Abstract
Animals use thermosensory systems to achieve optimal temperatures for growth and reproduction and to avoid damaging extremes. Thermoregulation is particularly challenging for small animals like the fruit fly Drosophila melanogaster, whose body temperature rapidly changes in response to environmental temperature fluctuation. Recent work has uncovered some of the key molecules mediating fly thermosensation, including the Transient Receptor Potential (TRP) channels TRPA1 and Painless, and the Gustatory Receptor Gr28b, an unanticipated thermosensory regulator normally associated with a different sensory modality. There is also evidence the Drosophila phototransduction cascade may have some role in thermosensory responses. Together, the fly’s diverse thermosensory molecules act in an array of functionally distinct thermosensory neurons to drive a suite of complex, and often exceptionally thermosensitive, behaviors.
Temperature sensation in Drosophila
Temperature affects all aspects of physiology, and animals use their thermosensory systems to achieve optimal temperatures for growth and reproduction and to avoid damaging thermal extremes1. Thermoregulation is particularly challenging for small animals like the fruit fly Drosophila melanogaster (weighing < 2 mg), which can reach undesirable body temperatures within seconds of environmental temperature fluctuation or sunlight exposure2, 3. Drosophila thermosensory responses are sensitive and robust, with milli-degree per second temperature changes triggering readily assayed behavioral responses4. The availability of clear behavioral readouts, together with Drosophila’s genetic toolkit and the quantitative nature of temperature as a stimulus, make fly thermosensation a powerful system for studying fundamental issues in sensory processing and behavior. In fact, the developing fly (the larva) and the adult fly provide two complementary systems. Both are exquisitely thermosensitive5, 6, but the cells, neural pathways and loco-motor strategies involved are different. Each has unique features: the larva’s simpler anatomy aids analysis of circuitry and behavioral strategy, while the adult’s rich behavioral repertoire facilitates studying the integration of temperature with other inputs.
Like other animals, flies possess multiple classes of thermoreceptors7-9. Thermoreceptors fall into four basic classes: warm receptors respond to innocuous (moderate) warming, cool receptors to innocuous cooling, high-temperature nociceptors to noxious (damaging) heat, and low-temperature nociceptors to noxious cold. Flies have at least three classes of thermoreceptors7-9 (cold nociception awaits analysis), and multiple subtypes of some classes10. This complexity raises several questions. What molecular mechanisms underlie the thermosensitivity of various thermoreceptors? How is thermoreceptor input processed to yield coherent responses? How can thermosensation be modified by experience, physiological status or other sensory input to sculpt appropriate responses? In this review, we focus on progress made toward answering these questions.
Innocuous thermosensation in the adult: multiple sensors driving diverse responses
Flies continually encounter innocuous temperature variations that affect their body temperature and can have significant long-term survival impacts 11. Four sets of neurons that act as innocuous thermosensors have been identified in the adult fly, two warm-responsive and two cool-responsive, and together they mediate multiple aspects of thermosensory behavior 8-10, 12 (Fig. 1).
