Abstract
Chronic infection the food-borne liver fluke, Opisthorchis viverrini, frequently induces cancer of the bile ducts, cholangiocarcinoma. Opisthorchiasis is endemic in Thailand, Lao PDR, Cambodia and Vietnam, where eating undercooked freshwater fish carrying the juvenile stage of this pathogen leads to human infection. Because inhibition of apoptosis facilitates carcinogenesis, this study investigated modulation by thioredoxin from O. viverrini of apoptosis of bile duct epithelial cells, cholangiocytes. Cells of a cholangiocyte line were incubated with the parasite enzyme after which they were exposed hydrogen peroxide. Oxidative stress-induced apoptosis was monitored using flow cytometry, growth in real time and imaging of living cells using laser confocal microscopy. Immunolocalization revealed liver fluke thioredoxin within cholangiocytes. Cells exposed to thioredoxin downregulated apoptotic genes in the mitogen activated protein kinases pathway and upregulated anti-apoptosis-related genes including apoptosis signaling kinase 1, caspase 9, caspase 8, caspase 3, survivin and others. Western blots of immunoprecipitates of cell lysates revealed binding of thioredoxin to apoptosis signaling kinase 1. Together the findings indicated that thioredoxin from O. viverrini inhibited oxidative stress-induced apoptosis of bile duct epithelial cells, which supports a role for this liver fluke oxidoreductase in opisthorchiasis-induced cholangiocarcinogenesis.
Keywords: Opisthorchis viverrini, liver fluke, thioredoxin, carcinogenesis, apoptosis, apoptosis signal-regulating kinase-1, cholangiocyte, xCELLigence, oxidative stress, gene arrays
Graphical Abstract
Apoptosis of cholangiocytes modulated by thioredoxin from Opisthorchis viverrini
1. Introduction
Infection with the liver fluke Opisthorchis viverrini is endemic in Thailand, Lao PDR, Cambodia and central Vietnam (Petney et al., 2013; Sithithaworn et al., 2012; Sripa et al., 2011). Infection follows the ingestion of uncooked/undercooked cyprinoid, fresh-water fish infected with the metacercariae of the parasite. Opisthorchiasis causes a spectrum of biliary system disease, including cholangitis, obstructive jaundice, hepatomegaly, cholecystitis and cholelithiasis (Mairiang et al., 2012; Mairiang and Mairiang, 2003). More problematically, chronic opisthorchiasis infection frequently causes cholangiocarcinoma, bile duct cancer (IARC, 2012; Sripa et al., 2012; Sripa et al., 2007). Carcinogenesis of opisthorchiasis-induced bile duct cancer likely arises from several, interrelated molecular insults. These factors include inflammation associated reactive oxygen species (ROS) and reactive nitrogen species (RNS), lesions caused by the feeding and other mechanical activities of the worms within the bile ducts, and soluble mediators released by the parasites (IARC, 2012; Porta et al., 2011; Sripa et al., 2012; Sripa et al., 2007). They also may include perturbation of the biliary and intestinal microbiome due to liver fluke infection (Plieskatt et al., 2013), and reactive oxysterol- and catechol estrogen quinone-like metabolites released by the fluke that can form depurinating adducts with chromosomal DNA of epithelial cells of the infected persons (Correia da Costa et al., 2014; Jusakul et al., 2012). The fluke secretes and/or excretes metabolic products, immunogens and other mediators (Mulvenna et al., 2010; Smout et al., 2009; Sripa and Kaewkes, 2000; Wongratanacheewin et al., 1988) including proteins like thioredoxin peroxidase (Suttiprapa et al., 2008) and thioredoxin (Suttiprapa et al., 2012).
Thioredoxin (Trx) is an inflammation-inducible oxidoreductase of 12 kDa that is expressed ubiquitously in prokaryotes and eukaryotes. This enzyme is involved in numerous physiological roles. Within the cell, Trx exerts cyto-protective effects: it scavenges ROS and thereby relieves oxidative stress, and it regulates redox-sensitive signaling pathways as well as ROS-independent genes. Outside the cell, Trx has cytokine and growth factor like properties, it promotes cell and tissue growth, and exhibits activities involved with protein disulfide reduction, protein repair, molecular chaperone tasks, structural components of enzymes, redox regulation of transcription factors, immunomodulation, and apoptosis (Carvalho et al., 2006; Holmgren and Lu, 2010; Nakamura et al., 1997; Powis and Kirkpatrick, 2007). Moreover, Trx has been implicated in carcinogenesis. It is encoded by a proto-oncogene that is overexpressed in tumors and correlates with poor prognosis (An and Kang, 2014). Trx stimulates survival of cancer cells, promotes angiogenesis, and inhibits apoptosis; evasion of apoptosis is a cardinal hallmark of cancer (Hanahan and Weinberg, 2000). Anti-apoptotic activities of thioredoxin, in particular interaction with apoptosis signal-regulating kinase-1 (ASK-1), are well described (Zhang et al., 2004). ASK-1 is a member of the mitogen activated protein kinase kinase kinase family (MAP3Ks); this enzyme activates c-Jun N-terminal kinase and p38K in response to oxidative and other stresses, leading to apoptosis, inflammation and other responses (Hattori et al., 2009; Zhang et al., 2004).
We have speculated that O. viverrini Trx-1 may contribute to the tumorigenic nature of chronic opisthorchiasis in the biliary tree (Sripa et al., 2012; Suttiprapa et al., 2012). Recombinant Ov-Trx-1 was produced from E. coli and its activity as an oxidoreductase characterized. Expression of Ov-Trx-1 in developmental stages of the liver fluke has been reported, and antibodies against the enzyme revealed its presence in cholangiocytes of hamsters adjacent to the liver fluke (Suttiprapa et al., 2012). Inflammatory cells secrete ROS/ RNS that are potentially genotoxic to fluke and to bystander cells. Nonetheless, O. viverrini flukes can survive for years, bathed in bile (Kaewkes, 2003). Thioredoxin from O. viverrini may be involved as a key main redox protein that protects this trematode from ROS (Suttiprapa et al., 2012). Given that inhibition of homeostatic apoptosis is obstructed in carcinogenesis, here we investigated anti-apoptotic effects of liver fluke thioredoxin on human normal cholangiocytes under oxidative stress, and on the interaction with apoptosis signal-regulating kinase-1, ASK-1.
2. Materials and Methods
Recombinant thioredoxin-1 of O. viverrini
The nucleotide sequence encoding Ov-Trx-1 was amplified from a cDNA library constructed from adult O. viverrini liver flukes (Laha et al., 2007). The nucleotide sequence of the amplicon was verified by sequencing, after which it was ligated into the expression vector pET-15b (Novagen, California, USA). BL21 (DE3) strain E. coli cells were transformed with the ligation products, and recombinant clones obtained by antibiotic selection of transformed bacteria. Expression of recombinant Ov-Trx-1 was induced with isopropyl β-d-1-thiogalactopyranoside; expression was monitored by SDS-PAGE (20% separating gel and 5% stacking gel)/ Coomassie Brilliant Blue staining. Recombinant Ov-Trx-1 was affinity purified on Ni-NTA resin (QIAGEN Inc., Valencia, California, USA), dialyzed against 1xPBS, absorbed with Triton-X114 to remove residual lipopolysaccharide (Aida and Pabst, 1990; Suttiprapa et al., 2012) and Bio-Beads SM2 (Bio-Rad, Hercules, California, USA) to remove Triton-X114, and then filtered through a 0.2 µm pore size membrane. Yields were quantified by Bradford assay, after which Ov-Trx-1 was stored in aliquots at −80°C
Anti-Ov-Trx-1 sera
Mice were vaccinated subcutaneously with purified Ov-Trx-1, 25 µg per immunization. The first immunization with Ov-Trx-1 was formulated with Freund's complete adjuvant and second and third immunizations with recombinant protein in Freund's incomplete adjuvant. Blood and sera were collected two weeks after the third immunization. Protocols for these experiments were approved by the Animal Ethics Committee of Khon Kaen University, approval number AEKKU25/2554, according to the Ethics of Animal Experimentation of the National Research Council of Thailand.
