microcirculatory dysfunction, exacerbation of underlying inflammation, increased oxidative stress, and increased predisposition to nosocomial infections[2, 6–11]. During storage, several structural, biochemical, and metabolic alterations occur to the RBC. These changes include loss of metabolites (e.g. ATP), loss of RBC volume with accompanying formation of echinocytic forms and hemoglobin-containing microparticles, RBC degradation, and release of cell-free hemoglobin (hemolysis), iron, and cellular debris[12]. While potential toxic effects of each of these aspects of RBC storage have been studied (see below), little attention has been given to the potential of heme released during storage as a mediator of transfusion injury. Notably, recent studies suggest that heme is a potent inducer of inflammatory tissue injury in sickle cell disease and sepsis [13–18].
Loss of nitric oxide (NO) homeostasis has emerged as a mechanism underlying many features of transfusion toxicity associated with stored RBC, including microcirculatory dysfunction and inflammation. Stored RBC, including cells with altered morphology, hemoglobin-containing microparticles and hemolysis-derived cell-free hemoglobin, all display ferrous heme dependent NO-scavenging with kinetics that are faster compared to freshly isolated RBC[19–21]. This biochemical property also translates to a greater inhibition of NO-dependent signaling ex vivo and in vivo[19, 20, 22]. In addition to scavenging NO, older RBCs may also be less effective at stimulating endogenous NO formation. Stored RBCs display faster rates of nitrite oxidation in vitro, and transfusion of trauma patients with older cells, but not younger RBCs, results in lower circulating nitrite levels [20]. Since nitrite is a putative substrate for NO formation in hypoxic tissues, this reactivity would lower an endogenous substrate for NO formation, thereby further leading to inhibition of NO signaling. Moreover, RBC–derived ATP-dependent activation of endothelial nitric oxide synthase is lost in stored RBC[23], and RBC-S-nitrosothiols have also been postulated as potential mechanisms for loss of NO homeostasis [24, 25]. Collectively, these data provide mechanistic insights into how transfusions with stored RBC inhibit endogenous NO signaling in the vasculature and suggest that NO-repletion strategies, or approaches that remove components in stored RBC that inhibit NO signaling could be beneficial. In the latter context, RBC washing immediately before transfusion to remove microparticles, cell-free hemoglobin, and iron, prevented many of the negative effects of storage in a canine model of transfusion and infectious lung injury[26] and attenuated cytokine levels in a mouse model of trauma-hemorrhage[27] . In the current study, we show similar protective effects of washing towards acute lung injury and lethality in a murine model of trauma-hemorrhage. In addition, we show that NO-repletion therapy using nitrite protects against storage-induced toxicity. Finally we provide evidence that free-heme significantly increases during storage and is a key mediator of injury in this model.
Materials and Methods
TAK-242, a small molecule inhibitor of TLR4, was purchased from InvivoGen and dissolved in intralipid (Sigma). Hemopexin was purchased from Athens Research & Technology. All other materials were purchased from Sigma–Aldrich (St Louis, MO) except MahmaNONOate which was obtained from Axxora Platform (San Diego, CA). Sodium nitrite (Sigma) used in resuscitation was dissolved in saline. Adsol was from Baxter Health Care Corporation. Human oxyhemoglobin was purified for healthy donors according to UAB Institutional Review Board approved protocols and stored in the CO-ligated form as previously described[28]. Methemoglobin was synthesized and purified as previously described using potassium ferricyanide added at a 2-fold excess over oxyHb (in heme) and purifying metHb by gel-filtration using Sephadex G-25 columns[29]. Similar protocols were used for preparing mouse hemoglobin. Male C57BL/6 mice weighing 22 g to 30 g were purchased from Harlan Laboratories (Indianapolis, IN) at 8–10 weeks of age.
Preparation of hemin and hemoglobin standards for spectral deconvolution
To ensure no COHb remained in oxyHb solutions and that all oxyHb was oxidized to metHb, reduction with sodium dithionite and >99% conversion to deoxyHb was verified. oxyHb and metHb solutions were diluted to 25µM (in heme) into Adsol pH7.4 or pH 6.8. Adsol was collected under sterile conditions on the day of experiments and pH adjusted from 5 (adsol basal pH) to 6.8 or 7.4 using 0.1N NaOH. Hemin was dissolved in 0.1N NaOH at 10mM (concentrations verified using ε385nm = 58.4mM−1 cm−1). 5ml of a 25µM solution of hemin was then prepared in Adsol pH 5.0 and pH adjusted to pH 6.8 using 0.1N NaOH. For pH 7.4, hemin was diluted to 25µM in Adsol (pH 5) and pH adjusted to 7.4 using 0.1N NaOH. Standard spectra were acquired between 450–700nm at room temperature. For preparation of hemin and oxyHb or metHb mixtures for validation of deconvolution method, pH of stock solutions were adjusted to 6.8 or 7.4 using Adsol (pH 5) or 0.1N NaOH and then mixed to give final indicated concentrations.
Free heme measurement- Method development and validation
We developed a spectral deconvolution approach to simultaneously measure the concentrations of oxyhemoglobin, methemoglobin and free heme in solution as described in results.
Human and mouse RBC collection
Human RBCs stored for up to 42d were collected from segments attached to blood bags from the UAB blood bank, and processed according to UAB Institutional Review Board approved protocols. All human RBCs used in this study were leukoreduced and stored in Adsol-1. At the time of collection, RBCs (~0.5ml) were placed into 1.7ml Eppendorf tubes and pelleted by centrifugation (1500 x g, 10min, 4°C). The supernatant (~200µl) was removed and indices of storage related changes (described below) determined. For acquisition of spectra, the resultant supernatant fraction was centrifuged again (2000 x g, 30sec). 50µl of the supernatant was taken and diluted 10-fold with distilled water and absorbance spectrum (450–700nm) measured. For mouse RBCs, blood (~800uL) was collected from healthy male C57BL/6 mice via cardiac puncture in 50µl citrate buffer (trisodium citrate (22.0g/L), citric acid (8.0g/L), dextrose (24.5g/L)). Blood was filtered through neonatal Sepacell filters or through Sephadex G25 microcellulose column to remove leukocytes by gel filtration. Columns were washed with 10 times the volume of PBS or Adsol to eluate RBC (with no differences in hemolysis during processing observed (not shown). The eluent was centrifuged at 1500 x g for 5 min, 4°C. The erythrocyte pellet was washed 3 times with cold Adsol and concentrated to a hematocrit of 60% with Adsol and stored in 0.7mL Eppendorf tubes with a head space of approximately 300uL. All solutions and procedures were performed under sterile conditions and used CPD anticoagulant and AS1 storage solutions obtained from the blood donation kit (Fenwal Express System, Lake Zurich, IL) used form human blood collection. RBCs were stored at 4° C in the dark, for up to 10 days. LPS measurement in stored RBC using the Limulus Amebocyte lysate assay (Cambrex, MD) indicated levels were below detection limit (not shown).
Storage lesion characterization
At various storage times, mouse RBC hematocrit, levels of cell-free hemoglobin (Hb), microparticles, oxygen affinity, nitric oxide dioxygenation kinetics and nitrite oxidation kinetics were determined as previously described[20, 30]. Briefly, the rate of NO-dioxygenation by RBCs was determined using competition kinetics (relative to cell-free hemoglobin) using MahmaNONOate. Nitrite oxidation was determined using hemolysates (prepared by lysing 20µl pRBCs with 80µl ice-cold water) and then diluting hemolysates in PBS containing 100µM DTPA (pH 7.4) to 25µM hemoglobin final concentration. Nitrite (1mM) was added to hemolysates in PBS (37°C) and methemoglobin formation measured as a function of time. Nitrite oxidation rates were compared by measuring lag times for methemoglobin formation (lower lag times indicating high rates of nitrite oxidation) as described[30].
Flow Cytometry
RBC preparations before and after leukoreduction were labeled with FITC conjugated anti-CD45 antibody (0.17µg/ml) and white blood cells measured by FACS. Microparticles were measured by adding FITC conjugated TER-119 antibody (a protein associated with glycophorin A in mice) to washed or unwashed RBC. Both preparations were incubated for 30min in the dark at room temperature. Samples were then analyzed by FACS using a Becton Dickinson FACSCalibur (BD Biosciences, Franklin Lakes, NJ) or a Beckman Coulter 3500 (Brea, CA) and events acquired using CellQuest or Beckman Coulter native software. Approximately 100,000 events were collected per measurement. All analyses were done with FlowJo software (Tree Star, Inc., Ashland, OR) or Kaluza (Beckman Coulter Inc. Brea, CA). Microparticles were assessed as being TERR-119 positive events that were below 1µm in size.