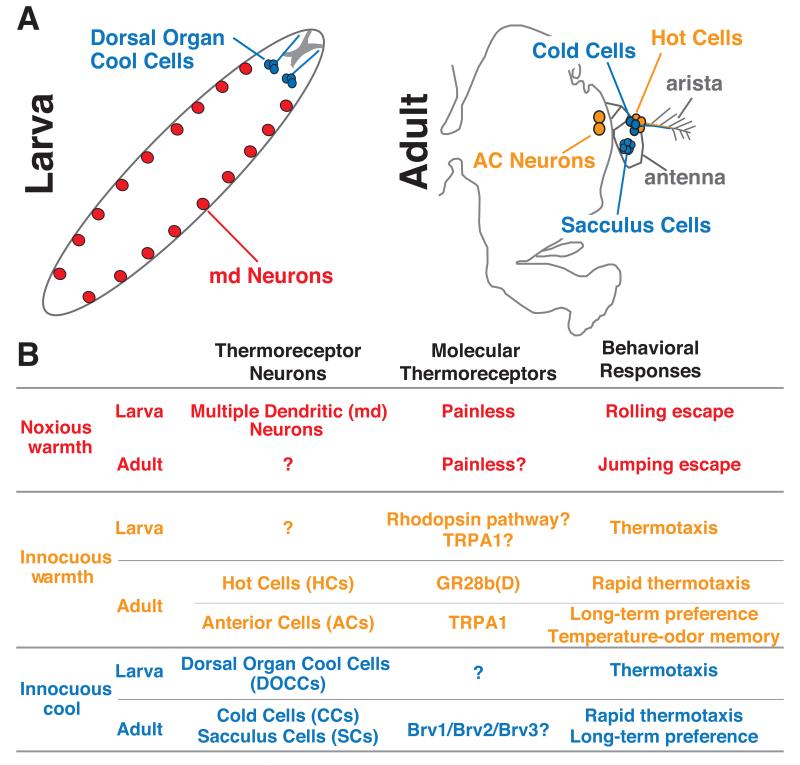
Figure 1. Drosophila thermosensory systems.
A. Thermosensory neurons of the larval (left) and adult (right) nervous systems. B. Molecular thermoreceptors and associated behavioral responses for each class of thermosensory neuron.
The Anterior Cell (AC) neurons inside the head8 and the Hot Cell (HC) neurons in the arista9 both function as innocuous warmth sensors, responding to temperatures above ~25°C (the fly’s preferred temperature)(Fig. 1). These cells sense temperature via different mechanisms and drive distinct behaviors. At the molecular level, the AC neurons detect warming using the Transient Receptor Potential (TRP) protein TRPA18. TRPs are an evolutionarily conserved family of cation channels, of which a subset, the thermoTRPs, function as thermoreceptors from flies to humans13-15. TRPA1 mediates the warmth-activation of AC neurons above ~25°C 8, and can act as a warmth-activated ion channel in heterologous cells8, 16, 17. The AC neurons also exhibit a second, TRPA1-independent warmth response at temperatures above ~32°C18. This second response is non-cell-autonomous and requires input from as yet unidentified antennal warmth sensors. Thus the AC neurons act both as thermosensors as well as interneurons that integrate thermosensory input.
The HC neurons sense warmth differently than the AC neurons, relying on the Gustatory Receptor (Gr) Gr28b(D), rather than a thermoTRP, to drive warmth responses10. Gr’s conventionally participate in insect chemoreception19, but ectopic Gr28b(D) expression confers warmth-sensitivity upon multiple cell types, indicating Gr28b(D) is a thermoreceptor10. At a mechanistic level, some Gr’s act as ion channels, but others signal through G proteins20, 21. The breadth of cell types in which Gr28b(D) mediates warmth-sensing suggests it may be a warmth-responsive channel, but more concrete proof awaits Gr28b(D)’s successful expression in heterologous cells.
The HC and AC neurons detect similar temperatures, but project to distinct brain regions8, 9, 22 and drive distinct behaviors. The peripheral HC neurons mediate rapid (<1 minute) warmth avoidance on steep thermal gradients (e.g., 5°C/cm)10, while the internal AC neurons mediate the slow (over ~20 minutes) warmth avoidance elicited by shallowerthermal gradients (e.g., 0.5°C/cm)10. Thus, HC neurons may primarily sense changing external thermal environments, and the AC neurons internal body temperature. In addition, the AC neurons mediate the aversive associative memory formed by pairing warmth with odor12. Such memory formation involves a cluster of ~20 dopaminergic neurons in the fly protocerebrum, which appear to be contacted and potentially regulated by the AC neurons12. Together, these findings begin to reveal surprising complexity in how warmth is sensed and affects behavior in the fly.