Uptake of liver fluke thioredoxin by human cholangiocytes
H69 is a cell line derived from an immortalized human cholangiocyte (Grubman et al., 1994; Park et al., 1999). H69 cells seeded at 20,000 cells on glass cover slips were cultured in 10% fetal bovine serum (FBS) in H69 medium (Grubman et al., 1994; Smout et al., 2009) for 48 h, and thereafter in H69 medium supplemented with 1% FBS and Ov-Trx-1 at 50 µg/ml for 30, 60 and 120 min. Cells were washed in cold 1x PBS, fixed in 2 % paraformaldehyde for 10 min, and re-washed in PBS. Cells were permeabilized with 0.5% Triton X-100/ 1x PBS for 5 min, washed with cold PBS, and probed with anti-Ov-Trx-1 sera, diluted 1:300 at 4°C for 18 h in a humidified atmosphere. After washing, cells were stained with goat anti-mouse IgG-Alexa Fluor 568 diluted 1:500. The cells were also stained with 4',6-diamidino-2-phenylindole (DAPI) for 2 h at 4°C. Last, the cells were washed with 1xPBS and fluorescence investigated using confocal laser scanning microscopy (CLSM) (Zeiss LSM 510 system, which includes an Axio Examiner Z1 microscope and a Quasar 32-channel spectral detector, Carl Zeiss, Oberkochen, Germany). Samples were scanned sequentially using a Plan-Apochromat 63x/1.40 Oil DIC objective (Zeiss). For acquisition of signals from the DAPI channel, a 405 diode laser line was used for excitation and emission was filtered in a band between 410 and 585 nm. Immunolabeling (Alexa Fluor 633) (Life Technologies, Pittsburgh, PA) was revealed by excitation with a diode 633 laser line, with emission recorded between 638-747 nm. Optical confocal sections were generated by adjusting the pinhole to one Airy unit using the most red-shifted channel, producing an optical section of about 0.7 µm in all channels. Confocal images were captured in sequential acquisition mode to avoid excitation bleed-through, particularly apparent with DAPI. Image frames measured 1024x1024 pixels with a pixel dimension of 0.132 µm. Image manipulation was undertaken with the assistance of Zen 2009 software (Zeiss); manipulations were limited to adjustment of brightness, cropping, insertion of scale bars and the like; image enhancement algorithms were applied in linear fashion across the image and not to selected aspects. Control images were adjusted similarly.
Imaging of living cells
H69 cells in H69 medium supplemented with 0 or 5 µg/ml Ov-Trx-1 were seeded at 40,000 cells on fibronectin (BD Biosciences, San Jose, California, USA) (10 µg/ml) coated chambers. Hydrogen peroxide was added to 300 µM, a concentration lethal for many cancer cell lines in (Chen et al., 2005). Subsequently, morphology of the cells was monitored and recorded continuously for 18 hours by CLSM (above). Confocal images of cells excited with 488 nm argon laser and 561 nm diode-pumped laser were captured through an Alpha Plan Apochromat 100×/1.46 objective lens with oil immersion. Images were analysed using Volocity 3D Image analysis software (Perkin Elmer, Waltham, Massachusetts, USA).
Flow cytometry analysis
H69 cells were transferred into H69 medium supplemented with 1 % FBS, and cultured for 24, 48 and 72 h with Ov-Trx-1 at 5 µg/ml. Thereafter, the medium was removed and hydrogen peroxide added to 300 µM. At 15 hours later, cells were harvested using a brief exposure to 2.5 % trypsin after which they were washed 1xPBS. Apoptosis was investigated by flow cytometry using annexin-V-Alexa 488 and propidium iodide (PI) in a FACSCalibur flow cytometry system (BD, Franklin Lakes, New Jersey, USA). Annexin-V-Alexa 488 (+ve)/PI (-ve) cells indicated early apoptosis, Annexin-V-Alexa Fluor 488 (Life Technologies) (+ve)/PI (+ve) indicated late apoptosis, whereas Annexin-V-Alexa488 (-ve)/PI (+ve) indicated cell necrosis (van Engeland et al., 1998). Cytometric data were analyzed with the assistance of the Flowjo software (Ashland, Oregon, USA).
Real time assessment by xCELLigence system of cell proliferation and apoptosis
Cell growth was evaluated using the xCELLigence DP system (ACEA Biosciences, San Diego, California, USA), designed to monitor events in real time by measuring electrical impedance across inter-digitated microelectrodes integrated on the bottom of tissue culture E-plates, see http://www.aceabio.com/main.aspx (Ke et al., 2011; Smout et al., 2009). H69 cells were seeded at 5,000 cells/well in E-plates (ACEA) in H69 medium supplemented with 10% FBS, 37°C and 5% CO2 and cultured for one day. The cells were rinsed in PBS and the medium was replaced with H69 medium diluted 1 in 20, i.e. 0.5% FBS. The cells were fasted in this reduced DBS containing medium for 4 to 6 hours after which the medium was supplemented with 0, 5 or 10 µg/ml Ov-Trx-1. Thereafter the cells were monitored for ~ 48 hours when H2O2 was added to 300 µM to induce oxidative stress. For some assays, Ov-Trx-1 that had been denatured by incubation at 85°C for 15 min was also included in some groups, and/or H2O2 was added to 30 or 75 µM. Subsequently, the cultures were monitored in real time for 24 hours or longer. The normalized cell index (CI), obtained by dividing the CI value at each time point by the CI at the time of addition of H2O2 was ascertained with the assistance of the RTCA Software 1.2 (ACEA). Raw normalized CI values were imported into Excel for analysis where the cellular growth percentages of cells exposed to H2O2 was expressed as the percentage of the normalized CI of cells cultured with Ov-Trx-1compared to (i.e. divided by) the normalized CI of control cells not incubated with the recombinant liver fluke protein (control cells growth rate = 100%). Statistical significance among the groups was assessed using Analysis of Variance (ANOVA) and Student’s t-test. P-values of ≤ 0.05 were considered to be significant.
Analysis of apoptosis signaling pathways by qPCR gene array
Gene expression profiles were investigated using total RNA (~800 ng) with the RT2-Profiler-PCR-Array, Qiagen catalog number PAHS 012Z, which targets the human apoptosis pathways with a panel of 84 cognate genes. Functional gene groups targeted include death domain receptors, extracellular apoptotic signals, DNA damage and repair, anti-apoptosis, negative regulation of apoptosis, positive regulation of apoptosis, caspases, caspase activation, and caspase inhibition. The system employs SYBR Green qPCR master mixes, and the manufacturer claims performance of amplification efficiency of 99%. H69 cells were seeded at 20,000 cell/well (6-wells plate) and cultured in H69 medium containing 0.5 % FBS and Ov-Trx-1 at 5 µg/ml for 24, 48 and 72 hours. H2O2 was added to 300 µM to induce apoptosis. Cells were harvested 18 hours later with 2.5% trypsin, after which mRNA was isolated from cell pellets (RNeasy mini kit, Qiagen). Purity and concentration of RNA were ascertained using the 2100 Bioanalyzer system (Agilent Technologies, Santa Clara, CA), after which cDNA was synthesized (RT2 first strand kit, Fermentas, Pittsburgh, Philadelphia, USA). The PCR cycling conditions included an initial denaturation of 95°C for 10 min followed by 40 cycles of 95°C for 15 sec, 60°C for 1 min, and melting curves of 55°C for 30 sec, 81 cycles. After thermal cycling in an iCycler fitted with an iQ5 real-time detector (Bio-Rad), data were uploaded for analysis at the RT2 Profiler PCR Array Data Analysis version 4 site, http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php. Three biological replicates were performed. Fold-change in gene expression for all the genes was calculated using the comparative cycle threshold CT (ΔΔCT) method (Le et al., 2011; Pfaffl, 2001). P values ≤ 0.05 were considered to be significant.
