SUMMARY
Foodborne illness is a major cause of morbidity and loss of productivity in developed nations. Although low socioeconomic status (SES) is generally associated with negative health outcomes, its impact on foodborne illness is poorly understood. We conducted a systematic review to examine the association between SES and laboratory-confirmed illness caused by eight important foodborne pathogens. We completed this systematic review using PubMed for all papers published between 1 January 1980 and 1 January 2013 that measured the association between foodborne illness and SES in highly developed countries and identified 16 studies covering four pathogens. The effect of SES varied across pathogens: the majority of identified studies for Campylobacter, salmonellosis, and E. coli infection showed an association between high SES and illness. The single study of listeriosis showed illness was associated with low SES. A reporting bias by SES could not be excluded. SES should be considered when targeting consumer-level public health interventions for foodborne pathogens.
Key words: Foodborne infections
INTRODUCTION
Socioeconomic status (SES) is an important predictor of disease. SES can be defined in different ways, but it is frequently measured based on individual- and community-level education, income, wealth, employment, and family background compared with other individuals or groups [1, 2]. Low SES is generally associated with greater morbidity and mortality [3]. For example, low SES is associated with greater susceptibility to, and worse outcomes when diagnosed with, chronic conditions such as diabetes, asthma, coronary artery disease, and certain malignancies and infectious diseases such as HIV, tuberculosis, and influenza [4–11]. However, the relationship between SES and foodborne illness is less well understood [12].
Foodborne illness is a major source of morbidity in developed countries. In the United States alone, there are an estimated 47·8 million cases annually [13, 14]. This estimate includes illness due to 31 major foodborne pathogens as well as cases due to unknown or unidentified causes. In sum, there are over 250 infectious and non-infectious agents that may contaminate food and many recognized food vehicles. Adding to the complexity of foodborne illness, foods can become contaminated at multiple points along the food's journey from production to consumption. It is also possible that different SES groups of individuals have different exposures because of dietary differences, or because of differences in individual food safety behaviours [15]. For example, behavioural studies have noted that high SES groups are more likely to eat undercooked foods, such as raw oysters and rare beef [16], and low SES groups are less likely to have sufficiently cool refrigerators [17]. However, these studies have failed to identify whether these differences are associated with differential rates of foodborne illness. A better understanding of the relationship between SES and foodborne illness is important for efficient public health policy and intervention targeting.
The purpose of this systematic review was to analyse the existing peer-reviewed research at the individual- and population-level associations between SES and laboratory-diagnosed cases of infection with pathogens commonly transmitted through food, including Campylobacter spp., Clostridium perfringens, shiga toxin- (or vero cytotoxin-) producing Escherichia coli (STEC/VTEC), Listeria, norovirus, non-typhoidal Salmonella spp., Shigella spp., Vibrio spp., and Yersinia enterocolitica.
METHODS
The review protocol has been registered in PROSPERO International Prospective Register of Systematic Reviews (crd.york.ac.uk/prospero/index.asp Identifier: CRD42013004359).
We searched PubMed/Medline for studies published between 1 January 1980 and 1 January 2013 using all combinations of the search and MeSH terms in the major categories (illness, SES metric, geography) outlined in Table 1. The objective of the search was to identify all papers examining the association between SES and infection with one of the nine pathogens of interest meeting our inclusion and exclusion criteria. The pathogens were selected because they were primarily foodborne (estimated >50% of cases transmitted through food) and cause significant morbidity (>50 000 estimated annual illnesses or >1000 estimated annual hospitalizations) [14]. Our inclusion criteria were that studies were published in English, were conducted in one of the 47 countries classified by the United Nations in 2011 to have ‘Very High Human Development’ [18], reported original data, included laboratory-confirmed cases of foodborne illness, and reported a quantitative measure of SES. We chose to limit the systematic review to highly developed countries because we assumed they would have comparable legal and commercial standards for food safety, better systems for disease surveillance and detection, and more comparable gradations of SES. Eligible measures of SES included both individual- and community-level measures of income and assets, poverty, educational status, utilization of social services, occupational status, and composite indexes of deprivation and disadvantage. We excluded studies reporting data from outbreaks and not written in English. This online search was followed by a review of eligible papers' bibliographies for eligible articles not included in the search terms.
Table 1.
Schematic of MeSH terms used in PubMed search string to identify studies of foodborne illness and socioeconomic (SES) status
| Illness terms | AND | SES metric terms | AND | Geography terms |
|---|---|---|---|---|
| Campylobacter | Class, social | Argentina | ||
| Clostridium perfringens | Educational status | Australia | ||
| Disease, foodborne | Income | Bahrain | ||
| Escherichia coli | Poverty | Barbados | ||
| Foodborne diseases | Residence characteristics | Canada | ||
| Gastroenteritis/microbiology | Socioeconomic factors | Chile | ||
| Norovirus | Europe | |||
| Listeria | Hong Kong | |||
| Shigella | Israel | |||
| Salmonella | Japan | |||
| Vibrio | New Zealand | |||
| Yersinia | Qatar | |||
| Singapore | ||||
| South Korea | ||||
| United Arab Emirates | ||||
| United States |
All titles were reviewed to include articles related to public health, foodborne illness, and SES. For those with appropriate titles, abstracts were reviewed. If the abstract appeared to meet inclusion/exclusion criteria, the full-text article was read until one or more exclusion criteria were found or until the end of the article, whichever occurred first. Papers that passed the selection process were then abstracted into a custom-made table that included categories for citation details, the study's location, design/approach, study period, data sources, SES variables, principal summary measures, results and P values, and other key findings. The paper selection and data extraction were conducted initially by K.L.N. then both were repeated by P.A.R. using the same methods. Any disagreements were resolved through review of the article in question and discussion among all authors.
We evaluated the risk of bias within studies using a version of the Cochrane Collaboration's tool for assessing risk of bias [19] modified to assess diverse study designs by grouping bias into the same general categories and using the same assessment criteria but adding subcategories specific to each study design. The studies were evaluated, as applicable, according to the potential for bias in the following domains: selection bias, measurement bias, analytical bias, and reporting bias. Case-control studies were evaluated based on selection of cases and controls; exposure, outcome, and confounder assessment; analysis; and reporting. Cohort studies were evaluated based on population sampling; exposure, outcome, and confounder assessment; follow-up; analysis; and reporting. Ecological studies were evaluated based on source population(s) selection; exposure, outcome, and confounder assessment; analysis; and reporting. Differences in study designs and in data sources precluded pooling the data for each pathogen and conducting a meta-analysis.