Drosophila also contain two sets of neurons responsive to innocuous cooling: the Cold Cells (CCs), which form thermosensor pairs with the HCs in the arista, and the Sacculus Cells (SCs), located in a pouch within the antenna called the sacculus9 (Fig. 1). These antennal cool sensors are important for innocuous cool responses, as their inhibition or surgical removal disrupts cool avoidance9,8. Three TRP channels, Brivido1 (Brv1), Brv2 and Brv3, participate in cool sensing, as brv family mutants disrupt cool avoidance and reduce CC neuron cool-sensitivity9. However, attempts to demonstrate thermoreceptor function of Brvs via ectopic expression were unsuccessful9, suggesting they may not be thermoreceptors. (Many TRPs are not thermoTRPs, but support neuronal function in other ways13.). At the circuit level, innocuous cooling stimulates dopaminergic neurons 23 and dopaminergic signaling in the mushroom bodies is critical for cool avoidance24, suggesting that cool-sensing and warmth-sensing both act through modulating dopaminergic signaling in the mushroom bodies. As thermal preference exhibits circadian variation, input from the circadian clock may also interact with these pathways25.
Innocuous thermosensation in the larva: from sensors to navigational strategies
As in the adult, innocuous warmth sensation in the larva relies on TRPA126. TRPA1 was initially found to mediate warmth avoidance of temperatures over ~30°C26, and subsequently as low as ~20°C 27, 28. Surprisingly, in the lower temperature range, mutants in Drosophila phototransduction proteins, including rhodopsin and its G protein/phospholipase C cascade, exhibited behavioral phenotypes similar to TrpA1 mutants27, 28. But as the thermoreceptor neurons controlling larval warmth avoidance are unknown, whether TRPA1 and/or rhodopsin function in thermoreceptors and to what extent they affect warmth detection versus another process is unclear.
While direct involvement in temperature detection remains uncertain, rhodopsin’s involvement in thermotaxis has generated interest in possible similarities between thermo- and photo-transduction. In one scenario, the rhodopsin pathway would detect temperature and TRPA1 would act as a transduction channel27, 28. However, this notion is controversial29. Not only are rhodopsins reported to be highly thermostable, thermally isomerizing an estimated once per ~1000 years at 24°C29, ectopic expression of rhodopsin has not been demonstrated to confer thermosensitivity or to activate TRPA1. An alternative would be TRPA1 as thermosensor. While TRPA1 can function as a warmth-responsive thermoTRP8, 16,17, the temperatures yielding robust responses in oocytes are several degrees warmer than the lowest temperature driving thermotaxis30. However, thermoTRPs do not have discrete or fixed temperature thresholds31, but rather exhibit graded responses to temperature modulated by cell signaling13. In addition, other factors contribute to a thermoreceptor neuron’s response properties, including the cell’s intrinsic excitability and its level of thermoTRP expression. Thus, TRPA1 could act as a thermoTRP in this system, with rhodopsin dynamically modulating its thermal sensitivity. Whether the rhodopsin pathway, TRPA1 or another molecule provides the thermosensor, interesting biology is emerging from the analysis of warmth sensing.
The molecular basis of larval innocuous cool detection is also largely unknown. Multiple TRPs, including TRPL and Inactive, are implicated in cool-responsive behavior, but none have been directly linked to the detection of cool temperatures32, 33. In contrast, a detailed understanding of larval responses to cooling is emerging at the behavioral and cellular levels4, 6, 34. At the behavioral level, larval thermotaxis involves the alternation between two behavioral states: forward movement (running) and head-sweeping (linked to turning)6, 34. Running is powered by peristaltic waves that propagate from tail to head, propelling the larva forward, while head-sweeping involves the asymmetric contraction of the animal’s anterior segments, sweeping the larval anterior from side to side34. When navigating on a gradient of cooler than preferred temperatures, larva tend to move toward more favorable (warmer) temperatures by regulating run length and by modulating the direction of the turns they make between runs6. Run lengths tend to be shorter when moving toward unfavorable (cooler) temperatures and longer when moving toward favorable (warmer) temperature. In this way, the larva executes a biased random walk driving net movement in the favorable direction6, similar to bacterial chemotaxis35. But unlike a bacterium, whose runs are punctuated by random reorientations, the larva also uses the head-sweeping program to execute biased turns that tend to reorient the animal in favorable run directions. These strategies also underlie larval avoidance of innocuously warmth6, and they resemble strategies driving larval phototaxis and chemotaxis36, suggesting multiple sensory pathways feed into similar neural pathways.
