Abstract
Background
Among providers who serve low-income and uninsured women, resistance to extending the cervical cancer screening interval following normal Pap and co-test results has been documented. Our objective was to examine provider characteristics and beliefs associated with guideline-consistent screening interval recommendations.
Method
We collected cross-sectional survey data between 2009 and 2010 from 82 primary care providers in six Federally Qualified Health Centers in Illinois, USA. The relationships between characteristics, beliefs, and screening interval recommendations (1 year vs. 3 years) were tested with Pearson chi-square, negative binomial and ordered logistic regression.
Results
Compared to providers who recommended annual intervals after a normal co-test, providers who recommended a guideline-consistent (i.e., 3 years) screening interval were significantly more likely to report the goodness, ease, and benefit of their recommendation and perceived encouragement for a 3-year interval from professional organizations and journals (p < .05). Providers who recommended a 3-year interval were also less likely to report that longer intervals increase patient risk for cervical cancer (p < .05). Interval recommendations were not associated with provider specialty, gender, or years in practice.
Conclusion
Messages that promote the benefits of longer screening intervals after a normal co-test, the natural history of human papillomavirus and cervical cancer, and low risk of developing cancer with a longer interval may be useful to promote evidence-based screening in this population of Federally Qualified Health Center providers. Dissemination of targeted messages through professional journals and specialty organizations should be considered.
Keywords: Cervical cancer screening, FQHC, Medically underserved, Screening intervals, Provider beliefs
Highlights
-
•
Annual cervical cancer screening is common, but not recommended by guidelines.
-
•
Pilot study assessed provider factors associated with screening recommendations.
-
•
Screening recommendations are associated with positive beliefs about screening tests.
-
•
Perceived risk of the patient developing cancer between screenings is significant.
-
•
Professional journals and organizations can disseminate screening interval messages.
Introduction
In the United States, screening for cervical cancer is a standard component of women's routine preventive healthcare, and has dramatically reduced cervical cancer incidence and mortality over the last six decades (Habbema et al., 2012). Current guidelines recommend screening intervals of 3 to 5 years following normal test results, based on the screening test used. Despite successful integration of cervical cancer screening into women's routine care, some uninsured and low-income women are screened less often than recommended and suffer disproportionate cervical cancer morbidity, mortality, and late-stage diagnosis (Benard et al., 2008, Fedewa et al., 2012, Spence et al., 2007). Conversely, too-frequent cervical cancer screening has also been documented in both the medically underserved and general population (Corbelli et al., 2014, King et al., 2014, Perkins et al., 2013, Roland et al., 2011, Teoh et al., 2015, Verrilli et al., 2014, Yabroff et al., 2009). Plausible explanations include opportunistic clinical service provision (Habbema et al., 2012), provider specialty (Corbelli et al., 2014, Yabroff et al., 2009), practice setting (Yabroff et al., 2009) and size (Perkins et al., 2013), provider concern for losing the patient to follow-up (King et al., 2014, Perkins et al., 2013, Verrilli et al., 2014), patient expectations (King et al., 2014, Perkins et al., 2013, Teoh et al., 2015, Verrilli et al., 2014), and provider knowledge (Teoh et al., 2015).
In 2009, the Centers for Disease Control and Prevention (CDC) launched the Cervical Cancer (Cx3) Study to examine both provider and patient knowledge, attitudes, and beliefs about co-testing (i.e., simultaneous Pap and human papillomavirus testing) and extended screening intervals in a medically underserved population (Benard et al., 2014, Hawkins et al., 2013, Roland et al., 2013). Baseline survey data collected for the study found that many providers recommended annual screening for women after a normal co-test despite guidelines at the time recommending a triennial interval. Providers also reported that extending the screening interval to three years with a normal co-test would result in the patent not returning annually for others tests and losing contact with the medical system (Roland et al., 2013). The purpose of the current analysis was to examine what provider characteristics and beliefs were associated with their screening interval recommendations (annual versus triennial interval) using the baseline survey data.
Method
The Cx3 Study was a pilot study conducted in 15 clinics associated with six Federally Qualified Health Centers (FQHCs) in Illinois, USA. In the United States, FQHCs are funded by the U.S. Department of Health and Human Services' Health Resources and Services Administration (HRSA) under Section 330 of the U.S. Public Health Service Act. FQHCs are safety-net clinics, and are mandated to serve an underserved area or population, offer a sliding fee scale, and provide preventive primary care services serve almost 22 million patients annually.