Mouse Trauma/Hemorrhage resuscitation
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham. Trauma hemorrhage was performed as previously described [31]. C57BL/6 male mice were anaesthetized by inhalation of 5% isoflurane in air. The concentration of isoflurane was then reduced to the minimal concentration for maintenance (<1%). The abdomen and groins were shaved and washed with 10% povidone-iodine. A 2 cm midline laparotomy was performed to induce soft-tissue trauma. The incision was closed in two layers (fascia/muscle and skin) and bathed in 1% lidocaine for analgesia. Both femoral arteries were cannulated with catheters (Braintree Scientific, MA). Systemic arterial pressure was continuously monitored through one arterial line while hemorrhage and transfusion performed via the other. Mice were bled over 30 min to a MAP (mean arterial pressure) of 25±5 mmHg (~60% blood loss, 800 µl). This blood pressure was maintained for a further 60 min by additional bleeding as required. At the end of the hemorrhagic shock period, if animals were receiving nitrite treatment, prior to transfusion, 100µl of nitrite dissolved in saline was administered intravenously in a bolus form (nitrite stock solutions were 0.1 mM, or 1 mM). Mice receiving TLR4 inhibitor were given TAK-242 (7.5 mg/kg) or vehicle (50µL intralipid) either prior to hemorrhage or 5–10 min prior to resuscitation with RBC. Hemopexin (0.5mg) was dissolved in PBS and delivered 10 min prior to transfusion in a volume of 100uL. All animals were resuscitated over 30 min with saline (room temperature) at 4X volume shed during hemorrhage, or with saline at the same volume of shed blood containing either 1 unit or 3 units of pRBCs (100ul or 300ul respectively of 60% Hct) stored for 0d (used the day of leukoreduction), 5d or 10d; initial studies determined different resuscitation volumes required to achieve pre-hemorrhage MAP’s immediately post-transfusion. In some experiments, RBC were washed 30min prior to transfusion 3 times with ice-cold Adsol (1500xg, 5 min). If the cells were washed, Adsol was added to equal volume of RBCs prior to washing. After washing, RBC were prepared and transfused in the same manner as unwashed RBC. For nitrite treatment, 5min prior to transfusion, 100µl of nitrite dissolved in saline was administered intravenously in a bolus form (nitrite stock solutions were 0.1 mM, or 1 mM resulting in 10µmol or 100µmol per mouse respectively). Hemopexin (0.5mg) was administered 10min prior to resuscitation. At 30 min post resuscitation, femoral cannulations were removed and isoflurane treatment ended. All experiments were carried out for at least 4h post-resuscitation with animals kept on a heated pad for this duration. Arterial blood samples were collected immediately before transfusion, at the completion of transfusion (30min), 60min and 4h after the initiation of transfusion. At 4h post initiation of transfusion, mice were sacrificed and acute lung injury indices assessed. Mice were also observed every 30min to evaluate for mortality by observing physical activity, righting reflex, body temperature drop over 4°C along with loss of righting reflex or failure to move after repositioning over 2 consecutive 10 min intervals and breathing pattern. Any mice displaying these symptoms were immediately euthanized. Control experiments included testing effects of saline or stored RBC transfusion in mice that underwent trauma only without hemorrhage.
Acute Lung injury assessment
Mice were euthanized with intraperitoneal ketamine and xylazine (100 and10 mg/kg body weight respectively). A final blood sample was taken by cardiac puncture and an incision was made at the neck to expose the trachea and a 3mm endotracheal cannula inserted. Lungs were lavaged with 3 × 1 ml of PBS. Recovered aliquots of lavage fluid were kept on ice, and centrifuged immediately at 300g for 10 minutes to pellet cells. Supernatants were removed and stored on ice for protein analysis using the Protein Assay Reagent Kit (Bio-Rad, Hercules, CA) compared to BSA standards. Cells were re-suspended in 100µl PBS and counted using a Neubauer hemocytometer. Cells were then placed on slides using a cellspin (Tharmac) and stained using Wright’s stain. Differential counts (specifically monocytes, neutrophils, and lymphocytes) were then performed on slides via light microscopy.
Statistical analysis
Storage time- or therapy dependent changes were analyzed by 1-way repeated measures ANOVA with Tukey’s post-test. For survival the two-tailed N-1 two proportion test was used. P-values less than 0.05 were considered significant.
Results
Characterization of Murine RBC storage
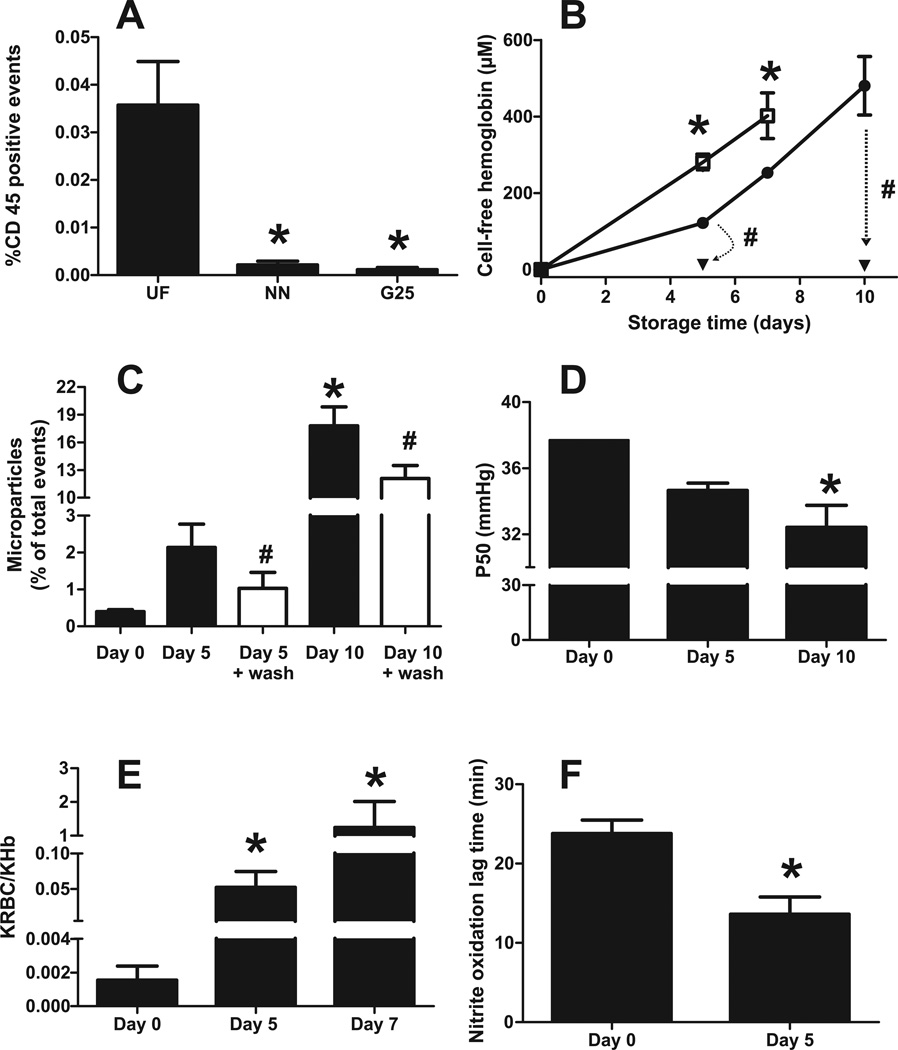
Initial studies were designed to validate methods to leukoreduce (LR) and store mouse RBC (mRBC) and compare this to human RBC (hRBC) stored for 35–42d. While recent studies have shown that storage of murine RBC for ~2 weeks is equivalent to human RBC storage for 42 days based on RBC stability and recovery post transfusion[10, 27, 32], the effects on NO-dioxygenation and nitrite oxidation kinetics are not known. C57BL/6 RBC were leukoreduced using either microcellulose Sephadex G25-columns or by neonatal Sepacell filters (Figure 1A). Each method significantly reduced leukocyte content by 94% and 97% respectively; further studies used microcellulose sephadex-G25 only. Leukoreduced mRBC were stored in Adsol for up to 10 days and markers of storage induced damage including hemolysis, microparticle formation, oxygen affinity, rates of nitric oxide dioxygenation and rates of nitrite oxidation measured (Figure 1B–F respectively). Hemolysis increased in a storage time-dependent manner, with the magnitude of hemolysis being lower in LR vs non-LR RBC. Sepacell filtered RBCs showed similar hemolysis to G25-filtered RBCs (not shown), indicating no adverse effects on RBC mechanical fragility by G25 filtration. Notably, the extent of hemolysis with mRBC was significantly greater compared to 35–42d hRBC; the latter was ~50–100µM (or ~0.5–1%) [19, 20] compared to ~400µM (or ~4%) for mRBC stored for 10d. These data are consistent with previous studies that also show a relatively high degree of hemolysis for stored mRBC[33]. TER-119 positive microparticles and oxygen affinity (decreasing P50) also increased with storage time (Figure 1C–D), although the magnitude of changes in P50 were relatively small compared to hRBC stored for >35d. The rate of NO-dioxygenation was accelerated 34- and 800-fold with day 5 and 7 mRBC respectively, compared to day 0 mRBC (Figure 1E); day 10 measurements were not possible due to extensive hemolysis during experiments. Similarly, the rate of nitrite oxidation was also increased with day 5 mRBC, as indicated by lower lag times (Fig 1F).
Figure 1. Characterization of murine RBC storage.
Panel A C57BL/6 blood was filtered by either Purecell NEO Neonatal High Efficiency Leukocyte Reduction Filter (Pall corporation) (NN) or by Sephadex G25: microcellulose column. (G25). Leukocyte content was determined by FACs and staining for the surface antigen CD45. * P< 0.05 compared to unfiltered (UF) by 1-way ANOVA with Tukey post test (n=3). Panel B: Storage time dependent hemolysis in control (non leukoreduced) RBC (□), leukoreduced (LR) RBC (●) or LR RBC after washing (▼), change indicated by dashed arrows *P<0.01 by 2-way ANOVA with Bonferroni post test (n = 3–9). #P<0.02 by paired t-test (n=3–6). Panel C: Storage time dependent formation of glycophorin positive microparticles in LR mRBC before and after washing. *P<0.01 by 1-way ANOVA with Tukey post test relative to day 0 unwashed RBC. #P<0.04 by paired t-test relative to respective unwashed RBC (n=3–6). Panel D Storage time dependent changes in P50. *P<0.05 relative to day 0 (n=3) by 1-way ANOVA with Tukey post test. Panel E shows relative rate constants for NO-dioxygenation by intra-erythrocytic (KRBC) hemoglobin vs. acellular hemoglobin (KHb) as a function of storage age. *P<0.05 relative to day 0 by 1-way ANOVA with Tukey post test (n=3–6). Panel F shows lag times for hemolysate mediated nitrite oxidation. *P<0.01 by t-test (n=5).