At the cellular level, initial studies suggested the cool receptors driving thermotaxis might reside in the terminal organ, a pair of sensory ganglia at the larval anterior37. However, the cell-specific promoter used to establish the terminal organ’s importance, GH86-Gal4, also drives gene expression in sensory neurons of the nearby dorsal organs, clouding the picture4. Surprisingly, severing nerves from the terminal organs to the brain has no effect on innocuous cool avoidance, while severing the nerves from the dorsal organs completely eliminates the response, suggesting the dorsal organ houses the relevant thermoreceptors4. Indeed, each dorsal organ contains three highly cool-responsive neurons (Fig. 1). Cell-specific inhibition of these neurons eliminates cool avoidance behavior, while optogenetic activation of these neurons evokes the thermotactic behavioral program4. Thus, these thermoreceptors are critical for innocuous thermosensation.
The dorsal organ’s cool receptors have two important physiological features. First, their thermosensitivity (they exhibit significant calcium responses upon ~0.005°C/sec fluctuations4) places them among the most sensitive biological thermoreceptors known, such as those of the rattlesnake pit organ38. In addition, while these neurons respond to cooling, they can operate at very warm temperatures4. When cooled from a starting temperature in the mid-30s, two of the three cells begin responding near 32°C, warmer than the first instar larva’s preferred range of 22-28°C. This places the cool receptors with many other thermoreceptors more sensitive to the direction and rate of temperature change than its absolute value39. Perhaps the name “cooling receptors” might be more apt.
High-temperature nociception: sensitive and sensitized to harm
The first thermoreceptors identified in Drosophila were the multiple dendritic (md) neurons (Fig. 1), a set of high-temperature nociceptors that tile the larval body wall and trigger a characteristic rolling escape response when activated by temperatures over ~39°C 7. Two TRP channels, TRPA1 and Painless are critical for this response7, 40. Painless appears to function as a high-temperature-responsive thermoTRP exhibiting robust activity at temperatures above ~42°C in heterologous cells41. In contrast, TRPA1’s role is enigmatic. The TRPA1 gene encodes not just one channel isoform, but rather multiple TRPA1 channel isoforms42, 43. These isoforms range from the highly warmth-responsive isoform present in the AC neurons to thermally insensitive isoforms present in TRPA1-dependent chemoreceptors42, 43. Interestingly, Zhong et al. found that a TRPA1 isoform with no detectable thermosensitivity could support high-temperature nociception when expressed in the md neurons, suggesting that TRPA1 is not acting as thermoreceptor in these neurons43. So while Painless appears to be a high-temperature receptor, TRPA1’s role in high-temperature nociception remains mysterious. Painless and TRPA1 are also implicated in high-temperature nociception in adult flies40, 44, but whether the mechanisms are similar to those in the larva is unknown.