RESULTS
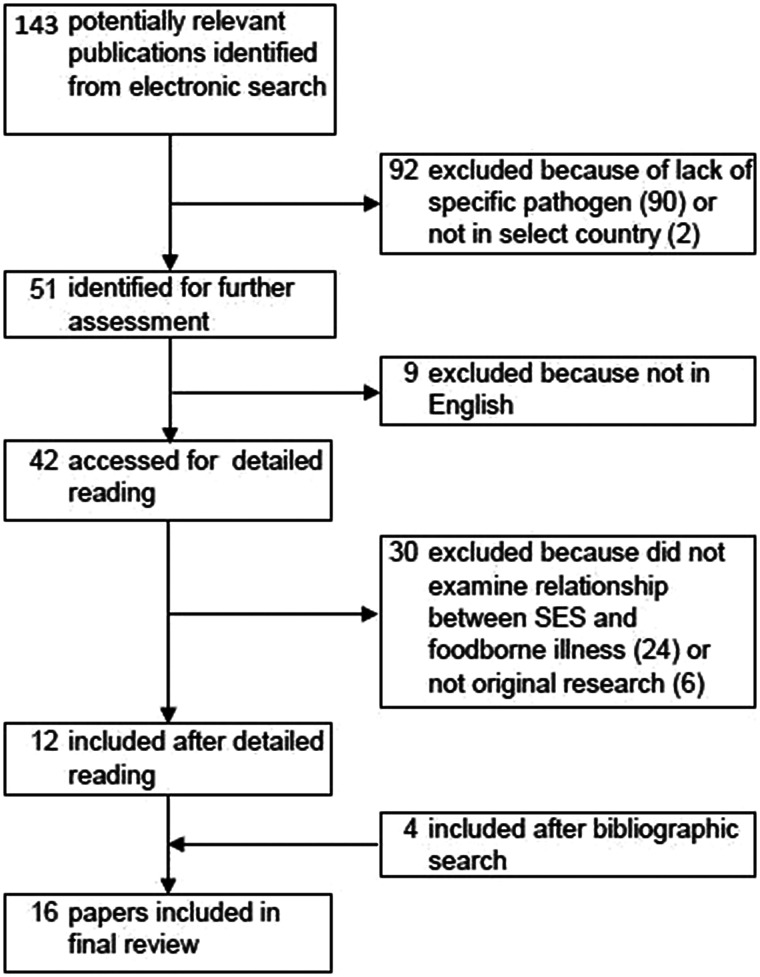
The initial search yielded a total of 143 articles (Fig. 1). After abstract and full-text review, the number was reduced to 12. From the bibliographies of these articles, four additional papers were identified, resulting in a total of 16 articles included in the systematic review. The most common reason for exclusion was due to the lack of a specific pathogen focus. Two were excluded because they were not in a target country [20, 21].
Fig. 1.
Flow chart of study selection for systematic review. Boxes indicate number of papers under consideration after each step in paper identification process. Steps in paper selection leading to inclusion or exclusion are identified by arrows.
The most commonly studied pathogen was Campylobacter (n = 7), followed by non-typhoidal Salmonella (n = 6), STEC or VTEC (n = 5), and Listeria (n = 1) (Table 2, Supplementary Table S1). No articles were identified for C. perfringens, norovirus, Shigella spp., Vibrio spp., or Y. enterocolitica. Most studies were conducted in the United States (n = 4) and Great Britain (n = 3) (Supplementary Table S1). The most common study design used was ecological analysis (n = 11), followed by case-control (n = 3).
Table 2.
Impact of socioeconomic status (SES) on incidence of pathogen-specific foodborne illness
| Pathogen | Ref. | First author | Study design | Study period | Key findings | Summary |
|---|---|---|---|---|---|---|
| Campylobacter | [24] | Nichols | Ecological | 1989–2011* | Lower deprivation associated with higher incidence | High SES associated with greater incidence of campylobacteriosis in all seven studies |
| [26] | Simonsen | Cohort | 1993–2004 | Greater income and education associated with higher incidence among adults | ||
| [25] | Green | Ecological | 1996–2004 | Higher SES associated with higher incidence | ||
| [23] | Rind | Ecological | 1997–2005 | Lower deprivation associated with higher incidence | ||
| [28] | Gillespie | Cohort | 2000–2003 | People in semi-routine occupations had the highest incidence. Incidence marginally higher in white-collar workers than blue-collar workers | ||
| [22] | Spencer | Ecological | 2001–2007 | In urban areas, lower deprivation associated with higher incidence | ||
| [27] | Pyra | Ecological | 2004–2007 | Higher income, education rate, and home ownership rate associated with higher incidence | ||
| E. coli | [26] | Simonsen | Cohort | 1993–2004 | No association with income or education | Some markers of high SES associated with increased incidence of E. coli O157 infection in three of five studies |
| [29] | Chang | Ecological | 1995–2002 | Higher poverty and higher education associated with increased incidence. No association with social service utilization | ||
| [32] | Jalava | Ecological | 1997–2006 | Higher education associated with higher incidence. No association by social service utilization | ||
| [30] | Sakuma | Ecological | 1999–2004 | Higher income associated with higher incidence | ||
| [31] | Pearl | Ecological | 2000–2002 | No association with income | ||
| Listeria | [33] | Gillespie | Ecological | 2001–2007 | Higher deprivation associated with higher incidence | Low SES associated with increased listeriosis incidence |
| Salmonella | [36] | Borgnolo | Case-control | 1989–1994 | Unemployed or blue-collar father associated with increased incidence in children | High SES associated with increased incidence of salmonellosis in four of six studies |
| [35] | Banatvala | Case-control | 1993 | Higher SES associated with higher incidence, but not for all species | ||
| [29] | Chang | Ecological | 1993–2002 | Low unemployment and high education associated with increased incidence | ||
| [26] | Simonsen | Cohort | 1993–2004 | Greater income and education associated with increased incidence among adults, but not for all species | ||
| [34] | Yonus | Case-control | 1997–2006 | Greater income and education associated with increased incidence | ||
| [37] | Yonus | Ecological | 2006–2007 | No association with individual income, local mean income, or parental education |
2007–2009 for SES data.