RBC storage results in increased free heme
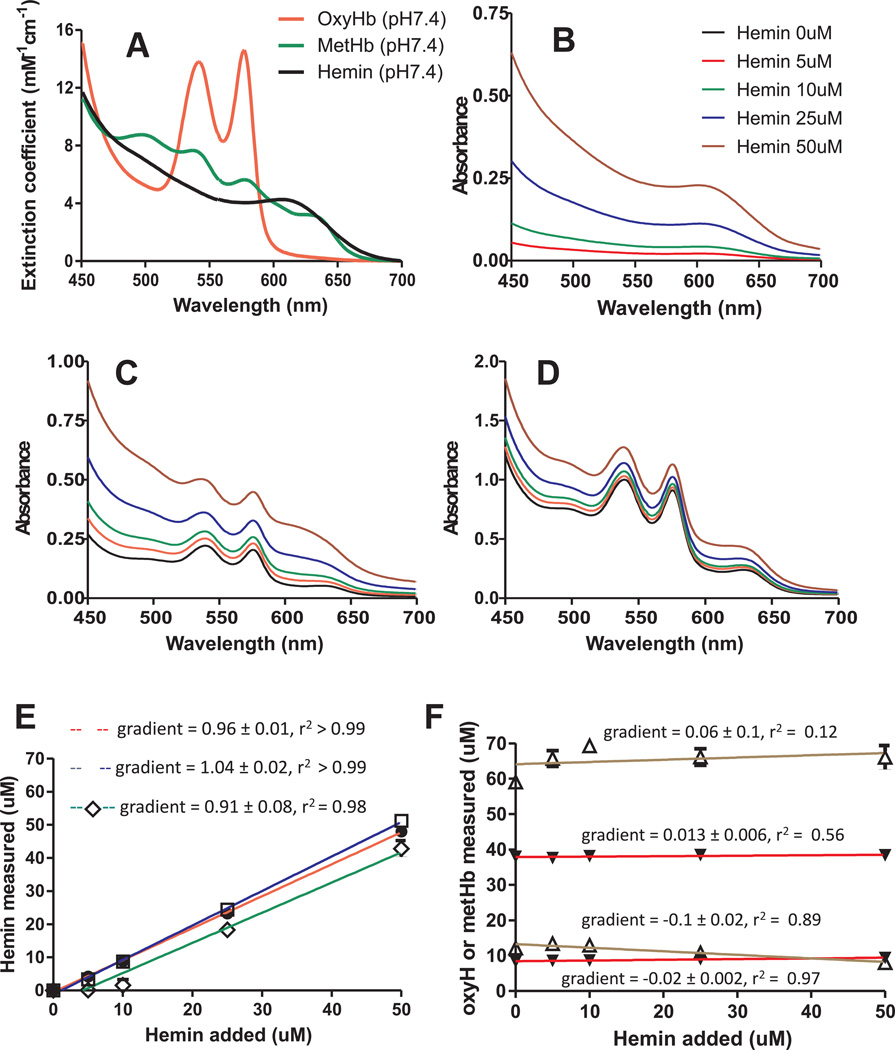
In order to measure free heme levels, we developed a spectral deconvolution approach that would allow simultaneous assessment of free heme and Hb in the same sample. This approach relies on individual species within a complex mixture having distinct absorbance spectra, and availability of spectra for a known concentration of each species alone. Concentrations of each individual species within a mixture are determined by deconvolution of experimental spectra against standards by multi-linear regression fitting. While hemoglobin can exist in a number of ligation and oxidation states, in the context of cell-free hemoglobin arising during RBC storage, only oxyhemoglobin and methemoglobin are detectable. Less is known on the oxidation or ligation state of any free heme that may arise. However, free heme is readily oxidized to the ferric oxidation state and bound by chloride (hemin) or hydroxide (hematin).We assumed any free heme present would be in the ferric oxidation state and used hemin to generate standard spectra. Since the spectrum of metHb is particularly pH sensitive, and pH of storage solution decreases with storage time (measured pH being 7.4 for day 7, and pH 6.8 for day 35) standards were generated in Adsol at both pH 7.4 and 6.8. Day 7 sample spectra were deconvoluted using pH7.4 standard spectra, and day 35 sample spectra using pH 6.8 standard spectra. The 3-component standard spectra (oxyhemoglobin, methemoglobin and hemin) at pH 7.4 or 6.8 used for deconvolution are shown in Figure 2A for pH 7.4, and supplementary Figure 1A for pH 6.8. To test and validate the method, spectra of hemin (0–50µM) alone or with oxyHb (10µM) and metHb (10µM), or with oxyHb (40µM) and metHb (60µM) were measured in Adsol at pH 7.4 or pH 6.8. Figure 2 (pH 7.4) and supplementary Figure 1 (pH 6.8) show spectra (Panel B–D), and measured concentrations of hemin (panels E) and oxyHb and metHb (Panel F) after deconvolution relative to the amount of hemin added (gradients indicating recovery across concentrations tested are shown on each graph). The measured hemin concentration was directly proportional to the hemin added for every condition except at low hemin concentration (<10µM) at pH7.4 in the presence of high oxyHb + metHb levels. This was due to metHb indicated by higher yields of metHb over 0–10µM added hemin. Importantly, metHb did not interfere with hemin measurements when present at lower concentrations at pH 7.4 or pH 6.8 and spectral deconvolution was able to distinguish between oxyHb and hemin in all conditions tested. Since metHb levels never exceeded 2µM in experimental samples, deconvolution is sensitive to detect changes in extra-erythrocytic hemin in stored RBC.
Figure 2.
Standard spectra for oxyhemoglobin, methemoglobin, and hemin in Adsol buffer, pH 7.4 (Panel A). Solutions of hemin alone (0–50µM) or in combination with oxyHb (10µM) + metHb 10µM or with oxyHb 40µM + metHb (60µM) were prepared in Adsol at pH 7.4 and spectra measured (Panel B–D respectively). Spectra were deconvoluted and calculated hemin (Panel E) and oxyHb and metHb (panel F) plotted vs hemin added. Deconvolution analysis was performed using standard spectra shown in Figure 2. Data in panels E–F were fitted by linear regression and are mean ± STDEV (n=3). Gradients determined from regression fit are indicated on respective plots. Key: Panel E: ● = hemin alone group, □ = hemin + oxyHb (10µM) + metHb (10µM) group; ◊ = hemin + oxyHb (50µM) + metHb (50µM) group. Panel F: ▼ = oxyHb, Δ= metHb.
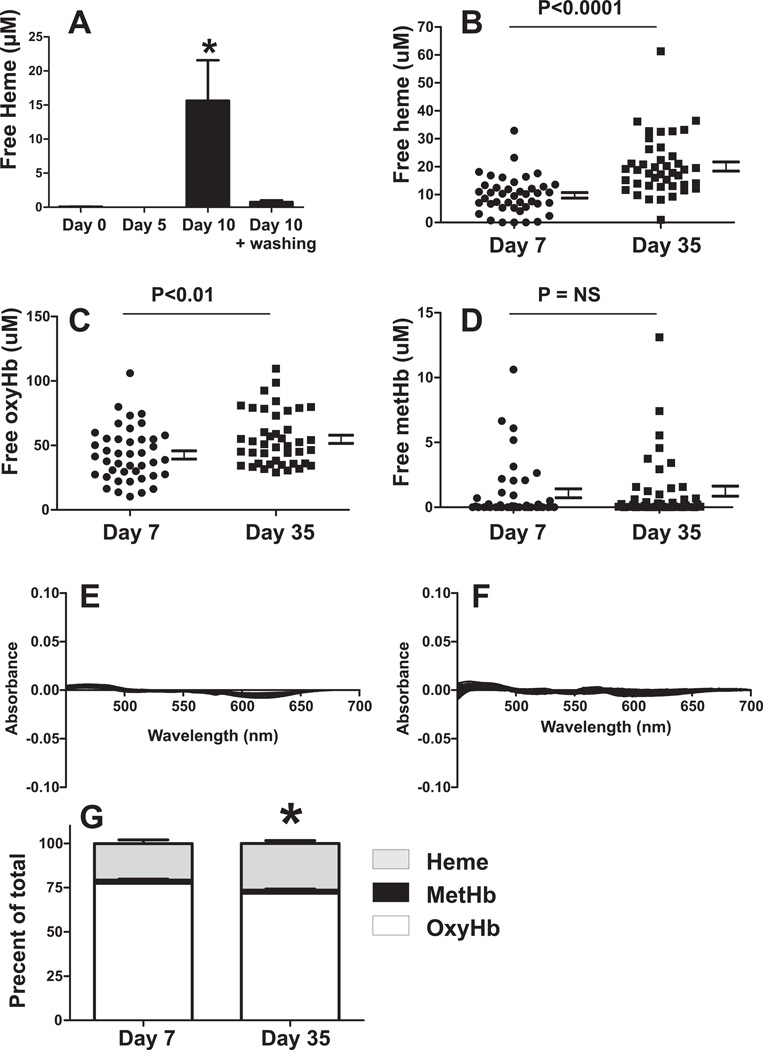
Figure 3A shows that after 10d of mRBC storage, significant increases in free heme are observed which are significantly lowered after washing. Figure 3B–D compares free heme, free oxyhemoglobin, and free methemoglobin levels measured in the same samples of human RBC stored for 7d or 35d; Figure 3E–F shows residuals for spectral deconvolution fits. Free heme increased ~2-fold from 9.7 ± 1µM to 20 ± 1.6µM (mean ± SEM) with storage. Total cell-free hemoglobin also increased with storage, with this being exclusively mediated by increases in oxyhemoglobin (Fig 3C); no changes in methemoglobin were observed (Fig 3D). Figure 3G plots the percentage of free heme, oxy or methemoglobin as a function of total extracellular heme, and shows that the relative contribution of free heme vs. Hb increases with storage age.