From a biomedical perspective, nociceptor sensitization after tissue damage contributes to pain and inflammation in mammals45. Sensitization to noxious stimulation can be classified in terms of allodynia, a decrease in the stimulus threshold sensed as noxious, and hyperalgesia, an increase in the magnitude of the response to a normally noxious stimulus. The md nociceptors have been used as a potential model for investigating these processes. Fly larvae exhibit both types of sensitization to heat after UV-elicited tissue damage46, and two evolutionarily conserved signaling pathways, the Tumour Necrosis Factor (TNF)46 and Hedgehog (Hh)47 pathways, are known to be involved in this sensitization. TNF signaling, also involved in mammalian nociceptor sensitization 48, was specifically implicated in larval allodynia. UV damage to epithelial cells elicited production of the Drosophila TNF Eiger, which sensitizes the md neurons via the TNF receptor Wengen46. Hedgehog signaling, on the other hand, promotes both allodynia and hyperalgesia47. Intriguingly, allodynia induction by both Hh and TNF signaling appear to specifically involve Painless, while Hh-induced hyperalgesia specifically involves TRPA147. The complexities of the Painless and TrpA1 genes, which each encode multiple channel isoforms42, 43, 49, raise the possibility that gene regulatory mechanisms could contribute to these sensitization responses.
Looking ahead
The last few years have seen significant advances in identifying the molecules, cells, and behavioral strategies mediating thermosensation in Drosophila. Still, our understanding of thermosensation, in flies and in other animals, lags behind our knowledge of other senses like vision, olfaction and gustation. In the fly, many of the key thermosensory neurons and thermoreceptor molecules remain unidentified, and for those that have been identified, we have little insight into how they perform their thermosensory functions. For example, whether the exceptional sensitivity of thermosensors found in the larval dorsal organ (or the rattlesnake pit organ) reflects specific signal amplification pathways, as in vertebrate phototransduction50, is unknown, as are the mechanisms by which temperature activates molecular thermoreceptors31. Insights are needed on multiple levels, from molecular biology to sensory physiology to biophysics. At the circuit level, while some interneurons responsive to thermoreceptor activation have been characterized, the explosion of genetic tools for marking and manipulating identified neurons in the fly brain51 promises to transform our understanding of how thermosensory information is processed and interfaces with other sensory inputs to modulate behavior. In an ecological context, an understanding of thermosensation Drosophila melanogaster provides an entry point for studying variation within and between the many species of Drosophila, from latitudinal clines of Drosophila melanogaster, to extremophiles like the desert-dwelling Drosophila mojavensis. Such work will increase our knowledge of how animal distributions emerge, and provide insights and tools to help monitor, understand and perhaps predict the impacts of climate change on other insects, from disease vectors to agricultural pests52. Although Drosophila has little thermal mass, the study of its thermosensory behavior permits experimental access to many weighty topics.
Acknowledgments
B.B. is supported by NINDS 5T32NS007292-28. PAG is supported by NIMH R01MH094721 and NIGMS P01 GM103770.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dell AI, Pawar S, Savage VM. Systematic variation in the temperature dependence of physiological and ecological traits. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10591–10596. doi: 10.1073/pnas.1015178108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garrity PA, Goodman MB, Samuel AD, Sengupta P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes & development. 2010;24:2365–2382. doi: 10.1101/gad.1953710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heinrich B. The Hot-Blooded Insects: Strategies and Mechanisms of Thermoregulation. Harvard University Press; 1993. [Google Scholar]
- 4.Klein M, Afonso B, Vonner A, Hernandez-Nunez L, Berck M, Tabone CJ, Kane EA, Pieribone V, Nitabach MN, Cardona A, Zlatic M, Sprecher SG, Gershow M, Garrity P, Samuel ADT. Sensory determinants of behavioral dynamics in Drosophila thermotaxis. Proceedings of the National Academy of Sciences of the United States of America. doi: 10.1073/pnas.1416212112. in press. ** In this paper, the authors identify cool-responsive thermosensory neurons in the larval dorsal organ, and demonstrate that these neurons exhibit exceptional thermosensitivity which closely matches the sensitivity and dynamics of thermotactic behavior. Cell-specific inhibition and optogenetic activation experiments, combined with reverse-correlation analysis, demonstrate that these neurons are essential for driving cool avoidance behaviors
- 5.Sayeed O, Benzer S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6079–6084. doi: 10.1073/pnas.93.12.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo L, Gershow M, Rosenzweig M, Kang K, Fang-Yen C, Garrity PA, Samuel AD. Navigational decision making in Drosophila thermotaxis. J Neurosci. 2010;30:4261–4272. doi: 10.1523/JNEUROSCI.4090-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tracey WD, Jr., Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 8.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallio M, Ofstad TA, Macpherson LJ, Wang JW, Zuker CS. The coding of temperature in the Drosophila brain. Cell. 2011;144:614–624. doi: 10.1016/j.cell.2011.01.028. **This manuscript describes populations of thermosensory neurons in the antenna that drive thermotactic behavior in the adult fly. The authors demonstrate that the warm-responsive Hot Cells are specifically required for warmth responses, while the cool-responsive Cold Cells and Sacculus Cells are specifically required for cool responses.