Impact of SES by pathogen
Campylobacter
High SES was associated with a higher incidence of disease in six of the seven studies that examined the association between SES and laboratory-confirmed Campylobacter infections (Table 2, Supplementary Table S2). Of these, four examined the impact of SES using a composite deprivation index that incorporated area-level measures of variables such as car ownership, home ownership, and unemployment taken from national censuses [22–25]. Of the remaining two studies, Simonsen et al. in Denmark found an increased incidence of campylobacteriosis in persons with higher income and higher educational level [26]. The other remaining study, Pyra et al., found that United States census tract-level median income greater than $20 101 annually, high educational attainment, and home ownership greater than 50% were associated with increased incidence of Campylobacter infection [27]. The only equivocal study result was from Gillespie et al., who measured SES using occupation [28]. Gillespie found that although incidence was marginally higher in white-collar workers compared to blue-collar workers, semi-routine occupations (e.g. retail clerk, taxi driver, cosmetologist) had the highest incidence.
STEC/VTEC
Studies examining STEC (or VTEC), including STEC O157, reported inconsistent findings with regard to the impact of SES on disease incidence (Table 2, Supplementary Table S3). In the United States Chang et al. identified a positive association between STEC O157 disease incidence and higher levels of education and lower levels of poverty at the census tract level. In Japan Sakuma et al. identified an inverse relationship between average income and VTEC incidence [29, 30]. Other studies failed to find any association or found conflicting results. Pearl et al. [31] found no association between average income and rates of foodborne STEC O157 in Canada, similar to Simonsen et al. [26], who found no association between income and education and STEC in Denmark. In Finland, Jalava et al. found at the municipal level, an association between higher incidence of STEC O157 and higher levels of adult education [32].
Listeria
Using surveillance data from England and Wales, Gillespie et al. identified a strong association between low SES and high incidence of listeriosis using an index of multiple deprivation that included income, crime and disorder, living environment, health deprivation and disability, and unemployment [33].
Salmonella
In general, studies found an increasing incidence of salmonellosis with increasing SES, although there are some exceptions (Table 2, Supplementary Table S4). Studies conducted in the United States by Chang et al. and Yonus et al. found associations between high SES and salmonellosis incidence [29, 34]. Chang found low unemployment and high educational attainment were positively associated with incidence. Yonus also found a positive association with high educational attainment, as well as a positive association with high income. European studies by Bantvala et al. (UK) [35] and Simonsen et al. (Denmark) [26] also found positive associations between SES and salmonellosis incidence, but not for all species. Bantvala et al. found an association between high SES, as measured by the Townsend index of deprivation, and incidence of S. enteriditis but no association between SES and S. Typhimurium. Simonsen et al. also found increased incidence of S. enteriditis with high income, but they found an increased incidence of S. Typhimurium with low income and low educational attainment. Furthermore, Simonsen et al. found no association between SES and other Salmonella species.
One study of salmonellosis incidence and SES found an association with low SES, and one study reported null results. A 1996 case-control study conducted in Italy by Borgnolo et al. reported that children with non-typhoidal salmonellosis were more likely to have unemployed fathers and fathers who worked in non-blue-collar jobs than controls [36]. A 2010 case-control study conducted in the United States by Yonus et al. found no association between SES and salmonellosis [37].
SES indicators
The SES variables used in the studies ranged from simple metrics, such as home ownership or education to indexes of deprivation, such as the Oxford Index of Multiple Deprivation and the New Zealand Deprivation Index (Table 3). The most commonly used measure of SES was income, which was included in the analyses of seven studies. Higher income was associated with increased incidence of Campylobacter (2/2 studies) and Salmonella (2/4 studies). However, two studies of Salmonella incidence [29, 37] found no association by income. Studies of STEC/VTEC that measured income found mixed results – one study of VTEC incidence found increased incidence with lower income [30], one found increased STEC incidence with higher income [29], and two found no association between STEC incidence and income [26, 31].
Table 3.
Type of association by socioeconomic status (SES) variable and pathogen type
| SES measure | Pathogen | Ref. | Study areas | Change in incidence with increase in SES* (number of studies) |
|---|---|---|---|---|
| Income | Campylobacter | [26, 27] | Denmark, United States | Increase (2) |
| E. coli | [26, 29, 30, 31] | Denmark, Canada, Japan, United States | Increase (1); decrease (1); no association (2) | |
| Salmonella | [26, 29, 34, 37] | Denmark, United States | Decrease (2); no association (2) | |
| Education | Campylobacter | [26, 27] | Denmark, United States | Increase (2); decrease (1)‡ |
| E. coli | [26, 32], [29] | Denmark, Finland, United States | Increase (2); no association (1) | |
| Salmonella | [26, 29, 34, 37] | Denmark, United States | Increase (2); decrease (1); no association (1) | |
| Index of deprivation* | Campylobacter | [22–25] | Canada, England and Wales, New Zealand | Increase (4) |
| Listeria | [33] | England and Wales | Decrease (1) | |
| Salmonella | [35] | England | Increase (1) | |
| Employment/occupation† | Campylobacter | [28] | England and Wales, Ireland | Increase (1) |
| E. coli | [29] | United States | No association (1) | |
| Salmonella | [29, 36] | Italy, United States | Increase (1); decrease (1) | |
| Home ownership | Campylobacter | [27] | United States | Increase (1) |
| Social services | E. coli | [29, 32] | Finland, United States | No association (2) |
| Salmonella | [29] | United States | No association (1) |
High index of deprivation indicates low SES.
High employment or occupation indicates ‘white-collar’ worker categories.
Simonsen et al. [26] showed an increase in incidence for adults with high SES and decrease in incidence for children with high SES.
Six studies analysed the relationship between incidence of pathogens and educational attainment. The results were mixed for all three pathogens studied. An increase of Campylobacter incidence was observed in adults with higher education [26, 27] but for children with higher levels of education, the incidence was lower, even after controlling for age [26]. STEC/VTEC incidence was higher in those with greater education in two Scandinavian studies [26, 32], but there was no association found in a study conducted in the United States in 2009 by Chang et al. [29]. There were also mixed results in the analyses of the impact of education on salmonellosis. A Danish study by Simonsen et al. found higher incidence in those with low education [21], two US studies found a lower incidence in those with low education [29, 34], and one US study found no association between incidence and education [37].