Figure 3. Cell-free heme increases with RBC storage.
Panel A: Mouse RBC were stored for 0, 5, or 10d and cell-free heme measured by spectral deconvolution before and after washing. Data show mean ± SEM (n=3–8). *P<0.05 vs. all other groups by 1-way ANOVA with Tukey post test. Panel B,C,D show respectively levels of cell-free heme, cell-free oxyhemoglobin and cell-free methemoglobin measured in the same sample of human RBC collected from bag associated segments after 7d or 35d of storage. Each point represents distinct RBC sample. Indicated P-values calculated by unpaired t-test (N=42). Panel E–F shows residuals for fits of experimental spectra (d7 and d35 respectively) by deconvolution. Panel G shows percent of each species relative to total extracellular heme (free or present in hemoglobin). Data are mean ± SEM (n=42) *P<0.05 for hemin and oxyHb relative to day 7. Deconvolution analysis were performed with mouse (panel A) or human hemoglobin (panel B–E) standard spectra for respectively.
Effects of stored RBC on trauma-hemorrhage induced toxicity
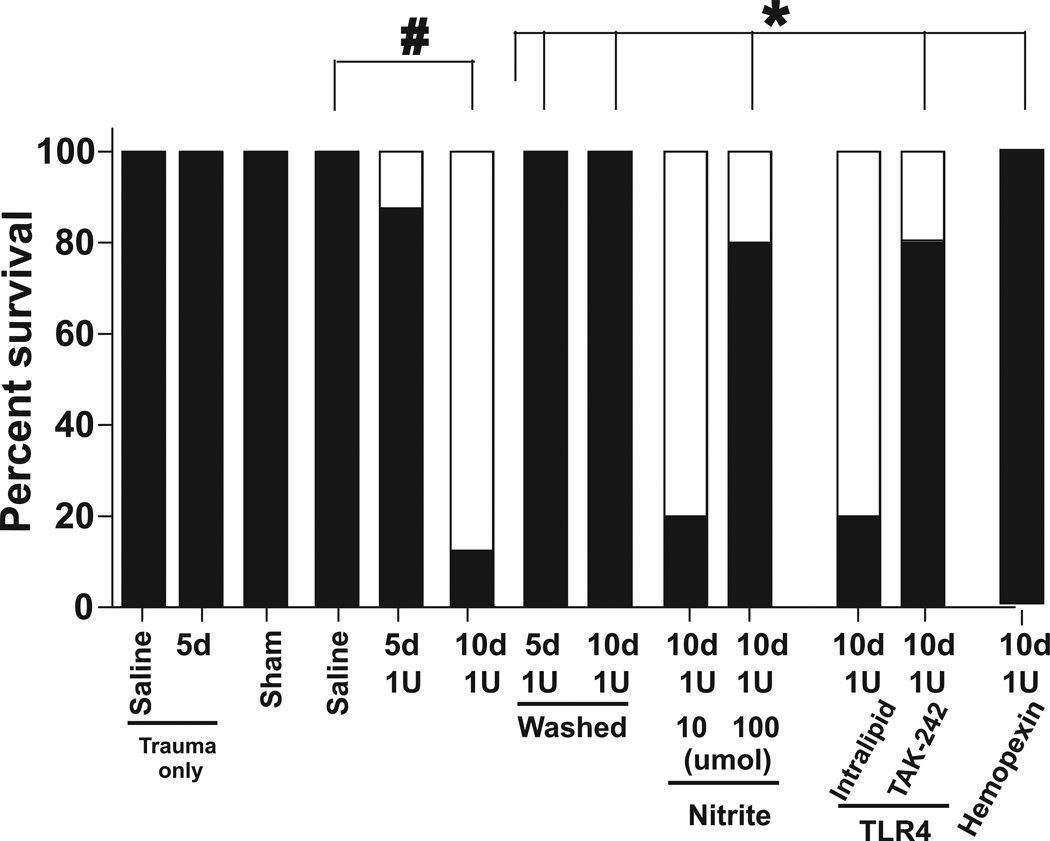
Figure 4 shows that there was no or minimal mortality in sham surgery group, in trauma only or trauma-hemorrhage mice transfused with saline or with 1 unit of mRBC stored for 5d. However, transfusion with 1 unit of 10d RBC caused ~90% mortality over the 4h observation period post-transfusion. Figure 5A–B show respectively, changes in BALF protein and inflammatory cell levels with 5d RBC transfusions. A trend (P=0.06) towards increase in BALF protein in saline alone transfused mice compared to sham was observed indicating injury mediated by trauma-hemorrhage. Transfusion with 1unit of d0 mRBC had no effect beyond saline alone, but d5 mRBC significantly increased BAL protein further consistent with a storage dependent gain of toxicity. To assess if the ALI end points display a RBC dose effect, the effects of transfusion with 3 units of d0 or d5 mRBC were determined. Interestingly, 3 units of d0 mRBC decreased BAL protein levels suggesting a protective effect. However, 3 units of d5 mRBC significantly increased BAL protein relative to 3 units d0 mRBC (P <0.04), but the magnitude of this increase was still lower compared to 1 unit of d5 mRBC.
Figure 4. Effects of RBC storage on survival after trauma-hemorrhage.
Shown is the percent of mice that survived or died during trauma-hemorrhage resuscitation. Lethality was only observed post resuscitation. Indicated are n-values per group. *P<0.05 or #P<0.01 by two-tailed N-1 two proportion test. (□ = dead; ▪ = live)
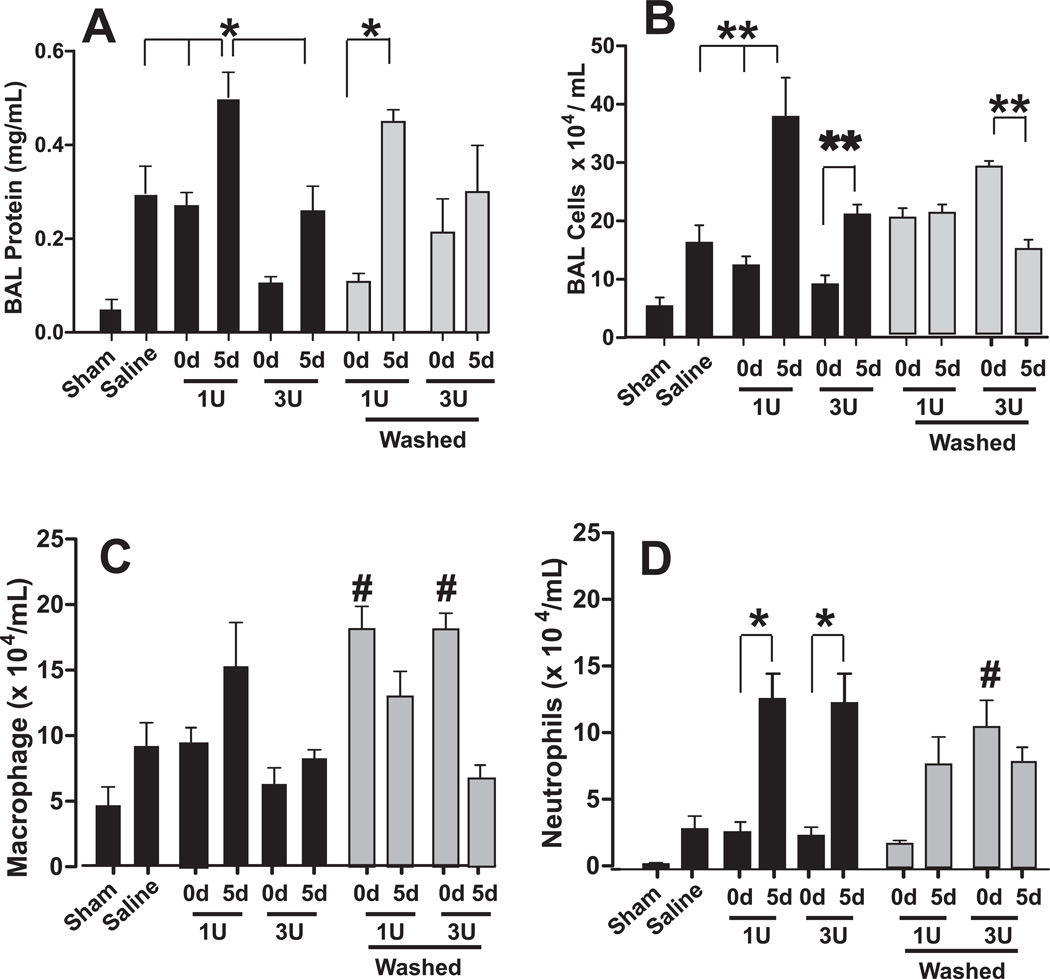
Figure 5. Effects of RBC storage and washing on acute lung injury after trauma-hemorrhage.
Mice were exposed to trauma-hemorrhage and resuscitated with either saline or RBCs that were stored for either 0d or 5d and BAL levels of protein (Panel A) or inflammatory cell (Panel B), with differential analysis (Panel C–D) determined. As indicated, tested variables included transfusing with 1unit (1U) or 3 units (3U), and washing RBC prior to transfusion (grey bars). Black bars indicate pRBC transfusion without washing. *P<0.05 or **P<0.01 as indicated by 1-way ANOVA with Tukey post test. #P<0.01 relative to respective unwashed d0 group by 1-way ANOVA with Tukey post test. n= 3–12 per group.