RT-PCR analysis targeting MAP3K/ASK-1/JNK- P38K signaling pathway
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis of mRNA expression of 12 members of the MAP3K/ASK-1/JNK- P38K signaling pathway was undertaken. The p38 kinase, c-Jun N-terminal kinase (JNK) and mitogen-activated protein kinase (MAPK) constitute intracellular signal transduction pathways that are responsive to stimuli such as oxidative stress, carcinogens and chemokines, and are involved in apoptosis, autophagy, cell differentiation and diverse other processes (Hattori et al., 2009; Nikoletopoulou et al., 2013; Soga et al., 2012). H69 cells were cultured with Ov-Trx-1, 5 µg/ml, for 24, 48 and 72 h after which H2O2 was added to 300 µM to induce oxidative stress and apoptosis. Cells were harvested 18 h later with 2.5% trypsin, and RNA isolated using the RNeasy Mini Kit (Qiagen), quantity determined as above, and cDNA synthesized (iScript cDNA Synthesis Kit) (Bio-Rad). Specific primers for downstream genes of the MAPK /ASK-1 pathways, specifically targeting the genes encoding ASK-1, P38K-α, JNK-1, -2, MAP2K-3, -4, -6, -7 (Hattori et al., 2009), were designed. Primers for two reference genes, GADPH and β-actin, were included. The primers were designed with the assistance of the Beacon designer software (Premier Biosoft, Palo Alto, CA). Primer sequences are provided in Supplementary Table S1. Total RNA (~ 800 ng) was employed to synthesize cDNA that was employed as template in real time qPCR (as above) using the SYBR system of Bio-Rad. Output data were analyzed with the assistance of the Bio5 RT2-qPCR software. Three biological replicates were performed.
Immunoprecipitation and western blotting
H69 cells cultured to a density of 20,000 cells per well were harvested and lysed in 25 mM Tris-HCl pH 7.4, 150 mM NaCl, 1mM EDTA, 1% NP-40 and 5% glycerol (Pierce IP Lysis Buffer, Thermo Scientific) for 30 min at 4°C. The lysate was incubated with Ov-Trx-1 at 1 mg/ml (1:1, volume: volume) for 18 h at 4°C. Complexes of cellular proteins were pulled down on His-Magnetic Sepharose (GE Healthcare), eluted and subjected to SDS-PAGE (20% separating gel, 5% stacking gel). After electrophoresis, products were transferred to nitrocellulose membranes, and membranes blocked with 5% skimmed milk powder in 1xPBS/0.05% Tween-20 (PBS-T) for 2 hours at 23°C. Thereafter, membranes were probed separately with four primary antibodies, anti-human ASK-1 (Abcam, Cambridge, Massachusetts, USA), anti-His (Abcam), anti-beta actin (USBiological, Salem, MA) and anti-Ov-Trx-1 (above), overnight at 4°C. Following washes in PBS-T, membranes were probed with goat anti-mouse or goat-rabbit IgG-conjugated to horseradish peroxidase (HRP) (Abcam) (secondary antibody) for 2 hours at 23°C. Reactive bands were detected using chemiluminescence (ECL Plus, GE Healthcare) in a 2000R Kodak Image Station, after which antibodies were stripped from the membranes (Restore western stripping buffer, Thermo Scientific). Membranes were re-probed sequentially with each primary antibody.
Results
Thioredoxin of O. viverrini enters human cholangiocytes
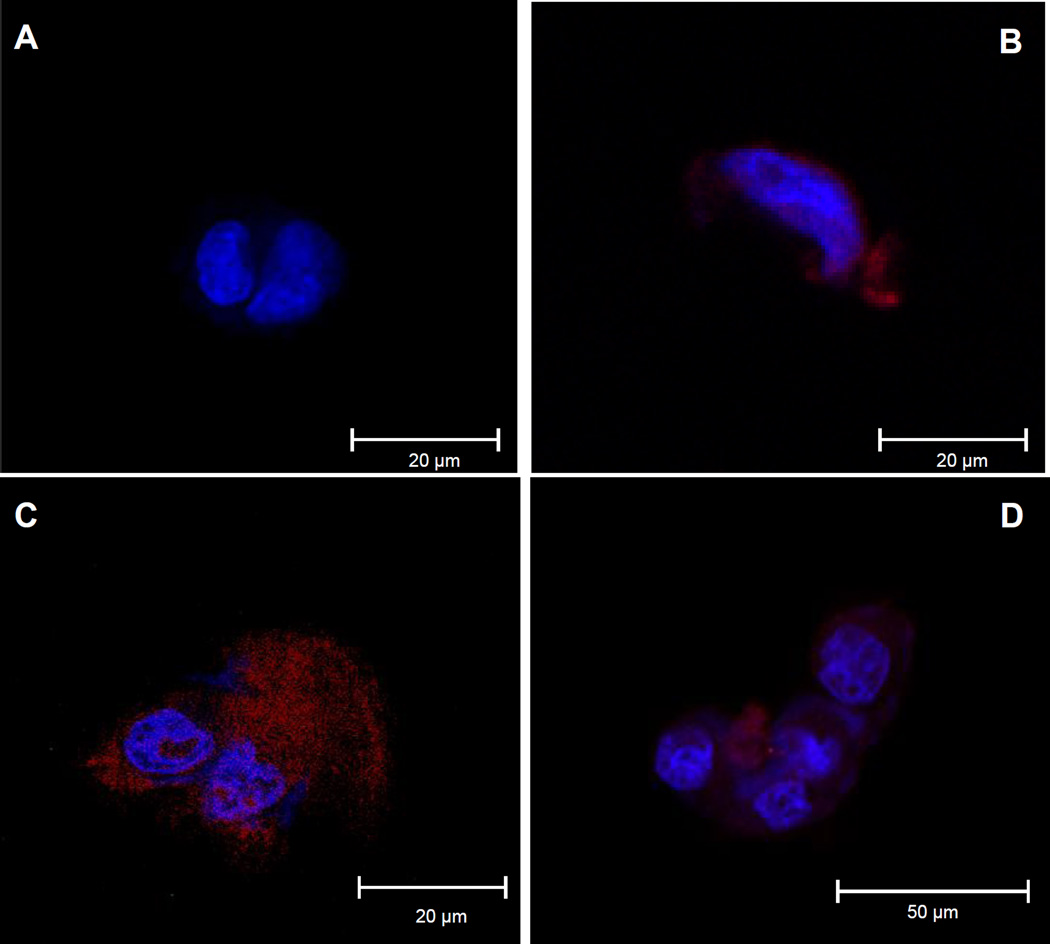
H69 cells were co-cultured with recombinant Ov-Trx-1 at 50 µg/ml for 30, 60 and 120 minutes. Immunofluorescence signals became evident at 30 min (Fig. 1A vs. B), and increased with time (Fig. 1B and C, respectively). No signal was evident in the absence of Ov-Trx-1 (negative control) (Fig. 1A). The fluorescence was apparent in the cytoplasm of the cholangiocytes but not evident in the nuclei of these cells, which counter-stained strongly with DAPI.
Figure 1. Fluke thioredoxin enters cytoplasm of human cholangiocytes.
Confocal imaging of cholangiocytes incubated with liver fluke thioredoxin. Control cells in medium without Ov-Trx-1, probed with primary (mouse anti-Ov-Trx-1 sera) and secondary (goat anti-mouse IgG-Alexa Fluor 568) (red) antibodies (panel A), and counter-stained with DAPI (blue). Panels B, C and D show cells incubated in Ov-Trx-1 for 30 min, 60 min and 120 min, respectively.