The four studies of campylobacteriosis and one study of salmonellosis that used an index of deprivation to measure SES uniformly found an association between low levels of deprivation and high incidence [22–25, 35]. The one study of listeriosis that used an index of deprivation found an association between high levels of deprivation and high incidence [33].
Three studies measured the association between employment or occupation and disease incidence. A 2008 study by Gillespie et al. in England and Wales found a slightly higher incidence of campylobacteriosis for white-collar workers compared to blue-collar workers (relative risk 1·06, P = 0·01) [28]. A 2009 study by Chang et al. in the United States found no association between E. coli incidence and employment status [29]. The two studies that examined employment status and salmonellosis found divergent results [29, 36]. The US-based study reported a weak negative association between neighborhood unemployment and salmonellosis (R2 = 0·0194) [29], and the Italy-based study reported a strong odds ratio (OR) for the association between paternal unemployment or blue-collar job and non-typhoidal childhood Salmonella (OR 2·56 for cases compared to inpatient controls) [36].
Other SES variables that were used in the reviewed studies included home ownership and a collection of variables measuring the utilization of social services such as public daycare, school lunch programmes, and Medicare. Home ownership was associated with increased incidence of campylobacteriosis in a 2012 US study by Pyra et al. [27]. Metrics of social service use were not associated with E. coli or Salmonella incidence in either study that considered them [29, 32].
Evaluation of bias
Using a modified version of the Cochrane Collaboration's tool for assessing risk of bias [19], we assessed half of the studies (n = 8) as having at least one domain in which they had a high likelihood of bias, and only one study [26] was rated as having no areas with more than a low likelihood of bias (data not shown). The domain most commonly evaluated as having a high likelihood of bias was in the evaluation of exposure (i.e. SES status). Eight of the ecological analysis studies were considered as having a high likelihood of bias in their evaluation of exposure [23, 24, 27–31, 34]. An additional three ecological studies and the three case-control studies were evaluated as having a moderate likelihood of bias in their evaluation of exposure [22, 25, 33, 35–37]. Ten of the ecological studies were evaluated as having a moderate likelihood of bias in their assessment of potential confounders [23–25, 27–33, 38].
DISCUSSION
Overall key findings
The goal of our systematic review was to compare existing quantitative research on the association between SES and foodborne illness at the individual and at the population level. We had three key findings. First, there was no uniform effect of SES on foodborne illness across all laboratory-confirmed pathogens. Second, within pathogen categories, there was some uniformity – high SES was associated with increased incidence of campylobacteriosis and salmonellosis in the majority of studies, but the effect of SES on STEC/VTEC incidence varied by study. Third, different SES metrics did not provide consistent results.
Contrary to the association commonly seen between low SES and worse health outcomes [4–11], we found that in most of the reviewed studies of Campylobacter and Salmonella, low SES was associated with a lower burden of illness (Table 2) in countries classified by the United Nations in 2011 as having ‘Very High Human Development’ [18]. Studies of STEC and VTEC incidence had mixed results, with some studies indicating that low SES was associated with lower incidence and others that it was associated with higher incidence (Table 2). The one study of Listeria incidence found higher rates of listeriosis among low SES groups (Table 2). In general, however, most studies identified in our initial database search did not consider the association between SES and disease incidence (Fig. 1). Furthermore, of the nine common foodborne pathogens included in our search string, only four of these pathogens had been studied with regard to SES.
The differences in results by pathogen are consistent with a diverse biology and risk factor profile for each bacterium. In considering questions of differential exposure, known individual-level risk factors for each pathogen suggest causes for observed differences in individual- and population-level incidence, although there may be additional population-level risk factors that account for the observed differences. High SES was associated with increased incidence of campylobacteriosis and salmonellosis (Table 2). Risk factors for Campylobacter include eating restaurant-prepared food, having contact with farm animals, drinking untreated surface water, eating undercooked food, and drinking raw milk [39, 40]. Many of these risk factors are associated with higher SES groups, including eating restaurant-prepared food, drinking raw milk, and consuming undercooked foods [16, 41–47]. Risk factors for Salmonella include eating undercooked raw eggs, international travel, and eating in restaurants [48]. As with risk factors for campylobacteriosis, these activities are more common in high SES groups. We found no consistent association between SES and STEC/VTEC incidence. Risk factors for STEC/VTEC include eating undercooked hamburgers, eating at non-fast-food chain restaurants, drinking untreated surface water, contact with small children, and contact with farm animals [49]. While undercooked beef and restaurant-prepared food consumption are more common in high SES groups, other STEC/VTEC risk factors are less clearly associated with any specific SES group, perhaps contributing to the mixed results reported in this review.
Low SES was associated with increased incidence of listeriosis [33]. Risk factors for Listeria include eating cold processed meats, unpasteurized milk products, and being immunosuppressed [50]. Individuals of low SES are more likely to consume delicatessen meat and other cold processed meats, although they are less likely to consume raw milk [42, 51]. The finding of increased listeriosis in low SES groups may be driven by meat or unpasteurized cheese consumption patterns or may be through an increased immunological vulnerability, as seen in viral respiratory infection incidence [52].
It is also important to recognize how population-level SES may be associated with different community risks for foodborne illness. Risk factors like surface water contamination, farm animals, and low availability of fresh, non-prepared food, such as in food desserts [53], can impact large groups of people through environmental risk rather than individual actions. The findings from ecological analyses, such as those included in this review, suggest that there may be risk factors working at an even larger scale [54], such as national food safety policies, the availability of publicly funded healthcare, and regional public health activities [12]. For instance, pregnant and elderly individuals in low SES areas have less access to healthcare and therefore may receive less guidance about avoiding foods that can put them at risk for contracting listeriosis, thus possibly contributing to the increased risk of listeriosis in low SES groups [33]. Comparing associations between SES and foodborne illness rates between countries may also suggest population level risk factors. Studies conducted in Denmark and Canada found no association between income and E. coli infection [26, 31], but researchers in Japan and Finland found that higher income was associated with greater risk of E. coli [30, 32]. These differences may be the result of national differences in food safety regulations or enforcement rather than individual choice alone. However, further research is needed to be able to identify which policies may be at work. Overall, there is a need for investigation of the impact of population level, systematic determinants of foodborne illness.