Similar to protein levels in the BAL, more inflammatory cells were observed in saline vs. sham groups (P=0.05) (Figure 5B). Also, mice transfused with 1 unit of d5 mRBC had greater numbers of inflammatory cells compared to saline or d0 mRBC, and transfusion of 3 units of d5 mRBC did not increase injury further compared to 1 unit transfusion, but did show higher levels of BAL inflammatory cells compared to 3 units of d0 mRBC (P<0.01). Differential analysis of BAL cells demonstrated that 1 or 3 units of day 5 mRBC induced accumulation of neutrophils, with no significant changes in macrophage content (Figure 5C–D). Supplementary Figure 2 shows that 1unit of d5 RBC had no effect on BAL protein or cells in mice that underwent trauma only (i.e. no hemorrhage) consistent with the concept that stored RBC provide a second hit that exacerbates underlying inflammatory stress.
Effects of RBC washing on trauma-hemorrhage induced toxicity
The concept that transfusion of microparticles or hemolysis derived products (cell-free Hb, iron) leads to inhibition of endogenous NO-signaling and oxidative stress raises the possibility that removal of these by washing RBCs immediately prior to transfusion may alleviate storage lesion toxicity[26, 34, 35]. On the other hand, washing may sensitize RBCs to subsequent hemolysis (depending on the RBC storage age[25, 26]) and we have shown that stored RBCs that remain after washing still scavenge and inhibit NO-signaling more so than younger RBC[20]. Thus, washing may not completely prevent loss of endogenous NO bioavailability and paradoxically may even promote NO-scavenging. To test this phenomenon, RBCs were washed by centrifugation immediately prior to transfusion, and effects on mortality using d10 mRBC and ALI using d5 mRBC assessed. Figure 1B–C and Figure 3A shows that washing was effective at removing cell-free Hb, heme, and microparticles. Washing modestly decreased hematocrit from 59.7% to 59.4% for 5d RBC and 59% to 58.3% for 10d RBC. Figure 4 shows that washing significantly improved survival after transfusion with 10d mRBC. In the sub-lethal model however, transfusion with washed d5 mRBCs (both 1 and 3 units) had no effect on BAL protein levels relative to respective d5 unwashed groups (Figure 5A). Interestingly, while a significant storage-dependent increase in injury was noted between washed d0 and d5 mRBC, this effect was not observed with 3 unit transfusions, in part due to a trend towards increased BAL protein in mice receiving washed d0 mRBCs. Figure 5B shows that for 1 unit transfusions, washing did not protect against BAL inflammatory cell accumulation induced by 5d mRBC. In fact, no storage age-dependent effect was observed at all in large part due to an increased BAL cell accumulation in mice receiving washed d0 mRBC, which was significantly higher than unwashed d0 RBC (P<0.03 by unpaired t-test). This increase elicited by washing of d0 mRBC was due to increase levels of macrophages (Fig 5C). Moreover, increased injury induced by washing of fresh (0d) mRBC was more pronounced with 3 unit transfusions (P<0.01 between 0d 3unit washed and unwashed), which again was mediated largely by increased macrophages and, to a lesser extent, neutrophils (Fig 5C–D). Also, for 3 unit transfusions, washing 5d mRBC did not provide any protection relative to the respective unwashed condition, although a trend was noted. With 3 unit transfusions, washing 5d mRBCs induced less injury compared to 3 units of washed 0d mRBCs; a result mediated by decreased macrophage numbers (Fig 5C).
Effect of nitrite on RBC storage and trauma-hemorrhage induced toxicity
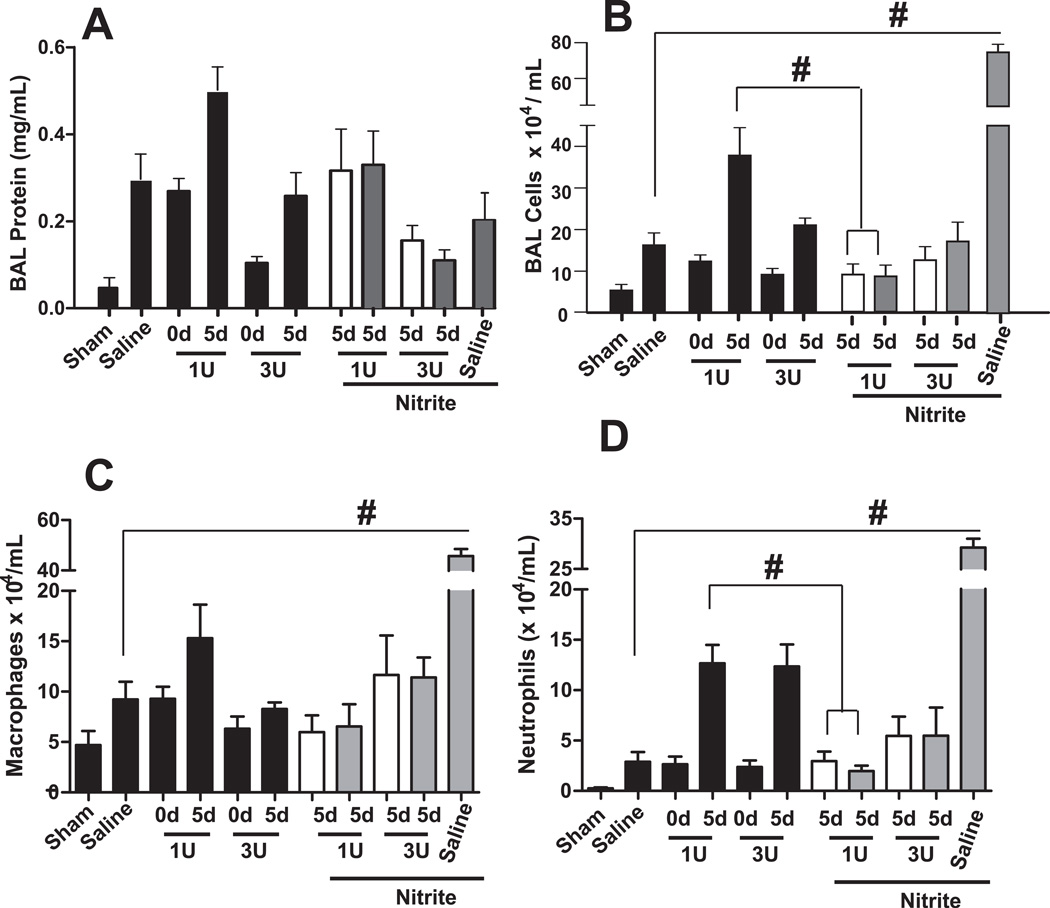
Nitrite at the highest dose tested significantly improved survival in mice transfused with 1unit of 10d mRBC (Figure 4). Figure 6A shows that compared to respective saline or d5 mRBC transfusion with 1 or 3units, nitrite therapy at either 10µmol or 100µmol had no effect on BAL protein levels, although a trends (P=0.07) towards decreased BAL protein with 3unit transfusions was noted. At 100µmol, nitrite increased BAL cell accumulation in trauma-hemmorhage and saline alone transfused mice. In contrast, a significant protective effect of nitrite was observed at both doses tested against 1 unit mRBC transfusions (Fig 6B) that was mediated by decreased neutrophils (Fig 6D), with trends towards decreased macrophages noted (Fig 6C).
Figure 6. Effects of nitrite therapy on stored RBC mediated acute lung injury after trauma-hemorrhage.
Mice were exposed to trauma-hemorrhage and resuscitated with either saline or pRBC that were stored for either 0d or 5d and BAL levels of protein (Panel A) or inflammatory cell (Panel B), with differential analysis (Panel C–D) determined. Nitrite therapy was injected once immediately prior to transfusion at either 10µmol (□) or 100µmol (▪) injected amount per mouse. Note black bars are the same as data presented in Figure 5 and plotted here to improve ease of comparison of nitrite effects on pRBC-dependent acute lung injury test. #P<0.01 by 1-way ANOVA with Tukey post test. n= 3–12 per group.
Role of free heme and TLR-4 signaling on RBC storage and trauma-hemorrhage induced toxicity
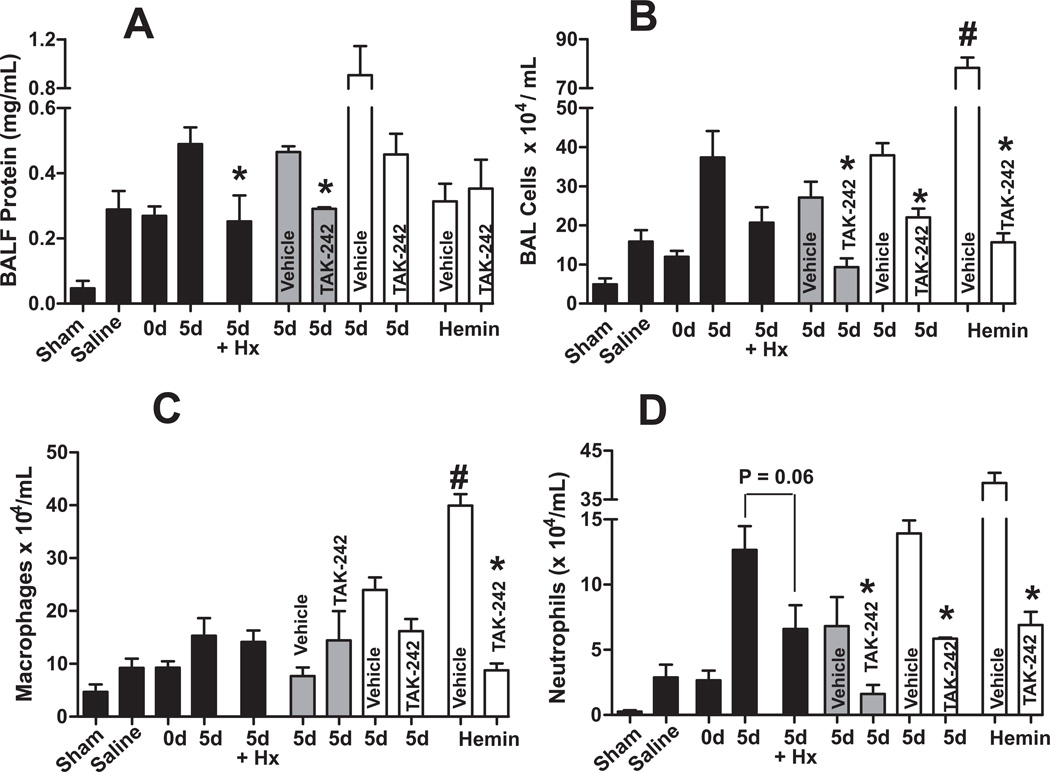
Both TAK-242 and hemopexin significantly improved survival in mice receiving 10d mRBC (Figure 4). Figure 7A–B shows that similarly, TAK-242 administered just prior to trauma or prior to resuscitation attenuated d5 mRBC induced protein and neutrophil accumulation in the BAL; although we note that for BAL protein, the effects of TAK-242 administered 5 min prior to resuscitation relative to vehicle control, was not significant, although a trend towards protection was observed (P=0.09). Hemopexin also attenuated protein accumulation with a trend (P=0.06) for decreased neutrophils also observed. Resuscitation with 100µl of hemin (15µM) alone had no effect on BAL protein but did significantly increase BAL cells (both macrophages and neutrophils), which was prevented TAK-242 (Figure 7).