Liver fluke thioredoxin modulated apoptosis of cholangiocytes under oxidative stress
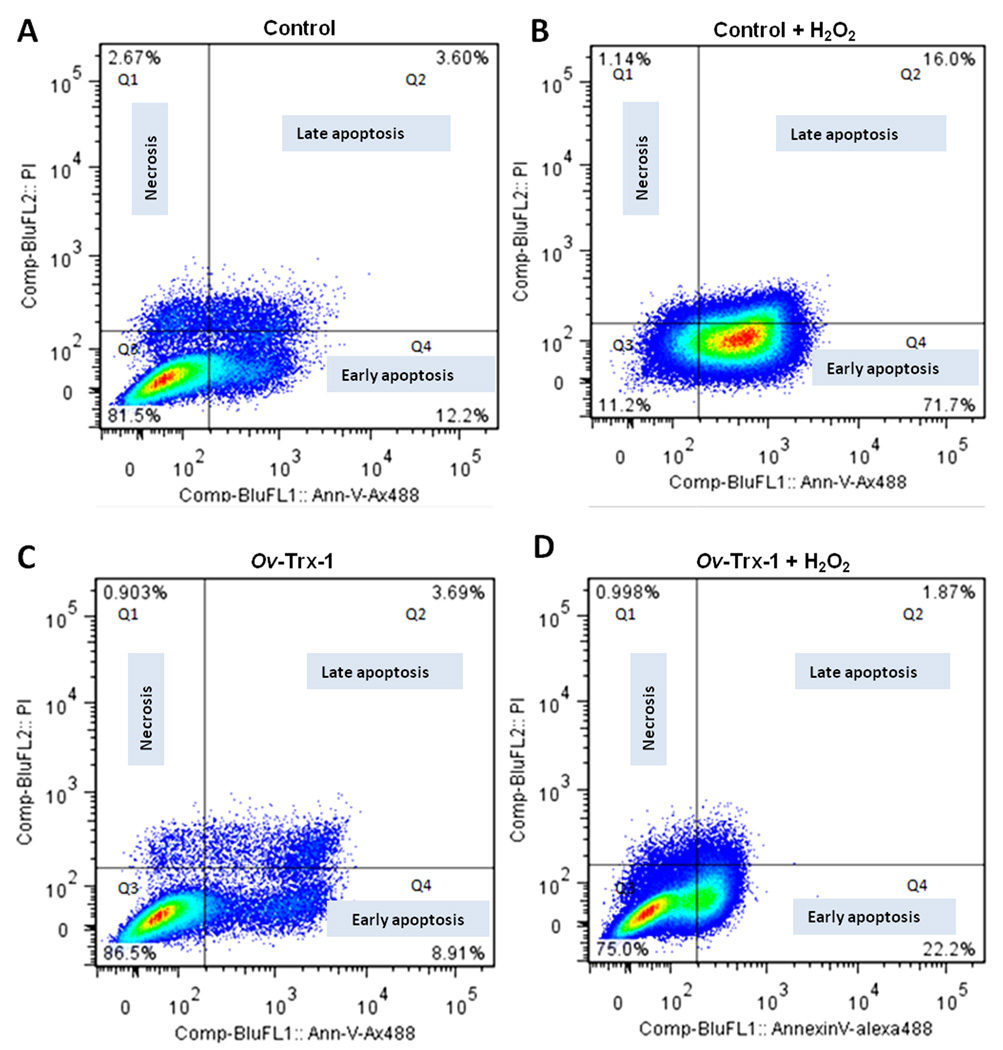
At 48 hours, similar percentages of live cells were seen in control H69 cells and H69 cells cultured in Ov-Trx-1 groups, 81.5% and 86.5%, respectively (Fig. 2A, C). Whereas most of the cells exposed to peroxide entered early apoptosis and late apoptosis, 71.7% and 16%, respectively, for cells pretreated with Ov-Trx-1 less apoptosis was induced by hydrogen peroxide with 22.2% and 1.87% of early and late apoptosis, respectively (Fig. 2B, D). Similar trends were apparent in a biological replicate at each of 24, 48 and 72 hours (not shown).
Figure 2. Fluke thioredoxin protects human cholangiocytes from oxidative stress induced apoptosis.
Flow cytometric analysis using staining with Annexin-V/Alexa488 and propidium iodide (PI) to investigate apoptosis of H69 cells; panel A, control H69 cells after 48 hours in culture; H69 cells exposed to hydrogen peroxide (B); H69 cells cultured in Ov-Trx-1, 5 µg/ml (C); H69 cells cultured in Ov-Trx-1, 5 µg/ml H69 cells, exposed to H2O2 (D).
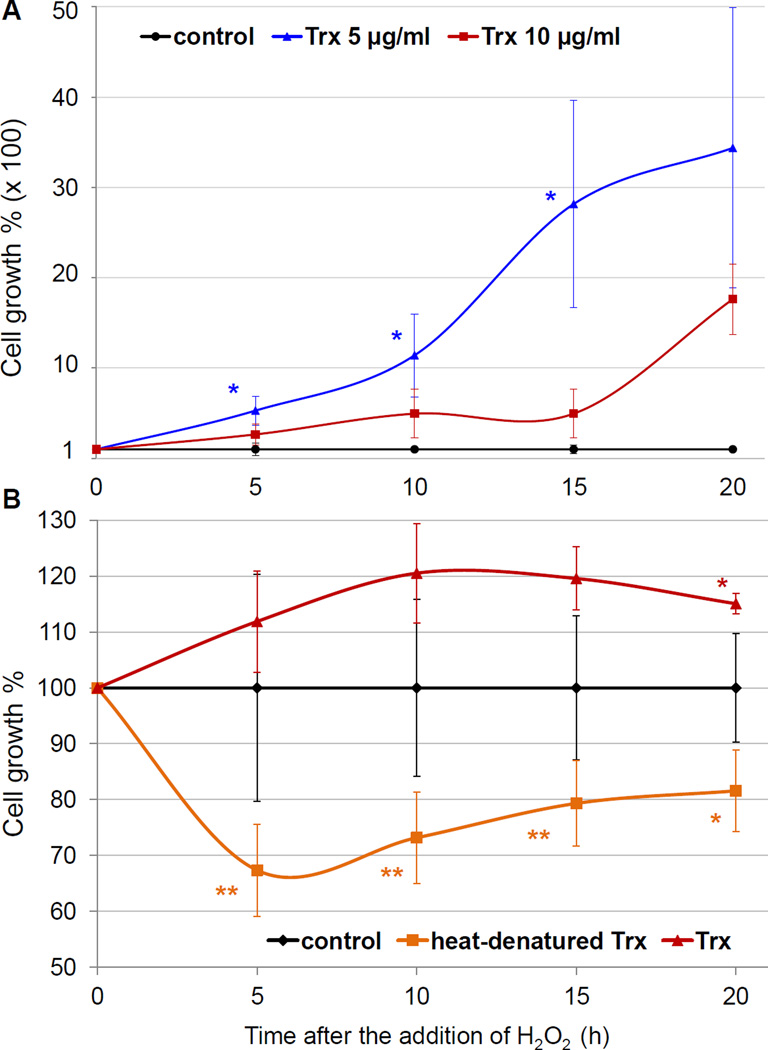
Figure 3. Liver fluke thioredoxin partially rescued cholangiocytes from H2O2-induced apoptosis as measured by xCELLigence real time cell assay.
Panel A: Cellular growth percentage of cells exposed to 300 µM of H2O2 is expressed as the percentage of the Normalized Cell Index of cells pre-incubated with 5 or 10 µg/ml of recombinant Ov-Trx-1 compared to the Normalized Cell Index of control cells pre-incubated without Ov-Trx-1 (growth rate = 100%). Blue asterisks denote significance at P ≤ 0.05 between the growth percentage of cells cultured with 5 µg/ml of Ov-Trx-1 and control cells. Panel B: Cellular growth percentage of cells exposed to 300 µM of H2O2 expressed as the percentage of the Normalized Cell Index of cells pre-incubated with 10 µg/ml of active recombinant Ov-Trx-1 or heat-denatured recombinant Ov-Trx-1 compared to the Normalized Cell Index of control cells pre-incubated without Ov-Trx-1 (growth rate = 100%). Red asterisks denote significance at P ≤ 0.05 between the growth percentage of cells cultured with 10 µg/ml of Ov-Trx-1 and control cells. Orange asterisks ndicate levels of significance (*, P ≤ 0.05, **, P ≤ 0.01) between the growth percentage of cells cultured with 10 µg/ml of active Ov-Trx-1 cells and the growth percentage of cells cultured with 10 µg/ml of heat-denatured Ov-Trx-1.