- 10.Ni L, Bronk P, Chang EC, Lowell AM, Flam JO, Panzano VC, Theobald DL, Griffith LC, Garrity PA. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature. 2013;500:580–584. doi: 10.1038/nature12390. **This manuscript identifies the Gustatory Receptor Gr28b(D) as new class of warmth receptor essential for Hot Cell function. The paper also demonstrates that flies possess two molecularly and functionally distinct pathways for warmth detection, one peripheral and one internal. The Gr28b-dependent Hot Cells in the periphery drive rapid warmth avoidance in response to steep thermal gradients. The TRPA1-dependent Anterior Cell neurons, located internally, drive slower responses to shallower thermal gradients.
- 11.Dillon ME, Wang G, Huey RB. Global metabolic impacts of recent climate warming. Nature. 2010;467:704–706. doi: 10.1038/nature09407. [DOI] [PubMed] [Google Scholar]
- 12.Galili DS, Dylla KV, Ludke A, Friedrich AB, Yamagata N, Wong JY, Ho CH, Szyszka P, Tanimoto H. Converging circuits mediate temperature and shock aversive olfactory conditioning in Drosophila. Current biology: CB. 2014;24:1712–1722. doi: 10.1016/j.cub.2014.06.062. **This manuscript finds that the TRPA1-dependent Anterior Cell neurons, required for long-term warmth avoidance, also mediate the formation of a long-term aversive associative memory created when odor is paired with warming. The authors also demonstrate that the Anterior Cell neurons contact dopaminergic neurons in the protocerebrum and identify a specific subset of ~20 dopaminergic neurons required for the response.
- 13.Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacological reviews. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zakharian E, Cao C, Rohacs T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J Neurosci. 2010;30:12526–12534. doi: 10.1523/JNEUROSCI.3189-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron. 2013;77:667–679. doi: 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, Patapoutian A, Jegla T. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Schupp M, Zurborg S, Heppenstall PA. Residues in the pore region of Drosophila transient receptor potential A1 dictate sensitivity to thermal stimuli. The Journal of physiology. 2013;591:185–201. doi: 10.1113/jphysiol.2012.242842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang X, Platt MD, Lagnese CM, Leslie JR, Hamada FN. Temperature integration at the AC thermosensory neurons in Drosophila. J Neurosci. 2013;33:894–901. doi: 10.1523/JNEUROSCI.1894-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benton R. Chemical sensing in Drosophila. Current opinion in neurobiology. 2008;18:357–363. doi: 10.1016/j.conb.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Sato K, Tanaka K, Touhara K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11680–11685. doi: 10.1073/pnas.1019622108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Ward A, Gao J, Dong Y, Nishio N, Inada H, Kang L, Yu Y, Ma D, Xu T, Mori I, Xie Z, Xu XZ. C. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog. Nature neuroscience. 2010;13:715–722. doi: 10.1038/nn.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shih HW, Chiang AS. Anatomical characterization of thermosensory AC neurons in the adult Drosophila brain. Journal of neurogenetics. 2011;25:1–6. doi: 10.3109/01677063.2011.571323. [DOI] [PubMed] [Google Scholar]
- 23.Tomchik SM. Dopaminergic neurons encode a distributed, asymmetric representation of temperature in Drosophila. J Neurosci. 2013;33:2166–2176a. doi: 10.1523/JNEUROSCI.3933-12.2013. *Along with ref. 24, this manuscript investigates the role of dopaminergic signaling in cool responses. The author demonstrates that a subset of the PPL1 cluster of dopaminergic neurons (which innervate the mushroom body vertical lobes) respond to cooling, but not to warming.