Figure 7. Role of free-heme in stored RBC mediated acute lung injury after trauma-hemorrhage.
Mice were exposed to trauma-hemorrhage and resuscitated with either saline or pRBC that were stored for either 0d or 5d, or hemin (15µM stock, 100µl transfusion) and BAL levels of protein (Panel A) or inflammatory cell accumulation (Panel B), with differential analysis (Panel C–D) determined. Hemopexin (Hx), intralipid (vehicle) or TAK-242 (TLR-4 inhibitor) therapy was administered once prior to hemorrhage (grey bars) or once 5–10 min prior to resuscitation (white bars). Note black bars are the same as data presented in Figure 5 and plotted here to allow comparison of TLR-4 inhibition and hemin effects to saline and RBC-dependent acute lung injury. *P<0.05 relative to respective vehicle by t-test. #P<0.05 by t-test relative to saline (N= 3–12 per group).
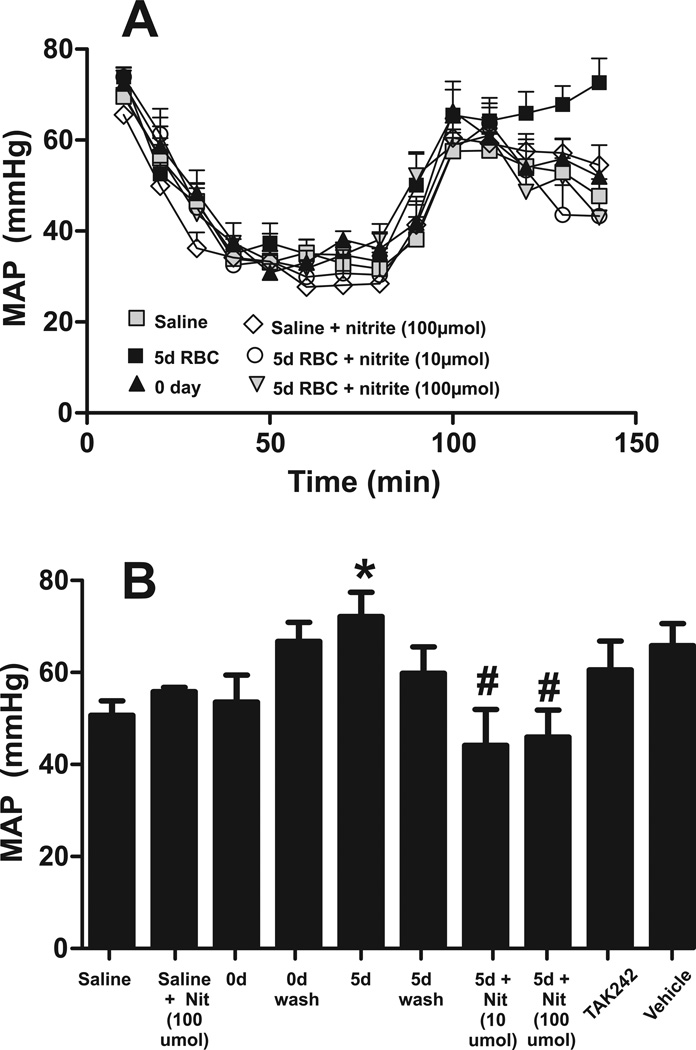
Effects of washing, nitrite and TLR-4 treatment on blood pressure
No differences in blood pressure between groups during hemorrhage and shock periods were observed (Figure 8A); for the sakes of clarity, only traces for saline, 0d, 5d RBC with and without nitrite are presented. Figure 8B shows that 30 min post transfusion MAP was significantly higher with 5d mRBC transfusions, but not when the same cells were washed prior to transfusion. Nitrite therapy prevented the 5d mRBC-dependent increase in MAP. No other treatment significantly affected MAP relative to RBC transfusion group. Also nitrite alone had no effect on saline transfused mice.
Figure 8. Effects of RBC storage on blood pressure post transfusion.
Shown are MAP measured 30min post transfusion of saline or 1unit of RBC stored for 0d or 5d. Data are mean ± SEM (n=3–12 per group). *P<0.05 relative to saline by t-test. #P<0.02 relative to 5d RBC.
Discussion
Our goals in this study were to determine mechanisms by which transfusion with stored RBCs mediate toxicity, and to test potential therapeutics. A mouse model of trauma hemorrhage coupled with transfusion of freshly isolated or leukoreduced stored RBC was used. Consistent with recent studies, storage of mouse RBCs for 10d was sufficient to promote significant biochemical and functional changes similar to that observed in human RBC stored for 42d. These changes included hemolysis, microparticle formation, and formation of RBC that displayed increased rates of NO-dioxygenation and nitrite oxidation[10, 27, 32]. In addition, we showed that significant increases in free heme also occur during storage of both mouse and human RBC, underscoring its potential role in storage-related toxicities. We developed a spectral deconvolution method for measuring cell-free heme simultaneously with cell-free Hb and showed that during storage, both significantly increase, but the relative increase was higher for free heme. In other words, the relative contribution of free heme to total extracellular heme increases with storage. This may reflect either direct heme release from RBCs and/or heme release from degradation of cell-free Hb. We speculate the latter, since our recent studies suggest that cell-free hemoglobin undergoes more oxidative damage relative to erythrocytic hemoglobin[36]. Moreover, we speculate that free heme levels may be underestimated as its hydrophobicity is likely to have led to membrane localization; we measured only heme that was dissolved in the extracellular fraction. A limitation in our measurements is that for human RBCs, we quantitated storage-dependent free heme and Hb formation from segments that are attached to RBC bags. Recent studies have shown that segments have higher levels of hemolysis relative to the paired bag, and our data demonstrated differences in complement levels also, but with levels being higher in the bag relative to the segment [37, 38]. Further studies evaluating cell-free heme formation in bags vs. segments is required.
To evaluate mechanisms and potential therapies for stored RBC toxicity, we employed a mouse model of trauma-hemorrhage to incorporate the two-hit concept of transfusion toxicity and to allow comparison with insights of the storage lesion gained from trauma patients. Transfusion with stored RBCs, but not fresh RBCs significantly induced acute lung injury or mortality with the severity of injury being proportional to the age of the transfused RBCs; these associations are similar to our studies with trauma patients in which poorer microcirculatory function and tissue oxygenation, higher incidence of end organ injury and mortality were noted in patients who received older vs. younger RBCs[2, 39, 40]. Our data also support the two hit model in which stored RBC constitute a second hit causing injury only when underlying stress (first hit) from trauma, hemorrhage or surgery is present. Injury was exacerbated with stored, but not freshly isolated RBC transfusions, and transfusion with stored RBC had no effect on mice that underwent trauma only. This observation is consistent with recent experimental studies that show increased lung injury or infection following transfusion with stored RBCs or stored RBC-derived microparticles[10, 11, 41, 42]. The general applicability of the two-hit hypothesis is also indicated by the fact that the first hit comprised diverse stimuli (LPS injection, pneumonia, high fat diet or trauma hemorrhage) and was observed in different animal models (mice, rats or dogs). We highlight an important limitation however, at least in the model employed in this study, where injury parameters were not proportional to the number of RBC units transfused. This is an important and often over-looked factor. Our prior work showed that in addition to storage age, the number of RBC units transfused also correlate with morbidity and mortality in trauma patients[43]. While a RBC dose effect was evident in relation to mouse survival, this correlation was not observed with acute lung injury end points using d5 RBCs. In fact, transfusion of 3 units resulted in less injury compared to 1 unit of stored RBCs. We did not pursue the mechanism of this effect and speculate that limitations of the model (transfusion of 3 units concurrently, rather than sequentially) and / or potential protective effects of RBC (via antioxidant activities or chemokine scavenging) may have played a role. Notwithstanding, the need for models that recapitulate clinical associations between RBC age and the number of units transfused with toxicity are required. With this in mind, we note a limitation in the in vivo system employed. We did not observe protection by transfusion with fresh (d0) RBC relative to saline alone. Since our end point of lung injury is sensitive to blood volume (and pressure), we opted to use a volume repletion protocol. We used saline alone or saline + RBC (d0, d5 or d10). Since the volume was constant, the control did in fact contain more saline than any of the RBC containing groups, perhaps precluding an appropriate control. Also we note that 1U of RBC is unlikely to be sufficient to see protection in this model of trauma-hemorrhage where 60% of blood is lost. Consistent with this, resuscitation with 3U of d0 RBC did show a trend (P=0.07) towards protection compared to saline alone wrt BAL protein (Fig 5A). This limitation notwithstanding, it is important to note that injury was increased by stored RBC (d5 or d10) relative to fresh (d0) RBC for either 1U or 3U comparisons, where saline content is the same. We also note that a recent study also using trauma-hemorrhage showed that resuscitation with a 1:1 mixture of fresh RBC : plasma decreased inflammatory cytokines and microvascular permeability compared to plasma alone[44]; our ongoing studies are testing whether RBC age affects injury in this model.