Modulatory effects of Ov-Trx-1 on cholangiocytes with respect to oxidative stress were also investigated in real time using the xCELLigence system (Ke et al., 2011). H69 cells were cultured in the presence of 0, 5 or 10 µg/ml of Ov-Trx-1 for two days, after which were exposed to H2O2 at 300 µM. In some assays, cells cultured in Ov-Trx-1 that had been heat denatured were included. The cells exposed to peroxide exhibited a rapid drop in the normalized CI, whether they were incubated or not with Ov-Trx-1, in comparison to control H69 (non-apoptotic) cells (not shown). However, from about six hours after the collapse, cells that had been incubated with Ov-Trx-1 appeared to recover and proliferation thereafter in comparison to control H69 cells exposed to H2O2 (Fig. 3A). The effect of Ov-Trx-1 at 5 µg/ml was more marked than for 10 µg/ml in this assay. The difference may reflect toxicity of the thioredoxin protein to the cells at large. We have previously reported that optimal concentration of liver fluke secretory/excretory products and reported inhibition of proliferation of H69 cells at concentrations higher than optimal concentration (Smout et al., 2009). Similar effects may occur with increasing concentration of Ov-Trx-1. In some assays we included denatured Ov-Trx-1, aiming to determine whether the activity of Ov-Trx-1 in modulation of apoptosis was dependent of the enzymatic activity of this thioredoxin. Unlike intact Ov-Trx-1, heat denatured Ov-Trx-1 failed modulate peroxide induced stress induced apoptosis (Fig. 3B and not shown). Similar trends were seen when H2O2 concentrations of 30 µM and 75 µM were used to induce oxidative stress, although significant modulatory effect of Ov-Trx-1 (and lack of modulatory effect of heat denatured Ov-Trx-1) was not apparent as quickly as with 300 µM H2O2 (Supplementary Fig. S1, panels A, B).
In addition, morphology of the H69 cells incubated with or without 5 µg/ml Ov-Trx-1 was monitored and recorded continuously for 18 hours by CLSM after addition of H2O2 at 300 µM, and the images were analyzed using Volocity 3D software. In brief, in the presence of Ov-Trx-1, morphology of cholangiocytes appeared to withstand the changes seen in the control cells exposed to peroxide; the cells exhibited less blebbing and less condensed nuclei (Supplementary Movie S1 and S2; and not shown).
Ov-Trx-1 down-regulates expression of apoptosis-associated genes
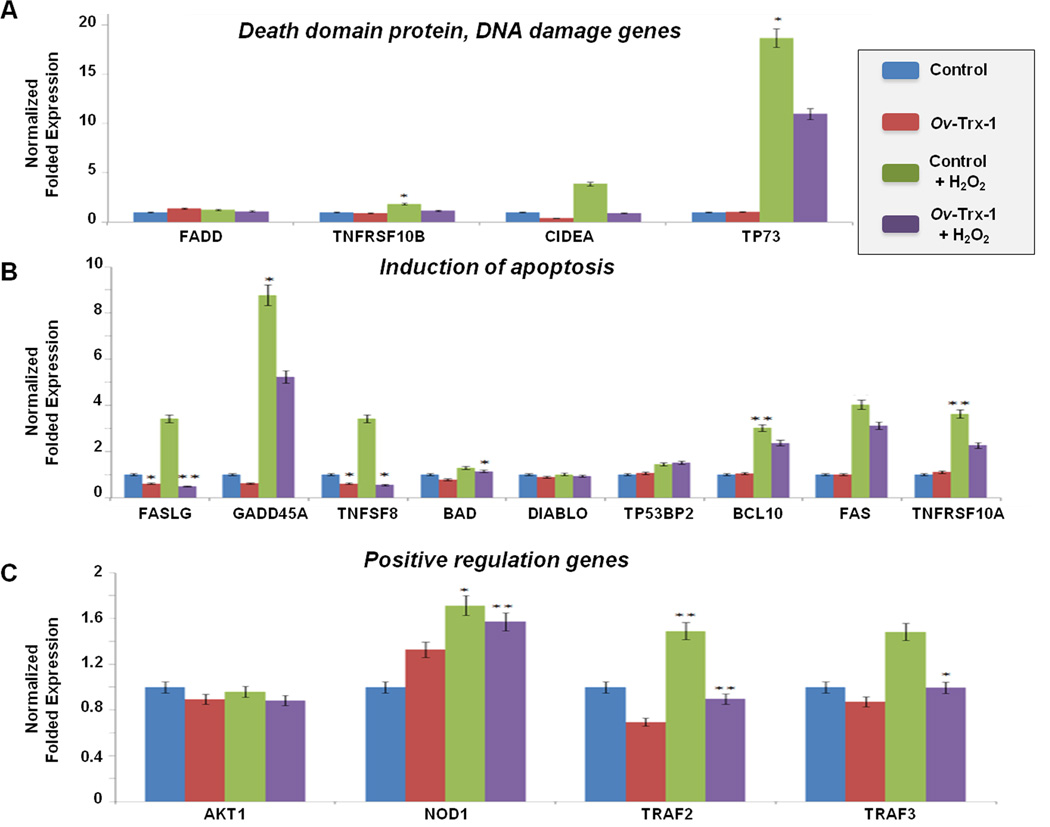
The modulatory potential of Ov-Trx-1 on cholangiocytes under oxidative stress was investigated by transcriptional changes using Qiagen’s RT2-Profiler-PCR-Array that targets the human apoptosis and programmed cell death pathways. Functional gene groups targeted included death domain receptors, extracellular apoptotic signals, DNA damage and repair, anti-apoptosis, negative regulation of apoptosis, positive regulation of apoptosis, caspases, caspase activation, and caspase inhibition. The transcriptional profile of H69 cells cultured with Ov-Trx-1 was generally similar to control H69 cells. Following addition of peroxide, at least 23 genes of the 84-gene array were upregulated, including APAF1, BCL10, BCL2A1, BCL2L10, BCL2L2, BIK, BICR5, CASP14, CASP5, CASP6, CD40LG, CIDEA, DAPK1, FAS, FASLG, GRADD45A, HRK, IL10, LTA, TNF, TNFRSF10A, TNFSF8, and TP73. By contrast, six of these genes were downregulated the addition of hydrogen peroxide to H69s cultured with Ov-Trx-1 -- BIK, BIRC5, DAPK1, FAS, GADD45A, TP73, along with two others, BNIP3 and TNFRSF9 (Supplementary Figure S2). Major changes in gene expression of this pathway induced by H202, and/or modulated by Ov-Trx-1, are illustrated in Figure 4. The array groups the genes in sub-categories, and changes in three of these sub-category pathways – death domain and DNA damage (Fig. 4, panel A), induction of apoptosis (Fig. 4B), and positive regulation (Fig. 4C) were apparent. Among the significant changes was a multi-fold increase in expression of CIDEA, GADD45A, BCL10, FASLG, TP73 and TNFRSF10A (among others) due to oxidative stress but, by contrast, comparative modulation of expression of these genes in the H69 cultured with the fluke thioredoxin. Two biological replicates of this assay were undertaken, with similar findings (not shown).
Figure 4. Gene expression during apoptosis signaling array in response to oxidative stress.
Gene expression levels of representative genes of the apoptosis signaling pathway analyzed by PCR pathway gene array; death domain protein and DNA damage genes, FADD, TNFRSF10B, CIDEA, TP73 (panel A), introduction of apoptosis genes, FASLG, GADD45A, TNFSF8, BAD, DIABLO, TP53BP2, BCL10, FAS, TNFRSF10A (panel B), and positive regulation genes, AKT1, NOD1, TRAF2, TRAF3 (panel C). Four groups of cells and their treatments were compared: control H69 cells (blue bars), H69 cells cultured with Ov-Trx-1 (red), H69 cells cultured with Ov-Trx-1 and exposed to H2O2 (purple) and H69 cells exposed to H2O2 (green). Significant differences among groups for various are indicated; * denotes p ≤ 0.05, **p ≤ 0.005.