- 24.Bang S, Hyun S, Hong ST, Kang J, Jeong K, Park JJ, Choe J, Chung J. Dopamine signalling in mushroom bodies regulates temperature-preference behaviour in Drosophila. PLoS genetics. 2011;7:e1001346. doi: 10.1371/journal.pgen.1001346. * Along with ref. 23, this manuscript investigates the role of dopaminergic signaling in cool responses. The authors demonstrate a requirement for dopaminergic signaling in long-term cool avoidance behavior, and find that the mushroom body Kenyon cells are an important target for dopamine in the response.
- 25.Kaneko H, Head LM, Ling J, Tang X, Liu Y, Hardin PE, Emery P, Hamada FN. Circadian rhythm of temperature preference and its neural control in Drosophila. Current biology: CB. 2012;22:1851–1857. doi: 10.1016/j.cub.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes & development. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon Y, Shim HS, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nature neuroscience. 2008;11:871–873. doi: 10.1038/nn.2170. [DOI] [PubMed] [Google Scholar]
- 28.Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science. 2011;331:1333–1336. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- 29.Minke B, Peters M. Cell biology. Rhodopsin as thermosensor? Science. 2011;331:1272–1273. doi: 10.1126/science.1203482. [DOI] [PubMed] [Google Scholar]
- 30.Fowler MA, Montell C. Drosophila TRP channels and animal behavior. Life sciences. 2013;92:394–403. doi: 10.1016/j.lfs.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clapham DE, Miller C. A thermodynamic framework for understanding temperature sensing by transient receptor potential (TRP) channels. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19492–19497. doi: 10.1073/pnas.1117485108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenzweig M, Kang K, Garrity PA. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14668–14673. doi: 10.1073/pnas.0805041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon Y, Shen WL, Shim HS, Montell C. Fine thermotactic discrimination between the optimal and slightly cooler temperatures via a TRPV channel in chordotonal neurons. J Neurosci. 2010;30:10465–10471. doi: 10.1523/JNEUROSCI.1631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lahiri S, Shen K, Klein M, Tang A, Kane E, Gershow M, Garrity P, Samuel AD. Two alternating motor programs drive navigation in Drosophila larva. PloS one. 2011;6:e23180. doi: 10.1371/journal.pone.0023180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macnab RM, Koshland DE., Jr. The gradient-sensing mechanism in bacterial chemotaxis. Proceedings of the National Academy of Sciences of the United States of America. 1972;69:2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez-Marin A, Partoune N, Stephens GJ, Louis M, Brembs B. Automated tracking of animal posture and movement during exploration and sensory orientation behaviors. PloS one. 2012;7:e41642. doi: 10.1371/journal.pone.0041642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Yermolaieva O, Johnson WA, Abboud FM, Welsh MJ. Identification and function of thermosensory neurons in Drosophila larvae. Nature neuroscience. 2003;6:267–273. doi: 10.1038/nn1009. [DOI] [PubMed] [Google Scholar]
- 38.Bullock TH, Diecke FP. Properties of an infra-red receptor. The Journal of physiology. 1956;134:47–87. doi: 10.1113/jphysiol.1956.sp005624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gingl E, Hinterwirth A, Tichy H. Sensory Representation of Temperature in Mosquito Warm and Cold Cells. Journal of neurophysiology. 2005;94:176–185. doi: 10.1152/jn.01164.2004. [DOI] [PubMed] [Google Scholar]