We tested three potential therapies to attenuate stored RBC toxicity. Washing of RBCs is based on the speculation that a single wash will remove smaller RBC degradation products (hemolysis, microparticles) or other potential pro-inflammatory effectors (e.g. cytokines, lipid peroxidation products) immediately prior to transfusion[26, 27, 34]. Recent data have shown that washing stored RBCs protects against hypertension, lung injury and infection, while washing of younger RBCs increased injury consistent with concerns over washing resulting in increased susceptibility of the RBC to subsequent hemolysis[26]. In addition to the rationale just discussed, we also tested washing to evaluate potential longer-term toxicity of intact stored RBCs that remain after washing. Our previous data noted that stored intact RBCs inhibited NO-signaling more so than fresh RBCs, and prior studies have shown that intact stored RBC are less able to bind chemokines and inhibit inflammation[10, 20]. Since these RBCs are likely to have longer circulatory half-life than cell-free Hb or microparticles, and be present at higher concentrations, we reasoned that they may sustain an inhibition of endogenous NO-signaling and mediate a persistent pro-inflammatory stimulus. Interestingly, the effects of washing were varied. Mortality induced by 10d mRBC was clearly prevented by washing RBCs consistent with a toxicity mediated by lower MWt components. However, under sub-lethal conditions, washing had no effect on stored RBC dependent increases in BAL protein or inflammatory cells, although trends were noted. This suggests that washing may be more effective, the more severe the injury caused by stored RBC transfusion. Another factor is the potential differential effect of washing on younger vs. older RBC. Our data suggest that transfusion of washed d0 mRBC increased BAL cells to levels that were equal or higher relative to washed 5d mRBC. In addition, a trend towards higher MAP in washed versus unwashed d0 RBC was noted (Fig 8B), overall suggesting a detrimental effect of washing on younger RBCs, a conclusion similar to a recent study using canine RBC[26]. Moreover, the method of washing may also differentially affect RBC sensitivity to hemolysis[34]. Collectively, these data underscore the need for detailed understanding of how washing affects RBCs and subsequent stability post-transfusion. In summary, our data suggest that while washing protects against injury, this is only evident when the injury is severe. Moreover, we hypothesize that while washing RBCs does remove mediators of transfusion toxicity, older and intact RBCs that remain may still provide a second hit to exacerbate the inflammatory component of acute lung injury.
In addition to increased scavenging of NO, stored RBCs also display increased rates of nitrite oxidation in vitro and loss of nitrite in vivo after RBC transfusion is associated with poorer microcirculatory function[20]. Since nitrite is a substrate for NO-formation with many studies showing therapeutic efficacy against circulatory and inflammatory diseases including trauma hemorrhage, sepsis, and acute lung injury[31, 45, 46], we tested if nitrite repletion may protect against storage dependent toxicity[20, 39]. Interestingly, inhaled NO also attenuated stored RBC induced oxidative damage and inflammation, an affect that may be due to increased circulating nitrite[42]. Nitrite therapy prevented stored RBC dependent neutrophilic accumulation in the BAL and a trend towards protection against protein accumulation also. These results resemble our previously published observations that nitrite therapy-dependent effects on BAL protein and cell accumulation differ[47]. Exactly how nitrite can mitigate stored RBC-dependent toxicity is unclear. One mechanism is oxidation of cell-free oxyhemoglobin that would prevent NO-scavenging. However, the resultant methemoglobin could mediate oxidative stress. Other potential mechanisms include improving tissue blood flow and preventing cell death. Further studies are required to better elucidate the nitrite-dependent protective mechanisms as well as other parameters that may affect effectiveness including timing of administration relative to the stage of shock. Of note, in trauma-hemorrhage mice transfused with saline alone, nitrite in fact increased BAL cells due to increases in both neutrophils and macrophages. Importantly however, when RBC were transfused, nitrite therapy prevented acute lung injury. These data are consistent with the hypothesis that the biological effects of nitrite are controlled by interactions with RBC[48], further reflected by data presented in Fig 8 showing that nitrite prevented the increase in MAP in d5 RBC groups, but had no effect on MAP in saline alone transfused mice.
To evaluate if free heme can mediate stored RBC toxicity, we tested the effects of preventing heme-toxicity by inhibition of TLR-4 or using hemopexin. The latter prevented stored RBC induced lethality and ALI underscoring the potential for toxic effects of heme. Moreover, transfusion of free heme alone at a dose found in d10 mRBC, recapitulated some aspects of stored RBC toxicity (increased BAL cells). Free heme-dependent activation of TLR4 and downstream signaling mediates multiple organ dysfunction in sickle cell disease and sepsis[13, 14]. TLR4 inhibition completely protected against all injury end points elicited by stored RBCs. The protective effects of hemopexin and TLR4 inhibition were surprising results as neither are expected to affect inhibition of NO-signaling mediated by cell-free hemoglobin, microparticles, or intact RBCs. Moreover, there was no detectable cell-free heme in mRBCs stored for 5d, but hemopexin and TLR4 antagonism still afforded protection. These data suggest that ongoing generation of cell-free heme and/or other TLR4 ligands after transfusion mediates toxicity, although we cannot exclude the possibility that TLR4 ligands produced by trauma-hemorrhage independent of RBC transfusion are the primary targets that result in protection. Interestingly, a recent study documented that in the absence of eNOS and NO-signaling, the severity of TLR4 dependent necrotizing enterocolitis was increased[49]. This suggests an intriguing scenario in stored RBC toxicity, whereby loss of NO-signaling may synergize with TLR4 activation to promote tissue injury. This is further suggested by the fact that hemin alone only increased BAL cells. The potential interaction between free heme and other stored RBC components, and subsequently between NO-inhibition and TLR4-dependent pathways to mediate stored RBC toxicity is currently under investigation.
In summary, we provide data highlighting the role of free heme to mediate stored RBC toxicity in trauma-hemorrhage and show that there is potential for RBC washing, nitrite, hemopexin and anti-TLR4 therapy to attenuate injury in this setting.
Supplementary Material
Highlights.
RBC storage results in increased free hemoglobin and free heme
Transfusion with stored but not fresh RBC, causes acute lung injury (ALI) in trauma-hemorrhage
Stored RBC toxicity is prevented by pre-transfusion washing, nitrite therapy, heme sequestration or blocking TLR4 signaling
Acknowledgements
This study was supported by grants from the National Institutes of Health (HL095468 to RPP. JW) and RS is supported by a NIH T32 HL007918 research training grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship contributions
All authors contributed to designing the studies. RS, CR, JYO, AB collected data. RS, CR, JYO, AB, RPP analyzed data. All authors contributed to writing and editing the manuscript.