Another possible reason for mixed results across pathogens is reporting bias. All included studies used laboratory-confirmed cases of illness, and all but one study relied upon national surveillance systems for case ascertainment [36]. Because not all illnesses are diagnosed, surveillance systems for foodborne illness only capture a small fraction of the total number of cases of illness that occur [55]. Illnesses typically causing more mild symptoms may be under-reported, and different SES groups have different rates and patterns of healthcare utilization [56]. A UK-based study found that individuals from a lower social class as well as individuals with a higher level of educational attainment were both more likely to present to a general practitioner for diarrhoea [57]. In the United States, education is not associated with seeking medical care and submitting a stool sample, but health insurance is. Individuals with health insurance are three times as likely to submit a stool sample as individuals without health insurance [58]. For Campylobacter, the estimated number of true cases is 30 times higher than the number reported to surveillance. For STEC, Listeria, and non-typhoidal Salmonella, the estimated number of actual cases are 26, 2, and 29 times higher, respectively, than the number reported to surveillance [14].
To capture a broader population, some studies have investigated acute gastroenteritis diagnoses regardless of laboratory confirmation. However, studies of acute gastroenteritis have yielded inconclusive associations. A 1990 study by Alexander et al. found that children of lower SES groups, as measured by the Hollingshead index, were more likely to report having been sick with diarrhoea over the previous 2 weeks [59]. However, a study from the same year of infants found no effect of SES as measured by the same index [60]. More recent studies in Denmark and Australia found a significant association between low educational attainment and low income and increased incidence of acute gastroenteritis. The diagnosis of acute gastroenteritis is generally based on symptoms and does not require laboratory confirmation of a pathogen; therefore it is difficult to distinguish illnesses transmitted by food from other modes of transmission [61, 62].
Another possible approach to combat reporting bias would be to conduct a prospective cohort study where persons reporting a diarrhoeal illness were asked to submit a stool sample for testing. This would help to alleviate some of the bias associated with access to care.
We found no consistent effect across all pathogens for a single SES measure, either indicating weakness in the measure as an indicator of social class [63] or reflecting differential effects of SES by pathogen type. The one exception to this was studies that used a deprivation index score to assess SES. All four studies of Campylobacter incidence that calculated a deprivation index found a direct association between high SES and high disease incidence [22–25]. This consistency was remarkable because it also spanned studies conducted on three continents. As such, it suggests that indexes can be used as a more generalizable metric for international comparisons of foodborne illness in developed countries.
The challenge in assessing SES arises from the lack of a single metric and the variable interpretation of SES measures depending on other variables. Individual-level metrics like personal income, educational attainment, or home ownership can be useful for measuring SES, and they reduce the likelihood of misclassification of exposure. Yet, individual-level data can be difficult to collect and does not capture an individual's social milieu, which may be similarly important in determining health outcomes [61, 63]. Therefore, many studies rely upon community-level metrics such as mean household income, mean adult educational attainment, or percentage of residents who own their home. These data are readily available at the census-tract level and has the additional advantage of indicating the social milieu in which an individual lives. However, there is heterogeneity within census tracts, which may have populations as large as a few thousand persons. Furthermore, capturing just an individual- or community-level measure of SES does not complete the picture. Social class is frequently relative and similar measures at different scales may measure different effects. For example, Pearl et al. and Chang et al. studied the association between STEC and SES as measured by income. Pearl et al. used each case's household income data, and Chang et al. used the percentage of households in a census tract that had incomes below the poverty line [29, 31]. Although both measured income, Pearl et al. found no association at the individual level between SES and STEC incidence, whereas Chang et al. found an increased incidence of STEC in census tracts with low levels of poverty. It is possible that these differences are the result of other variables, such as study location, time period, or size, but they point to the challenge of comparing within categories of SES metrics. Indexes benefit from having a rigorous, multifaceted approach to assessing SES that goes beyond just income or education and attempts to measure material need [64]. By combining multiple data sources, they reduce the impact of variability in each individual SES metric included in the calculation. Still, they face challenges in calibration and validation [64].
An additional challenge in measuring SES is establishing meaningful thresholds for categorization across different environments. For instance, a category for income may characterize a person as likely to be in a low SES group in an urban setting, but the same category may not correspond to persons of low SES in rural settings, where cost of living is lower. Reliance on a fixed cut-off may explain why Spencer et al. [22] observed an association between low SES and low campylobacteriosis incidence in urban areas in New Zealand but not in rural ones. However, another possibility that they suggest is that disadvantaged urban communities may have lower reporting levels, creating observed lower incidence as a surveillance artifact. This hypothesis has been supported by the work of Wheeler et al. in England [65]. Nevertheless, it is also possible that differential Campylobacter infection rates by SES in urban and rural areas are a byproduct of SES categorization or a true effect from different transmission pathways in the different environments.
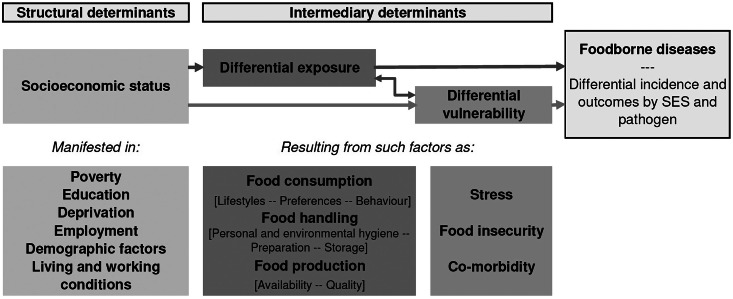
Based on this systematic review of findings in high income countries, we can adapt and extend the model Jouve et al. presents to illustrate the mechanism behind differences in foodborne illness by SES (Fig. 2) [66]. Structural determinants of health, in this case SES, lead to differential exposure and differential vulnerability. Exposure and vulnerability can interact, and both lead to differential incidence of foodborne illness. Exposure is a function of environmental and behavioural variables, whereas vulnerability is a function of environmental and physiological ones.
Fig. 2.
Social determinants of foodborne disease, adapted from Jouve et al. [66].