MAP3K/ASK-1/JNK-P38K pathways engaged by liver fluke thioredoxin
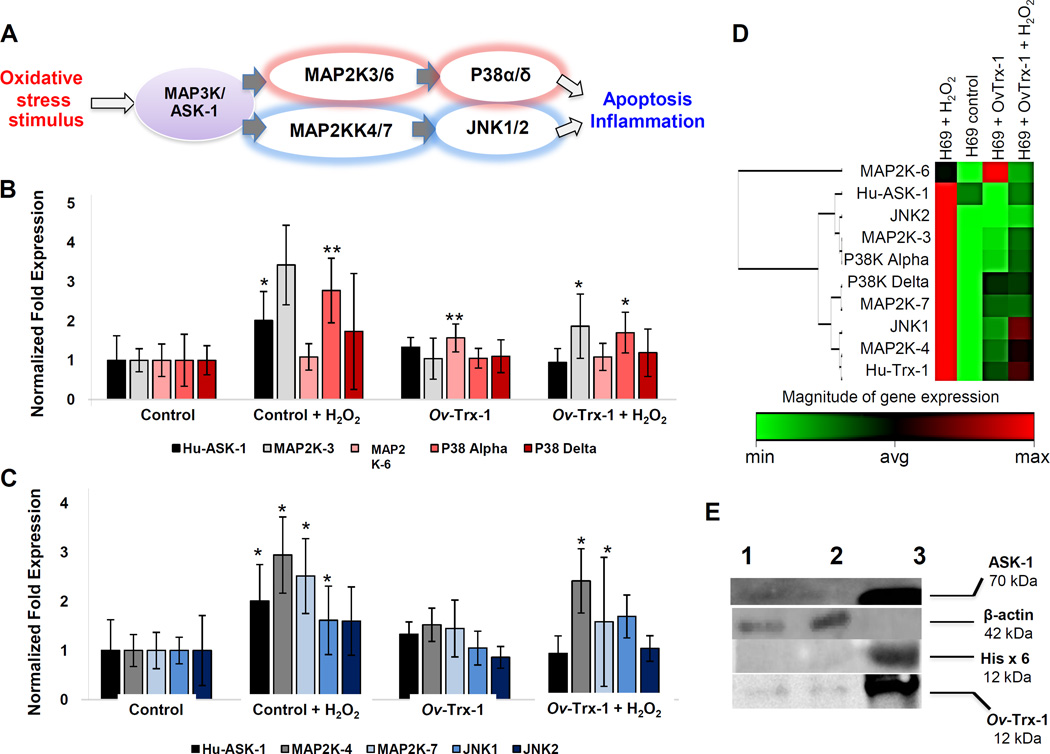
Apoptosis signal-regulating kinase 1 (ASK-1) is a member of the mitogen-activated protein kinase kinase kinase family that activates c-Jun N-terminal kinase and p38 in response to oxidative stress, endoplasmic reticulum stress and other insults. An array of regulatory mechanisms for ASK-1 are known, including activation by oxidative stress (Hattori et al., 2009). The expression profile of a panel of 12 genes that encode key members of the MAPK/ASK-1 JNK-P38K signaling cascade was investigated. Scatter plots of expression for the target genes were revealed that most were upregulated in response to H2O2-induced stress, whereas almost MAP3K/ ASK-1/JNK and P38K signaling genes were expressed similar as the normal condition (Supplementary Figure S3). The analysis revealed that expression levels of MAP3K/MAP2K, MAP3K/MAPK, MAPK/ASK-1-MAP2K3/6-P38K-α/δ and MAPK/ASK-1-MAP2K4/7-JNK1/2 downstream signaling pathways were modulated when H69 cells were co-cultured with the liver fluke thioredoxin in comparison to H69 cells stressed by exposure to H2O2 (Fig. 5, panels A-C). A clustergram for the expression of these MAPK/ASK-1 signaling genes is also presented (Fig. 5D), which revealed that the gene response profile of H69 cells cultured in Ov-Trx-1 before peroxide induced stress was not markedly dissimilar to that of the control cells.
Figure 5. Quantitative real time PCR and immunoprecipitation analyses of gene expression in the MAP3K/ASK-1 pathway.
Four treatment groups of H69 cells were compared: control H69 cells, H69 cells exposed to H202; H69 cells cultured with Ov-Trx-1; and H69 cells cultured with Ov-Trx-1 and exposed to H202. Gene expression in the MAP3K/MAP2K, MAP3K/MAPK, MAPK/ASK-1-MAP2K3/6-P38K-α/δ downstream pathway genes (panel A), and MAPK/ASK-1-MAP2K4/7-JNK1/2 downstream pathway genes (panel B). Schematic that indicates the gene pathway (panel C) (Aurelian, 2005). Differences among groups: * denotes p ≤ 0.05, **p ≤ 0.005. Panel D presents a clustergram of these MAPK/ASK-1 signaling genes; groups H69 control, H69 exposed to peroxide, H69 cells cultured in Ov-Trx-1, and H69 cells cultured in Ov-Trx-1 and exposed to H2O2 (red and green in the heat map denote maximal and minimal gene expression, respectively). Immunoprecipitation/ western blot analysis with anti-Ov-Trx-1, anti-Hisx6, anti-ASK-1, and anti-β-actin. H69 cells were cultured in Ov-Trx-1, 5 µg/ml. Lysate of the cells (lane 1); flow-through (lane 2); eluate (lane 3) (panel E).
Fluke thioredoxin interacts with human apoptosis signal regulating kinase-1 complex
Western blot analysis was carried out targeting the starting fraction, flow-through and eluate from the immunoprecipitation. Antigens in the eluate reacted strongly to each of His X6 at 12 kDa, Ov-Trx-1 at 12 kDa, and ASK-1 at 70 kDa. By contrast, the antibody to β-actin failed to react with the eluted fraction but reacted to the starting material and to the flow through material (Fig. 5E). These findings indicated that Ov-Trx-1 had bound to ASK-1 and the complex was enriched in the immunoprecipitation targeting the 6XHis motif of the recombinant liver fluke thioredoxin.
Discussion
Evasion of apoptosis is a cardinal hallmarks of cancer (Hanahan and Weinberg, 2000). An antiapoptotic activity of thioredoxin has been described, in particular the interaction with ASK-1, apoptosis signal-regulating kinase-1 (Zhang et al., 2004). Apoptosis is down regulated by thioredoxin (Yoshioka et al., 2006) and down regulated in opisthorchiasis-induced CCA (Chusorn et al., 2013; Sripa, 2014). Previously we characterized an antioxidant enzyme, thioredoxin-1 from the carcinogenic human liver fluke, O. viverrini (Suttiprapa et al., 2012). Ov-Trx-1 is detected in all developmental stages of the parasite especially in adult worm, and Ov-Trx-1 is ubiquitously expressed in tissues and organs of adult flukes. In addition, secretions/excretions (ES) of the adult flukes include Ov-Trx-1, suggesting that the enzyme performs additional roles outside of the liver fluke, in protection of this pathogen against reactive oxygen species (ROS) of host origin during inflammation. Since oxidative DNA damage have been reported whereas apoptosis in not common in O. viverrini infected liver/ biliary system tissues (Sripa, 2003, 2014), it is feasible that fluke thioredoxin may modulate apoptosis of the biliary epithelial cells in carcinogenesis of cholangiocarcinoma induced by chronic opisthorchiasis. The mechanisms utilized by the liver fluke O. viverrini to induce anti-apoptosis phenomena have not been reported. Findings that revealed anti-apoptotic characteristics of the liver fluke thioredoxin-1 are described here.
Immunolcalization detected by confocal microscopy revealed that liver fluke thioredoxin entered cholangiocytes, confirming earlier reports with immuno-stained liver sections and bile ducts from experimentally infected hamsters (Suttiprapa et al., 2012). Within cholangiocytes, the liver fluke enzyme modulated apoptosis, as determined by flow cytometric, real time growth analysis, gene pathway qPCR analysis, and immunoprecipitations. At the outset, we investigated the potential anti-apoptotic activity of Ov-Trx-1; flow cytometry revealed that Ov-Trx-1 inhibited the peroxide-induced apoptosis. Moreover, cells incubated in Ov-Trx-1 were partially rescued from apoptosis as shown by xCELLigence. The xCELLigence system has become established to monitor cellular behavior in real time, including proliferation, apoptosis and the effect of anti-apoptotic drugs (Sun et al., 2014). Liver fluke thioredoxin partially but, significantly, rescued the cells from cell death. The effect was ablated when the denatured thioredoxin was tested, which indicated the oxidoreductase activity of the enzyme scavenged ROS and thereby relieved the oxidative stress in the cholangiocytes exposed to hydrogen peroxide.