- 40.Neely GG, Keene AC, Duchek P, Chang EC, Wang QP, Aksoy YA, Rosenzweig M, Costigan M, Woolf CJ, Garrity PA, Penninger JM. TrpA1 regulates thermal nociception in Drosophila. PloS one. 2011;6:e24343. doi: 10.1371/journal.pone.0024343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sokabe T, Tsujiuchi S, Kadowaki T, Tominaga M. Drosophila painless is a Ca2+-requiring channel activated by noxious heat. J Neurosci. 2008;28:9929–9938. doi: 10.1523/JNEUROSCI.2757-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang K, Panzano VC, Chang EC, Ni L, Dainis AM, Jenkins AM, Regna K, Muskavitch MA, Garrity PA. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature. 2012;481:76–80. doi: 10.1038/nature10715. *This manuscript, along with ref. 43, identifies functionally distinct isoforms of TRPA1. The authors demonstrate that the cell-specific expression of a warmth-insensitive isoform of TRPA1 in chemosensory neurons permits TRPA1 to act as a noxious chemical detector in these neurons without interference from the warmth sensitivity of other TRPA1 isoforms. The unique N-terminus of the chemosensory neuron isoform is found to inhibit the channel’s thermosensitivity.
- 43.Zhong L, Bellemer A, Yan H, Honjo K, Robertson J, Hwang RY, Pitt GS, Tracey WD. Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP channel. Cell Reports. 2012 doi: 10.1016/j.celrep.2011.11.002. doi:10.1016/j.celrep.2011.11.002. *This manuscript, along with ref. 42, identifies functionally distinct isoforms of TRPA1. The authors show that a warmth-insensitive isoform of TRPA1 can rescue the high-temperature nociception defect of a TRPA1 mutant without interference from the responsiveness to milder warming exhibited by other TRPA1 isoforms. This manuscript identifies isoform-specific variation within TRPA1’s ankyrin repeats as a modulator of channel thermosensitivity.
- 44.Xu SY, Cang CL, Liu XF, Peng YQ, Ye YZ, Zhao ZQ, Guo AK. Thermal nociception in adult Drosophila: behavioral characterization and the role of the painless gene. Genes Brain Behav. 2006;5:602–613. doi: 10.1111/j.1601-183X.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 45.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babcock DT, Landry C, Galko MJ. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Current biology: CB. 2009;19:799–806. doi: 10.1016/j.cub.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babcock DT, Shi S, Jo J, Shaw M, Gutstein HB, Galko MJ. Hedgehog signaling regulates nociceptive sensitization. Current biology: CB. 2011;21:1525–1533. doi: 10.1016/j.cub.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leung L, Cahill CM. TNF-alpha and neuropathic pain--a review. Journal of neuroinflammation. 2010;7:27. doi: 10.1186/1742-2094-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwang RY, Stearns NA, Tracey WD. The ankyrin repeat domain of the TRPA protein painless is important for thermal nociception but not mechanical nociception. PloS one. 2012;7:e30090. doi: 10.1371/journal.pone.0030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arshavsky VY, Burns ME. Current understanding of signal amplification in phototransduction. Cellular logistics. 2014;4:e29390. doi: 10.4161/cl.29390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jenett A, Rubin GM, Ngo TT, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, Iyer N, Fetter D, Hausenfluck JH, Peng H, Trautman ET, Svirskas RR, Myers EW, Iwinski ZR, Aso Y, DePasquale GM, Enos A, Hulamm P, Lam SC, Li HH, Laverty TR, Long F, Qu L, Murphy SD, Rokicki K, Safford T, Shaw K, Simpson JH, Sowell A, Tae S, Yu Y, Zugates CT. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franks SJ, Hoffmann AA. Genetics of climate change adaptation. Annual review of genetics. 2012;46:185–208. doi: 10.1146/annurev-genet-110711-155511. [DOI] [PubMed] [Google Scholar]