Differences in food consumption patterns are driven by environmental and behavioural variables. Low SES individuals may have differential risk because of food availability. Fresh produce has increasingly been implicated as a cause of foodborne illness in the United States [67, 68] in addition to well-known sources like meat, poultry, eggs, and unpasteurized dairy [69]. In low SES neighbourhoods, there may be less access to some of these products [70], particularly fruit and vegetables [71]. This could create a protective effect because the reduced availability is not offset by a concurrent increase in the risk posed by available food sold in low SES areas [12, 15].
SES is also associated with food safety knowledge and food handling. Awareness of food safety labels regarding safe handling and preparation increases with education level [72], and individuals with less than a high-school level education are less likely to have heard of Salmonella or Listeria contamination as problems [44]. In addition, individuals with higher levels of education may be more willing to accept products that are modified to increase safety, such as irradiated beef [73]. However, low SES individuals are more likely to recognize that home food preparation can be a risky activity for foodborne disease, are more likely to think refrigerator temperature is important, and are more likely to have general knowledge about appropriate food-related hygiene [16, 44, 45].
Although better knowledge of proper food storage and preparation is associated with reduced incidence of food-associated illness, knowledge of specific elements, such as safety labels, does not always correlate with behaviour [72, 74]. Low SES is correlated with safer food handling and preparation. Individuals in low SES groups, as defined by income and education, are less likely to eat risky foods and more likely to practice good cross-contamination prevention, hand washing, and food storage techniques habits [16, 44, 75–7]. While the reason for this discrepancy has not been evaluated, one possibility is that individuals from lower SES groups may be more likely to work or have worked in food-preparation or other related industries in which they received specific hygiene instruction.
Strengths and limitations
Strengths of this study include adherence to the systematic review methodology, use of double data extraction, and availability of multiple studies for three of the four pathogens. Limitations include the predominance of ecological studies, difficulty in measuring SES, and reliance on laboratory-confirmed cases. There is a strong likelihood of bias within ecological analyses, which are generally considered less robust than case-control or cohort designs, when extrapolating to the individual-level. Because these analyses were not excluded from this review, it may have biased the results of our assessment when considering the ecological study results in light of known individual-level risk factors. However, ecological studies have the benefit of being able to suggest population-level factors that may cause disease, such as food, health, and social policies [54]. Publication bias may have also influenced our review, but for most studies we identified, SES was one of multiple exposures being analysed. Therefore, the decision to publish the results is less likely to have been influenced by the strength or direction of the association between SES and disease incidence. Last, another limitation is the exclusion of publications written in languages other than English (nine publications).
CONCLUSION
Foodborne illness a major contributor to morbidity in the developed world. High-risk groups vary by pathogen and for some pathogens risk of illness appears to be associated with SES. Improving understanding of the impact that SES has on the incidence of different foodborne infections is an important step towards efficient, targeted public health interventions to prevent unnecessary illness. However, major challenges for research on this topic include case ascertainment and measurement of SES status. Future areas of research include cohort and other robustly designed studies to better evaluate the impact of SES on foodborne illness and studying SES-targeted interventions to test their efficacy. At a time when foodborne illness is of increasing concern, understanding which populations are at greatest risk is critical for protecting individuals and their families.
ACKNOWLEDGEMENTS
This work was partially supported by the F30 grant (K.L.N., grant 1F30DK100097-01), the ARCS Foundation (K.L.N), the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (J.S.L., grant 1K01AI087724-01; P.A.R., grant T32AI074492), the National Institute of Food and Agriculture at the U.S. Department of Agriculture (J.S.L., grant 2010-85212-20608), and the Emory University Global Health Institute (J.S.L.). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the U.S. Department of Agriculture.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814003847.
click here to view supplementary material
REFERENCES
- 1.Oakes JM, Rossi PH. The measurement of SES in health research: current practice and steps toward a new approach. Social Science and Medicine 2003; 56: 769–784. [DOI] [PubMed] [Google Scholar]
- 2.Shavers VL. Measurement of socioeconomic status in health disparities research. Journal of the National Medical Association 2007; 99: 1013–1023. [PMC free article] [PubMed] [Google Scholar]
- 3.Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don't. Annals of the New York Academy of Sciences 1999; 896: 3–15. [DOI] [PubMed] [Google Scholar]
- 4.Adler NE, et al. Socioeconomic status and health. The challenge of the gradient. American Psychologist 1994; 49: 15–24. [DOI] [PubMed] [Google Scholar]
- 5.Jorgensen KT, et al. Socio-demographic factors, reproductive history and risk of osteoarthritis in a cohort of 4·6 million Danish women and men. Osteoarthritis and Cartilage 2011; 19: 1176–1182. [DOI] [PubMed] [Google Scholar]
- 6.Colhoun HM, Hemingway H, Poulter NR. Socio-economic status and blood pressure: an overview analysis. Journal of Human Hypertension 1998; 12: 91–110. [DOI] [PubMed] [Google Scholar]
- 7.Funch DP. Socioeconomic status and survival for breast and cervical cancer. Women Health 1986; 11: 37–54. [DOI] [PubMed] [Google Scholar]
- 8.Strike PC, Steptoe A. Psychosocial factors in the development of coronary artery disease. Progress in Cardiovascular Diseases 2004; 46: 337–347. [DOI] [PubMed] [Google Scholar]
- 9.Sumartojo E. Structural factors in HIV prevention: concepts, examples, and implications for research. AIDS 2000; 14 (Suppl. 1): S3–10. [DOI] [PubMed] [Google Scholar]
- 10.Lonnroth K, et al. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Social Science and Medicine 2009; 68: 2240–2246. [DOI] [PubMed] [Google Scholar]