Qantitative PCR findings revealed that H69 cells treated with Ov-Trx-1 down regulated pro-apoptotic genes, whereas anti-apoptotic genes were induced. Apoptosis signaling pathway genes that were down regulated included FASLG, CIDEA, TNFSF8, TNFRS and TRAF3. Moreover, downstream signaling molecules of ASK-1, including genes encoding key players of MAP2K 3,4,6,7 and MAPK P38K, JNK pathways were suppressed. Since mammalian thioredoxin binds and inactivates apoptosis signal-regulating kinase-1, ASK-1 (Liu and Min, 2002; Saitoh et al., 1998), we investigated whether Ov-Trx-1 might perform in a similar manner.
Immunoprecipitation revealed that Ov-Trx-1 bound to human ASK-1 in lysates of H69 cells. This conformed to the findings from the pathway expresion analyses where cholangiocytes undergoing apoptosis induced by H2O2 exhibited overexpression of genes in these pathways whereas in cholangiocytes in Ov-Trx-1 at the time of the H2O2 insult and oxidative stress, the expression was down regulated or modulated in both the MAPK/ASK-1-MAP2K3/6-P38K-α/δ and MAPK/ASK-1-MAP2K4/7-JNK1/2 signaling pathways.
As noted, human ASK-1 formed a hetero-oligomeric complex with Ov-Trx-1.. To protect the cell from oxidative stress-induced apoptosis, Ov-Trx-1 may directly scavenge oxygen radicals. Within the cytoplasm of the cholangiocyte, Ov-Trx-1 might increase the thioredoxin pool and supplement the endogenous cellular thioredoxin system to remove oxidants (Branco et al., 2014; Lu and Holmgren, 2014; Pannala and Dash, 2015). In addition, Ov-Trx-1 might indirectly neutralize pro-oxidizing agents by increasing redox potential by enhancement of internatization of L-cystine and elevation of intracellular glutathione (Iwata et al., 1997; Liu et al., 2000; Mitsui et al., 1992; Noguchi et al., 2005; Saitoh et al., 1998). On the other hand, the thio-dependent peroxidase, peroxiredoxin, exrts more potent activity against oxidation than thioredoxin (Nordberg and Arner, 2001); O. viverrini encodes a perioxredoxin (Young et al 2014. To conclude, liver fluke thioredoxin mediated anti-apoptosis phenomena in a model of oxidative stress in bile duct epithelium, apparently through the modulatory activity of thioredoxin for ASK-1. We speculate that Ov-Trx-1 antagonizes human-ASK-1 via the MAPK cascade, MAP3K-ASK-1/MAP2K-3, 4, 6, 7/MAPK-JNK, P38K, and hence may accelerate infection-induced carcinogenesis in the biliary tract. Future studies of hamsters experimental infected with O. viverrini in which the thioredoxin gene has been silenced (Thanasuwan et al., 2014) should facilitate functional studies of this hypothesis. Moreover, given that drugs that inhibit Trx/TrxR signaling systems have been developed (Mukherjee and Martin, 2008), similar approaches targeting liver fluke thioredoxin may provide leads for novel interventions for opisthorchiasis-induced bile duct cancer.
Supplementary Material
Highlights.
Secreted parasite enzyme may contribute to micro-environment conducive to helminth infection–induced carcinogenesis
Thrioredoxin-1 from liver fluke modulated oxidative stress-induced apoptosis in human cholangiocytes
Liver fluke thioredoxin bound to apoptosis signal-regulating kinase-1
Cholangiocytes cultured in liver fluke thioredoxin downregulated apoptotic genes in the MAP3K pathway, and upregulated anti-apoptosis-related genes
Acknowledgements
We thank Teresa Hawley and David Leitenberg for advice with flow cytometry, and Ian Toma for advice with chemiluminescence and imaging. PM is a Royal Golden Jubilee Ph.D. Program scholar, grant number PHD/0137/2550, in the laboratory of BS. Awards U01AI065871 (PJB, BS) and P50AI098639 (BS, PJB) from the National Institute of Allergy and Infectious Diseases, and CA155297 and CA164719 (GR, VHM, TL, PJB) from the National Cancer Institute, US National Institutes of Health (NIH) supported these studies. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. Journal of immunological methods. 1990;132:191–195. doi: 10.1016/0022-1759(90)90029-u. [DOI] [PubMed] [Google Scholar]
- An N, Kang Y. Thioredoxin and hematologic malignancies. Advances in cancer research. 2014;122:245–279. doi: 10.1016/B978-0-12-420117-0.00007-4. [DOI] [PubMed] [Google Scholar]
- Aurelian L. Cross talk of signaling and apoptotic cascades in the CNS: target for virus modulation. Frontiers in bioscience : a journal and virtual library. 2005;10:2776–2787. doi: 10.2741/1735. [DOI] [PubMed] [Google Scholar]
- Branco V, Godinho-Santos A, Goncalves J, Lu J, Holmgren A, Carvalho C. Mitochondrial thioredoxin reductase inhibition, selenium status, and Nrf-2 activation are determinant factors modulating the toxicity of mercury compounds. Free radical biology & medicine. 2014;73:95–105. doi: 10.1016/j.freeradbiomed.2014.04.030. [DOI] [PubMed] [Google Scholar]
- Carvalho AT, Fernandes PA, Ramos MJ. Theoretical study of the unusual protonation properties of the active site cysteines in thioredoxin. The journal of physical chemistry B. 2006;110:5758–5761. doi: 10.1021/jp053275f. [DOI] [PubMed] [Google Scholar]
- Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chusorn P, Namwat N, Loilome W, Techasen A, Pairojkul C, Khuntikeo N, Dechakhamphu A, Talabnin C, Chan-On W, Ong CK, et al. Overexpression of microRNA-21 regulating PDCD4 during tumorigenesis of liver fluke-associated cholangiocarcinoma contributes to tumor growth and metastasis. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34:1579–1588. doi: 10.1007/s13277-013-0688-0. [DOI] [PubMed] [Google Scholar]
- Correia da Costa JM, Vale N, Gouveia MJ, Botelho MC, Sripa B, Santos LL, Santos JH, Rinaldi G, Brindley PJ. Schistosome and liver fluke derived catecholestrogens and helminth associated cancers. Frontiers in genetics. 2014;5:444. doi: 10.3389/fgene.2014.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubman SA, Perrone RD, Lee DW, Murray SL, Rogers LC, Wolkoff LI, Mulberg AE, Cherington V, Jefferson DM. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. The American journal of physiology. 1994;266:G1060–G1070. doi: 10.1152/ajpgi.1994.266.6.G1060. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hattori K, Naguro I, Runchel C, Ichijo H. The roles of ASK family proteins in stress responses and diseases. Cell communication and signaling : CCS. 2009;7:9. doi: 10.1186/1478-811X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochemical and biophysical research communications. 2010;396:120–124. doi: 10.1016/j.bbrc.2010.03.083. [DOI] [PubMed] [Google Scholar]
- IARC. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100:1–441. [PMC free article] [PubMed] [Google Scholar]
- Iwata S, Hori T, Sato N, Hirota K, Sasada T, Mitsui A, Hirakawa T, Yodoi J. Adult T cell leukemia (ATL)-derived factor/human thioredoxin prevents apoptosis of lymphoid cells induced by L-cystine and glutathione depletion: possible involvement of thiolmediated redox regulation in apoptosis caused by pro-oxidant state. Journal of immunology. 1997;158:3108–3117. [PubMed] [Google Scholar]
- Jusakul A, Loilome W, Namwat N, Haigh WG, Kuver R, Dechakhamphu S, Sukontawarin P, Pinlaor S, Lee SP, Yongvanit P. Liver fluke-induced hepatic oxysterols stimulate DNA damage and apoptosis in cultured human cholangiocytes. Mutation research. 2012;731:48–57. doi: 10.1016/j.mrfmmm.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Kaewkes S. Taxonomy and biology of liver flukes. Acta Trop. 2003;88:177–186. doi: 10.1016/j.actatropica.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Ke N, Wang X, Xu X, Abassi YA. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods in molecular biology. 2011;740:33–43. doi: 10.1007/978-1-61779-108-6_6. [DOI] [PubMed] [Google Scholar]