Conflicts of Interest
RPP is a co-inventor on a patent for use of nitrite salts for the treatment of cardiovascular conditions
References
- 1.Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–1195. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberg JA, Barnum SR, Patel RP. Red blood cell age and potentiation of transfusion-related pathology in trauma patients. Transfusion. 2011;51:867–873. doi: 10.1111/j.1537-2995.2011.03098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinberg JA, McGwin G, Jr., Marques MB, Cherry SA, 3rd, Reiff DA, Kerby JD, Rue LW., 3rd Transfusions in the less severely injured: does age of transfused blood affect outcomes? The Journal of trauma. 2008;65:794–798. doi: 10.1097/TA.0b013e318184aa11. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg JA, McGwin G, Jr., Vandromme MJ, Marques MB, Melton SM, Reiff DA, Kerby JD, Rue LW., 3rd Duration of red cell storage influences mortality after trauma. The Journal of trauma. 2010;69:1427–1431. doi: 10.1097/TA.0b013e3181fa0019. discussion 1431–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner SJ, Glynn SA, Welniak LA, Products NWGoStORBC. Research opportunities in optimizing storage of red blood cell products. Transfusion. 2014;54:483–494. doi: 10.1111/trf.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baek JH, D'Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. The Journal of clinical investigation. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis RO, Jhang JS, Pham HP, Hod EA, Zimring JC, Spitalnik SL. Glucose-6-phosphate dehydrogenase deficiency in transfusion medicine: the unknown risks. Vox sanguinis. 2013 doi: 10.1111/vox.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gladwin MT, Kim-Shapiro DB. Storage lesion in banked blood due to hemolysis-dependent disruption of nitric oxide homeostasis. Current opinion in hematology. 2009;16:515–523. doi: 10.1097/MOH.0b013e32833157f4. [DOI] [PubMed] [Google Scholar]
- 9.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, Hendrickson JE, Zimring JC, Brittenham GM, Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mangalmurti NS, Xiong Z, Hulver M, Ranganathan M, Liu XH, Oriss T, Fitzpatrick M, Rubin M, Triulzi D, Choi A, Lee JS. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113:1158–1166. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belizaire RM, Prakash PS, Richter JR, Robinson BR, Edwards MJ, Caldwell CC, Lentsch AB, Pritts TA. Microparticles from stored red blood cells activate neutrophils and cause lung injury after hemorrhage and resuscitation. Journal of the American College of Surgeons. 2012;214:648–655. doi: 10.1016/j.jamcollsurg.2011.12.032. discussion 656–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laczko J, Feo CJ, Phillips W. Discocyte--echinocyte reversibility in blood stored in CPD over a period of 56 days. Transfusion. 1979;19:379–388. doi: 10.1046/j.1537-2995.1979.19479250174.x. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh S, Adisa OA, Chappa P, Tan F, Jackson KA, Archer DR, Ofori-Acquah SF. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. The Journal of clinical investigation. 2013;123:4809–4820. doi: 10.1172/JCI64578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassu AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, Marguti I, Cardoso S, Sepulveda N, Smith A, Soares MP. A central role for free heme in the pathogenesis of severe sepsis. Science translational medicine. 2010;2:51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 15.Janz DR, Bastarache JA, Sills G, Wickersham N, May AK, Bernard GR, Ware LB. Association between haptoglobin, hemopexin and mortality in adults with sepsis. Critical care. 2013;17:R272. doi: 10.1186/cc13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121:1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, Smith A, Nath KA, Hebbel RP, Vercellotti GM. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123:377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutra FF, Alves LS, Rodrigues D, Fernandez PL, de Oliveira RB, Golenbock DT, Zamboni DS, Bozza MT. Hemolysis-induced lethality involves inflammasome activation by heme. Proceedings of the National Academy of Sciences of the United States of America. 2014 doi: 10.1073/pnas.1405023111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stapley R, Owusu BY, Brandon A, Cusick M, Rodriguez C, Marques MB, Kerby JD, Barnum SR, Weinberg JA, Lancaster JR, Jr., Patel RP. Erythrocyte storage increases rates of NO and nitrite scavenging: implications for transfusion-related toxicity. The Biochemical journal. 2012;446:499–508. doi: 10.1042/BJ20120675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C, Liu X, Janes J, Stapley R, Patel RP, Gladwin MT, Kim-Shapiro DB. Mechanism of faster NO scavenging by older stored red blood cells. Redox biology. 2014;2:211–219. doi: 10.1016/j.redox.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander JT, El-Ali AM, Newman JL, Karatela S, Predmore BL, Lefer DJ, Sutliff RL, Roback JD. Red blood cells stored for increasing periods produce progressive impairments in nitric oxide-mediated vasodilation. Transfusion. 2013;53:2619–2628. doi: 10.1111/trf.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu H, Zennadi R, Xu BX, Eu JP, Torok JA, Telen MJ, McMahon TJ. Impaired adenosine-5'-triphosphate release from red blood cells promotes their adhesion to endothelial cells: a mechanism of hypoxemia after transfusion. Critical care medicine. 2011;39:2478–2486. doi: 10.1097/CCM.0b013e318225754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, Mulherin MA, Zhu H, Buck RD, Califf RM, McMahon TJ. Evolution of adverse changes in stored RBCs. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cortes-Puch I, Wang D, Sun J, Solomon SB, Remy KE, Fernandez M, Feng J, Kanias T, Bellavia L, Sinchar D, Perlegas A, Solomon MA, Kelley WE, Popovsky MA, Gladwin MT, Kim-Shapiro DB, Klein HG, Natanson C. Washing older blood units before transfusion reduces plasma iron and improves outcomes in experimental canine pneumonia. Blood. 2014;123:1403–1411. doi: 10.1182/blood-2013-11-539353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belizaire RM, Makley AT, Campion EM, Sonnier DI, Goodman MD, Dorlac WC, Friend LA, Lentsch AB, Pritts TA. Resuscitation with washed aged packed red blood cell units decreases the proinflammatory response in mice after hemorrhage. The journal of trauma and acute care surgery. 2012;73:S128–S133. doi: 10.1097/TA.0b013e3182606301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isbell TS, Gladwin MT, Patel RP. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. American journal of physiology. Heart and circulatory physiology. 2007;293:H2565–H2572. doi: 10.1152/ajpheart.00759.2007. [DOI] [PubMed] [Google Scholar]
- 29.Patel RP, Hogg N, Spencer NY, Kalyanaraman B, Matalon S, Darley-Usmar VM. Biochemical characterization of human S-nitrosohemoglobin. Effects on oxygen binding and transnitrosation. The Journal of biological chemistry. 1999;274:15487–15492. doi: 10.1074/jbc.274.22.15487. [DOI] [PubMed] [Google Scholar]
- 30.Owusu BY, Stapley R, Honavar J, Patel RP. Effects of erythrocyte aging on nitric oxide and nitrite metabolism. Antioxidants & redox signaling. 2013;19:1198–1208. doi: 10.1089/ars.2012.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez C, Vitturi DA, He J, Vandromme M, Brandon A, Hutchings A, Rue LW, 3rd, Kerby JD, Patel RP. Sodium nitrite therapy attenuates the hypertensive effects of HBOC-201 via nitrite reduction. The Biochemical journal. 2009;422:423–432. doi: 10.1042/BJ20090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimring JC, Spitalnik SL. On the appropriate use and interpretation of animal models in transfusion medicine research. Transfusion. 2013;53:2334–2339. doi: 10.1111/trf.12131. [DOI] [PubMed] [Google Scholar]
- 33.Makley AT, Goodman MD, Friend LA, Johannigman JA, Dorlac WC, Lentsch AB, Pritts TA. Murine blood banking: characterization and comparisons to human blood. Shock. 2010;34:40–45. doi: 10.1097/SHK.0b013e3181d494fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett-Guerrero E, Kirby BS, Zhu H, Herman AE, Bandarenko N, McMahon TJ. Randomized study of washing 40- to 42-day-stored red blood cells. Transfusion. 2014 doi: 10.1111/trf.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lannan KL, Sahler J, Spinelli SL, Phipps RP, Blumberg N. Transfusion immunomodulation--the case for leukoreduced and (perhaps) washed transfusions. Blood cells, molecules & diseases. 2013;50:61–68. doi: 10.1016/j.bcmd.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harper V, Oh JY, Stapley R, Marques MB, Wilson L, Barnes S, Sun CW, Townes TM, Patel RP. Peroxiredoxin-2 recycling is inhibited during erythrocyte storage. Antioxidants & redox signaling. 2014 doi: 10.1089/ars.2014.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurach JD, Hansen AL, Turner TR, Jenkins C, Acker JP. Segments from red blood cell units should not be used for quality testing. Transfusion. 2014;54:451–455. doi: 10.1111/trf.12303. [DOI] [PubMed] [Google Scholar]
- 38.Hu X, Barnum SR, Patel RP, Marques MB, Weinberg JA. The level of complement activation fragments is higher in red blood cell units than segments. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2013;49:692–693. doi: 10.1016/j.transci.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg JA, MacLennan PA, Vandromme-Cusick MJ, Angotti JM, Magnotti LJ, Kerby JD, Rue LW, 3rd, Barnum SR, Patel RP. Microvascular response to red blood cell transfusion in trauma patients. Shock. 2012;37:276–281. doi: 10.1097/SHK.0b013e318241b739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinberg JA, MacLennan PA, Vandromme-Cusick MJ, Magnotti LJ, Kerby JD, Rue LW, 3rd, Angotti JM, Garrett CA, Hendrick LE, Croce MA, Fabian TC, Barnum SR, Patel RP. The deleterious effect of red blood cell storage on microvascular response to transfusion. The journal of trauma and acute care surgery. 2013;75:807–812. doi: 10.1097/TA.0b013e3182a74a9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholson SE, Johnson RA, Craig T, Myers JG, Durante W, Stewart RM, Johnson FK. Transfusion-related acute lung injury in a rat model of trauma-hemorrhage. The Journal of trauma. 2011;70:466–471. doi: 10.1097/TA.0b013e3182032584. [DOI] [PubMed] [Google Scholar]
- 42.Lei C, Yu B, Shahid M, Beloiartsev A, Bloch KD, Zapol WM. Inhaled nitric oxide attenuates the adverse effects of transfusing stored syngeneic erythrocytes in mice with endothelial dysfunction after hemorrhagic shock. Anesthesiology. 2012;117:1190–1202. doi: 10.1097/ALN.0b013e318272d866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinberg JA, McGwin G, Jr., Griffin RL, Huynh VQ, Cherry SA, 3rd, Marques MB, Reiff DA, Kerby JD, Rue LW., 3rd Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. The Journal of trauma. 2008;65:279–282. doi: 10.1097/TA.0b013e31817c9687. discussion 282–274. [DOI] [PubMed] [Google Scholar]
- 44.Makley AT, Goodman MD, Belizaire RM, Friend LA, Johannigman JA, Dorlac WC, Lentsch AB, Pritts TA. Damage control resuscitation decreases systemic inflammation after hemorrhage. The Journal of surgical research. 2012;175:e75–e82. doi: 10.1016/j.jss.2011.11.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cauwels A, Buys ES, Thoonen R, Geary L, Delanghe J, Shiva S, Brouckaert P. Nitrite protects against morbidity and mortality associated with TNF- or LPS-induced shock in a soluble guanylate cyclase-dependent manner. The Journal of experimental medicine. 2009;206:2915–2924. doi: 10.1084/jem.20091236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickerodt PA, Emery MJ, Zarndt R, Martin W, Francis RC, Boemke W, Swenson ER. Sodium nitrite mitigates ventilator-induced lung injury in rats. Anesthesiology. 2012;117:592–601. doi: 10.1097/ALN.0b013e3182655f80. [DOI] [PubMed] [Google Scholar]
- 47.Samal AA, Honavar J, Brandon A, Bradley KM, Doran S, Liu Y, Dunaway C, Steele C, Postlethwait EM, Squadrito GL, Fanucchi MV, Matalon S, Patel RP. Administration of nitrite after chlorine gas exposure prevents lung injury: effect of administration modality. Free radical biology & medicine. 2012;53:1431–1439. doi: 10.1016/j.freeradbiomed.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr., Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yazji I, Sodhi CP, Lee EK, Good M, Egan CE, Afrazi A, Neal MD, Jia H, Lin J, Ma C, Branca MF, Prindle T, Richardson WM, Ozolek J, Billiar TR, Binion DG, Gladwin MT, Hackam DJ. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9451–9456. doi: 10.1073/pnas.1219997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.