- 11.Yousey-Hindes KM, Hadler JL. Neighborhood socioeconomic status and influenza hospitalizations among children: New Haven County, Connecticut, 2003–2010. American Journal of Public Health 2011; 101: 1785–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darcey VL, Quinlan JJ. Use of geographic information systems technology to track critical health code violations in retail facilities available to populations of different socioeconomic status and demographics. Journal of Food Protection 2011; 74: 1524–1530. [DOI] [PubMed] [Google Scholar]
- 13.Scallan E, et al. Foodborne illness acquired in the United States – unspecified agents. Emerging Infectious Diseases 2011; 17: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scallan E, et al. Foodborne illness acquired in the United States – major pathogens. Emerging Infectious Diseases 2011; 17: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Signs RJ, et al. Retail food safety risks for populations of different races, ethnicities, and income levels. Journal of Food Protection 2011; 74: 1717–1723. [DOI] [PubMed] [Google Scholar]
- 16.Patil SR, Cates S, Morales R. Consumer food safety knowledge, practices, and demographic differences: findings from a meta-analysis. Journal of Food Protection 2005; 68: 1884–1894. [DOI] [PubMed] [Google Scholar]
- 17.Johnson AE, et al. Food safety knowledge and practice among elderly people living at home. Journal of Epidemiology and Community Health 1998; 52: 745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klugman J. Human Development Report. New York, NY: United Nations, 2011. [Google Scholar]
- 19.Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2011. [Google Scholar]
- 20.Ellencweig AY, Slater PE. Demographic and socio-economic patterns of hospitalization for infectious diseases in Israel. European Journal of Epidemiology 1986; 2: 83–89. [DOI] [PubMed] [Google Scholar]
- 21.Achi R, Mata L, Lindberg AA. Serum antibody titres to Shigella lipopolysaccharides and invasion plasmid antigens in healthy Costa Rican and Swedish women. Scandinavian Journal of Infectious Diseases 1994; 26: 329–337. [DOI] [PubMed] [Google Scholar]
- 22.Spencer SE, et al. The spatial and temporal determinants of campylobacteriosis notifications in New Zealand, 2001–2007. Epidemiology & Infection 2012; 140: 1663–1677. [DOI] [PubMed] [Google Scholar]
- 23.Rind E, Pearce J. The spatial distribution of campylobacteriosis in New Zealand, 1997–2005. Epidemiology and Infection 2010; 138: 1359–1371. [DOI] [PubMed] [Google Scholar]
- 24.Nichols GL, et al. Campylobacter epidemiology: a descriptive study reviewing 1 million cases in England and Wales between 1989 and 2011. British Medical Journal Open 2012; 2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green CG, Krause DO, Wylie JL. Spatial analysis of campylobacter infection in the Canadian province of Manitoba. International Journal of Health Geographics 2006; 5: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simonsen J, Frisch M, Ethelberg S. Socioeconomic risk factors for bacterial gastrointestinal infections. Epidemiology 2008; 19: 282–290. [DOI] [PubMed] [Google Scholar]
- 27.Pyra M, et al. Determinants of campylobacteriosis notifications in New Zealand. Epidemiology & Infection 2012; 140: 2087–2088. [DOI] [PubMed] [Google Scholar]
- 28.Gillespie IA, et al. Demographic determinants for Campylobacter infection in England and Wales: implications for future epidemiological studies. Epidemiology & Infection 2008; 136: 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang M, et al. An ecological analysis of sociodemographic factors associated with the incidence of salmonellosis, shigellosis, and E. coli O157:H7 infections in US counties. Epidemiology & Infection 2009; 137: 810–820. [DOI] [PubMed] [Google Scholar]
- 30.Sakuma M, Urashima M, Okabe N. Verocytotoxin-producing Escherichia coli, Japan, 1999–2004. Emerging Infectious Diseases 2006; 12: 323–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearl DL, et al. A multi-level approach for investigating socio-economic and agricultural risk factors associated with rates of reported cases of Escherichia coli O157 in humans in Alberta, Canada. Zoonoses and Public Health 2009; 56: 455–464. [DOI] [PubMed] [Google Scholar]
- 32.Jalava K, et al. Agricultural, socioeconomic and environmental variables as risks for human verotoxigenic Escherichia coli (VTEC) infection in Finland. BMC Infectious Diseases 2011; 11: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillespie IA, et al. Human listeriosis in England, 2001–2007: association with neighbourhood deprivation. Eurosurveillance 2010; 15: 7–16. [DOI] [PubMed] [Google Scholar]
- 34.Younus M, et al. The role of neighborhood level socioeconomic characteristics in Salmonella infections in Michigan (1997–2007): assessment using geographic information system. International Journal of Health Geographics 2007; 6: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banatvala N, et al. Salmonellosis in North Thames (East), UK: associated risk factors. Epidemiology & Infection 1999; 122: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borgnolo G, et al. A case-control study of Salmonella gastrointestinal infection in Italian children. Acta Paediatrica 1996; 85: 804–808. [DOI] [PubMed] [Google Scholar]
- 37.Younus M, et al. The role of exposures to animals and other risk factors in sporadic, non-typhoidal Salmonella infections in Michigan children. Zoonoses and Public Health 2010; 57: e170–176. [DOI] [PubMed] [Google Scholar]
- 38.Towns RE, et al. Food safety-related refrigeration and freezer practices and attitudes of consumers in Peoria and surrounding counties. Journal of Food Protection 2006; 69: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 39.Friedman CR, et al. Risk factors for sporadic Campylobacter infection in the United States: a case-control study in FoodNet sites. Clinical Infectious Diseases 2004; 38 (Suppl. 3): S285–296. [DOI] [PubMed] [Google Scholar]
- 40.Danis K, et al. Risk factors for sporadic Campylobacter infection: an all-Ireland case-control study. Eurosurveillance 2009; 14(7). [PubMed] [Google Scholar]
- 41.Lachat C, et al. Eating out of home and its association with dietary intake: a systematic review of the evidence. Obesity Reviews 2012; 13: 329–346. [DOI] [PubMed] [Google Scholar]
- 42.Headrick ML, et al. Profile of raw milk consumers in California. Public Health Reports 1997; 112: 418–422. [PMC free article] [PubMed] [Google Scholar]
- 43.Altekruse SF, et al. A multi-state survey of consumer food-handling and food-consumption practices. American Journal of Preventive Medicine 1999; 16: 216–221. [DOI] [PubMed] [Google Scholar]