- Laha T, Pinlaor P, Mulvenna J, Sripa B, Sripa M, Smout MJ, Gasser RB, Brindley PJ, Loukas A. Gene discovery for the carcinogenic human liver fluke, Opisthorchis viverrini. BMC genomics. 2007;8:189. doi: 10.1186/1471-2164-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le XF, Mao W, He G, Claret FX, Xia W, Ahmed AA, Hung MC, Siddik ZH, Bast RC., Jr The role of p27(Kip1) in dasatinib-enhanced paclitaxel cytotoxicity in human ovarian cancer cells. Journal of the National Cancer Institute. 2011;103:1403–1422. doi: 10.1093/jnci/djr280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Nishitoh H, Ichijo H, Kyriakis JM. Activation of apoptosis signalregulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Molecular and cellular biology. 2000;20:2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circulation research. 2002;90:1259–1266. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- Lu J, Holmgren A. The thioredoxin antioxidant system. Free radical biology & medicine. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- Mairiang E, Laha T, Bethony JM, Thinkhamrop B, Kaewkes S, Sithithaworn P, Tesana S, Loukas A, Brindley PJ, Sripa B. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol Int. 2012:208–211. doi: 10.1016/j.parint.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairiang E, Mairiang P. Clinical manifestation of opisthorchiasis and treatment. Acta Trop. 2003;88:221–227. doi: 10.1016/j.actatropica.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Mitsui A, Hirakawa T, Yodoi J. Reactive oxygen-reducing and protein-refolding activities of adult T cell leukemia-derived factor/human thioredoxin. Biochemical and biophysical research communications. 1992;186:1220–1226. doi: 10.1016/s0006-291x(05)81536-0. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Martin SG. The thioredoxin system: a key target in tumour and endothelial cells. The British journal of radiology. 2008;81:S57–S68. doi: 10.1259/bjr/34180435. Spec No 1. [DOI] [PubMed] [Google Scholar]
- Mulvenna J, Sripa B, Brindley PJ, Gorman J, Jones MK, Colgrave ML, Jones A, Nawaratna S, Laha T, Suttiprapa S, et al. The secreted and surface proteomes of the adult stage of the carcinogenic human liver fluke Opisthorchis viverrini. Proteomics. 2010;10:1063–1078. doi: 10.1002/pmic.200900393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annual review of immunology. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochimica et biophysica acta. 2013;1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Takeda K, Matsuzawa A, Saegusa K, Nakano H, Gohda J, Inoue J, Ichijo H. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. The Journal of biological chemistry. 2005;280:37033–37040. doi: 10.1074/jbc.M506771200. [DOI] [PubMed] [Google Scholar]
- Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free radical biology & medicine. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Pannala VR, Dash RK. Mechanistic characterization of the thioredoxin system in the removal of hydrogen peroxide. Free radical biology & medicine. 2015;78:42–55. doi: 10.1016/j.freeradbiomed.2014.10.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Gores GJ, Patel T. Lipopolysaccharide induces cholangiocyte proliferation via an interleukin-6-mediated activation of p44/p42 mitogen-activated protein kinase. Hepatology. 1999;29:1037–1043. doi: 10.1002/hep.510290423. [DOI] [PubMed] [Google Scholar]
- Petney TN, Andrews RH, Saijuntha W, Wenz-Mucke A, Sithithaworn P. The zoonotic, fish-borne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. International journal for parasitology. 2013;43:1031–1046. doi: 10.1016/j.ijpara.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plieskatt JL, Deenonpoe R, Mulvenna JP, Krause L, Sripa B, Bethony JM, Brindley PJ. Infection with the carcinogenic liver fluke Opisthorchis viverrini modifies intestinal and biliary microbiome. Faseb J. 2013;27:4572–4584. doi: 10.1096/fj.13-232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta C, Riboldi E, Sica A. Mechanisms linking pathogens-associated inflammation and cancer. Cancer letters. 2011;305:250–262. doi: 10.1016/j.canlet.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Powis G, Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Current opinion in pharmacology. 2007;7:392–397. doi: 10.1016/j.coph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. The EMBO journal. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithithaworn P, Andrews RH, Nguyen VD, Wongsaroj T, Sinuon M, Odermatt P, Nawa Y, Liang S, Brindley PJ, Sripa B. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int. 2012;61:10–16. doi: 10.1016/j.parint.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smout MJ, Laha T, Mulvenna J, Sripa B, Suttiprapa S, Jones A, Brindley PJ, Loukas A. A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells. PLoS pathogens. 2009;5:e1000611. doi: 10.1371/journal.ppat.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soga M, Matsuzawa A, Ichijo H. Oxidative Stress-Induced Diseases via the ASK1 Signaling Pathway. International journal of cell biology. 2012;2012:439587. doi: 10.1155/2012/439587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B. Pathobiology of opisthorchiasis: an update. Acta tropica. 2003;88:209–220. doi: 10.1016/j.actatropica.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Sripa B. Infectious Diseases and Tropical Disease Pathology: SY16-3 Opisthorchiasis: from pathogenesis to control. Pathology. 2014;46(Suppl 2):S28. [Google Scholar]
- Sripa B, Bethony JM, Sithithaworn P, Kaewkes S, Mairiang E, Loukas A, Mulvenna J, Laha T, Hotez PJ, Brindley PJ. Opisthorchiasis and Opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop. 2011;120(Suppl 1):S158–S168. doi: 10.1016/j.actatropica.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, Bethony JM, Loukas A. The tumorigenic liver fluke Opisthorchis viverrini--multiple pathways to cancer. Trends Parasitol. 2012;28:395–407. doi: 10.1016/j.pt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S. Localisation of parasite antigens and inflammatory responses in experimental opisthorchiasis. International journal for parasitology. 2000;30:735–740. doi: 10.1016/s0020-7519(00)00054-0. [DOI] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B, et al. Liver fluke induces cholangiocarcinoma. PLoS medicine. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Lai X, Huang X, Zeng Y. Protective effects of ginsenoside Rg1 on astrocytes and cerebral ischemic-reperfusion mice. Biological & pharmaceutical bulletin. 2014;37:1891–1898. doi: 10.1248/bpb.b14-00394. [DOI] [PubMed] [Google Scholar]
- Suttiprapa S, Loukas A, Laha T, Wongkham S, Kaewkes S, Gaze S, Brindley PJ, Sripa B. Characterization of the antioxidant enzyme, thioredoxin peroxidase, from the carcinogenic human liver fluke, Opisthorchis viverrini. Molecular and biochemical parasitology. 2008;160:116–122. doi: 10.1016/j.molbiopara.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttiprapa S, Matchimakul P, Loukas A, Laha T, Wongkham S, Kaewkes S, Brindley PJ, Sripa B. Molecular expression and enzymatic characterization of thioredoxin from the carcinogenic human liver fluke Opisthorchis viverrini. Parasitology international. 2012;61:101–106. doi: 10.1016/j.parint.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanasuwan S, Piratae S, Brindley PJ, Loukas A, Kaewkes S, Laha T. Suppression of aquaporin, a mediator of water channel control in the carcinogenic liver fluke, Opisthorchis viverrini. Parasit Vectors. 2014;7:224. doi: 10.1186/1756-3305-7-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Wongratanacheewin S, Bunnag D, Vaeusorn N, Sirisinha S. Characterization of humoral immune response in the serum and bile of patients with opisthorchiasis and its application in immunodiagnosis. The American journal of tropical medicine and hygiene. 1988;38:356–362. doi: 10.4269/ajtmh.1988.38.356. [DOI] [PubMed] [Google Scholar]
- Yoshioka J, Schreiter ER, Lee RT. Role of thioredoxin in cell growth through interactions with signaling molecules. Antioxidants & redox signaling. 2006;8:2143–2151. doi: 10.1089/ars.2006.8.2143. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang R, Luo Y, D'Alessio A, Pober JS, Min W. AIP1/DAB2IP, a novel member of the Ras-GAP family, transduces TRAF2-induced ASK1-JNK activation. The Journal of biological chemistry. 2004;279:44955–44965. doi: 10.1074/jbc.M407617200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.