- 44.Anderson AL, Verrill LA, Sahyoun NR. Food safety perceptions and practices of older adults. Public Health Reports 2011; 126: 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fein SB, et al. Trends in U.S. consumers' safe handling and consumption of food and their risk perceptions, 1988 through 2010. Journal of Food Protection 2011; 74: 1513–1523. [DOI] [PubMed] [Google Scholar]
- 46.Gholami P, Lew SQ, Klontz KC. Raw shellfish consumption among renal disease patients. A risk factor for severe Vibrio vulnificus infection. American Journal of Preventive Medicine 1998; 15: 243–245. [DOI] [PubMed] [Google Scholar]
- 47.Timbo BB, et al. Raw shellfish consumption in California: the 1992 California Behavioral Risk Factor Survey. American Journal of Preventive Medicine 1995; 11: 214–217. [PubMed] [Google Scholar]
- 48.Domingues AR, et al. Source attribution of human salmonellosis using a meta-analysis of case-control studies of sporadic infections. Epidemiology & Infection 2012; 140: 959–969. [DOI] [PubMed] [Google Scholar]
- 49.Voetsch AC, et al. Risk factors for sporadic Shiga toxin-producing Escherichia coli O157 infections in FoodNet sites, 1999–2000. Epidemiology & Infection 2007; 135: 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swaminathan B, Gerner-Smidt P. The epidemiology of human listeriosis. Microbes and Infection 2007; 9: 1236–1243. [DOI] [PubMed] [Google Scholar]
- 51.Darmon N, Drewnowski A. Does social class predict diet quality? American Journal of Clinical Nutrition 2008; 87: 1107–1117. [DOI] [PubMed] [Google Scholar]
- 52.Cohen S. Social status and susceptibility to respiratory infections. Annals of the New York Academy of Sciences 1999; 896: 246–253. [DOI] [PubMed] [Google Scholar]
- 53.Quinlan JJ. Foodborne illness incidence rates and food safety risks for populations of low socioeconomic status and minority race/ethnicity: a review of the literature. International Journal of Environmental Research and Public Health 2013; 10: 3634–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz S. The fallacy of the ecological fallacy: the potential misuse of a concept and the consequences. American Journal of Public Health 1994; 84: 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allos BM, et al. Surveillance for sporadic foodborne disease in the 21st century: the FoodNet perspective. Clinical Infectious Diseases 2004; 38 (Suppl. 3): S115–120. [DOI] [PubMed] [Google Scholar]
- 56.Kaptan G, Fischhoff B. Diagnosing food-borne illness: a behavioral analysis of barriers to testing. Journal of Public Health Policy 2011; 32: 60–72. [DOI] [PubMed] [Google Scholar]
- 57.Tam CC, Rodrigues LC, O'Brien SJ. The study of infectious intestinal disease in England: what risk factors for presentation to general practice tell us about potential for selection bias in case-control studies of reported cases of diarrhoea. International Journal of Epidemiology 2003; 32: 99–105. [DOI] [PubMed] [Google Scholar]
- 58.Scallan E, et al. Factors associated with seeking medical care and submitting a stool sample in estimating the burden of foodborne illness. Foodborne Pathogens and Disease 2006; 3: 432–438. [DOI] [PubMed] [Google Scholar]
- 59.Alexander CS, et al. Acute gastrointestinal illness and child care arrangements. American Journal of Epidemiology 1990; 131: 124–131. [DOI] [PubMed] [Google Scholar]
- 60.Rubin DH, et al. Relationship between infant feeding and infectious illness: a prospective study of infants during the first year of life. Pediatrics 1990; 85: 464–471. [PubMed] [Google Scholar]
- 61.Ethelberg S, et al. Risk factors for diarrhea among children in an industrialized country. Epidemiology 2006; 17: 24–30. [DOI] [PubMed] [Google Scholar]
- 62.Hall GV, et al. Frequency of infectious gastrointestinal illness in Australia, 2002: regional, seasonal and demographic variation. Epidemiology & Infection 2006; 134: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Braveman PA, et al. Socioeconomic status in health research: one size does not fit all. Journal of the American Medical Association 2005; 294: 2879–2888. [DOI] [PubMed] [Google Scholar]
- 64.Morris R, Carstairs V. Which deprivation? A comparison of selected deprivation indexes. Journal of Public Health Medicine 1991; 13: 318–326. [PubMed] [Google Scholar]
- 65.Wheeler JG, et al. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. The Infectious Intestinal Disease Study Executive. British Medical Journal 1999; 318: 1046–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jouve JL, Aagaard-Hansen J, Aidara-Kane A. Food safety: equity and social determinants. In: Equity, Social Determinants and Public Health Programmes, 2010, pp. 95–114. [Google Scholar]
- 67.Painter JA, et al. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerging Infectious Diseases 2013; 19: 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lynch MF, Tauxe RV, Hedberg CW. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiology & Infection 2009; 137: 307–315. [DOI] [PubMed] [Google Scholar]
- 69.Lynch M, et al. Surveillance for foodborne-disease outbreaks – United States, 1998–2002. Morbidity and Mortality Weekly Report. Surveillance Summary 2006; 55: 1–42. [PubMed] [Google Scholar]
- 70.Walker RE, Keane CR, Burke JG. Disparities and access to healthy food in the United States: a review of food deserts literature. Health and Place 2010; 16: 876–884. [DOI] [PubMed] [Google Scholar]
- 71.Moore LV, Diez Roux AV. Associations of neighborhood characteristics with the location and type of food stores. American Journal of Public Health 2006; 96: 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang S, Angulo FJ, Altekruse SF. Evaluation of safe food-handling instructions on raw meat and poultry products. Journal of Food Protection 2000; 63: 1321–1325. [DOI] [PubMed] [Google Scholar]
- 73.Frenzen PD, et al. Consumer acceptance of irradiated meat and poultry in the United States. Journal of Food Protection 2001; 64: 2020–2026. [DOI] [PubMed] [Google Scholar]
- 74.Kennedy J, et al. Food safety knowledge of consumers and the microbiological and temperature status of their refrigerators. Journal of Food Protection 2005; 68: 1421–1430. [DOI] [PubMed] [Google Scholar]
- 75.Li-Cohen AE, Bruhn CM. Safety of consumer handling of fresh produce from the time of purchase to the plate: a comprehensive consumer survey. Journal of Food Protection 2002; 65: 1287–1296. [DOI] [PubMed] [Google Scholar]
- 76.Nesbitt A, et al. High-risk food consumption and food safety practices in a Canadian community. Journal of Food Protection 2009; 72: 2575–2586. [DOI] [PubMed] [Google Scholar]
- 77.Roseman MG. Food safety perceptions and behaviors of participants in congregate-meal and home-delivered-meal programs. Journal of Environmental Health 2007; 70: 13–21, 44. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814003847.
click here to view supplementary material