In recent years, HRSA-supported FQHCs have received funding to expand operations and provide clinical services to a greater number of medically underserved patients (http://bphc.hrsa.gov/about/healthcenterfactsheet.pdf). Providers working in FQHCs may face challenges related to increased patient load, and therefore FQHCs may experience higher staff turnover. Incentives to improve recruitment and retention of clinicians in underserved areas are being employed (Abrams et al., 2014).
All providers (physicians, nurse practitioners, certified nurse midwives, and physician assistants) in the participating clinics who routinely performed cervical cancer screening were eligible to participate (n = 109). Between 2009 and 2010, we sent self-administered, cross-sectional surveys and a $50 cash incentive to eligible providers with a stamped, self-addressed envelope for return; 98 providers completed the survey (89.9% response rate).
The survey collected provider demographic characteristics, practice characteristics, and screening practices and beliefs. A clinical vignette asked the provider when they would next screen a woman aged 35 years with a normal co-test result. Response options were 1 year, 2 years, 3 years, > 3 years. For this analysis, we used responses from the clinical vignette to compare the characteristics and beliefs of providers who recommended a 1-year interval (n = 57) to those of providers who recommended a 3-year interval (n = 25) (guideline-consistent at the time of the survey) as a method for defining guideline compliance and non-compliance. Significance of associations between these two interval recommendations were tested with Pearson chi-square, negative binomial and ordered logistic regression. All methods adjusted for the clustered sampling design. Missing data was handled with listwise deletion. CDC's Institutional Review Board approved the study. Additional details on study methods are published elsewhere Benard et al., 2014, Roland et al., 2013.
Results
Providers were primarily female (74%), physicians (67%) or nurse practitioners (21%), trained in OB/GYN (56%) or family medicine (35%), with an average of 8.9 years of providing clinical care (n = 82). Gender, specialty, provider type, and years in practice were not found to be significantly associated with screening interval recommendation (Table 1).
Table 1.
Demographic characteristics of study providers according to screening interval recommendations with the co-test a among 82 providers at Federally Qualified Health Centers, Illinois, 2009–2010.
| Sample description |
Recommended screening interval for patient aged 35 years with normal co-test this visit |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 year |
3 years |
p-Value | ||||||
| Col % | N | Row % | N | Row % | N | |||
| Primary specialty | Family medicine | 35% | 29 | 76% | 22 | 24% | 7 | 0.382 |
| OB–GYN/women's health | 56% | 46 | 63% | 29 | 37% | 17 | ||
| Other | 9% | 7 | 86% | 6 | 14% | 1 | ||
| Total | 100% | 82 | 70% | 57 | 30% | 25 | ||
| Type of provider | Physician | 67% | 55 | 71% | 39 | 29% | 16 | 0.442 |
| NP | 21% | 17 | 59% | 10 | 41% | 7 | ||
| Other | 12% | 10 | 80% | 8 | 20% | 2 | ||
| Total | 100% | 82 | 70% | 57 | 30% | 25 | ||
| Provider is OB/GYN physician | Other | 55% | 45 | 73% | 33 | 27% | 12 | 0.179 |
| OB/GYN MD | 45% | 37 | 65% | 24 | 35% | 13 | ||
| Total | 100% | 82 | 70% | 57 | 30% | 25 | ||
| Gender | Male | 26% | 21 | 71% | 15 | 29% | 6 | 0.822 |
| Female | 74% | 61 | 69% | 42 | 31% | 19 | ||
| Total | 100% | 82 | 70% | 57 | 30% | 25 | ||
| Years providing care (mean, sd) | 8.9 (9.9) | 9.33 (10.5) | 7.8 (8.5) | 0.513 | ||||
| Years providing clinical care, 4 categories | 1–4 | 46% | 38 | 68% | 26 | 32% | 12 | 0.836 |
| 5–9 | 22% | 18 | 61% | 11 | 39% | 7 | ||
| 10–15 | 12% | 10 | 80% | 8 | 20% | 2 | ||
| 16 + | 20% | 16 | 75% | 12 | 25% | 4 | ||
| Total | 100% | 82 | 70% | 57 | 30% | 25 | ||
Significance of associations with categorical variables was tested with design-adjusted Pearson chi-square.
Significance of the association with the count variable, number of years providing care, was tested with negative binomial regression.
All tests adjusted for the clustered sampling design.
Co-test (simultaneous Pap and human papillomavirus test).
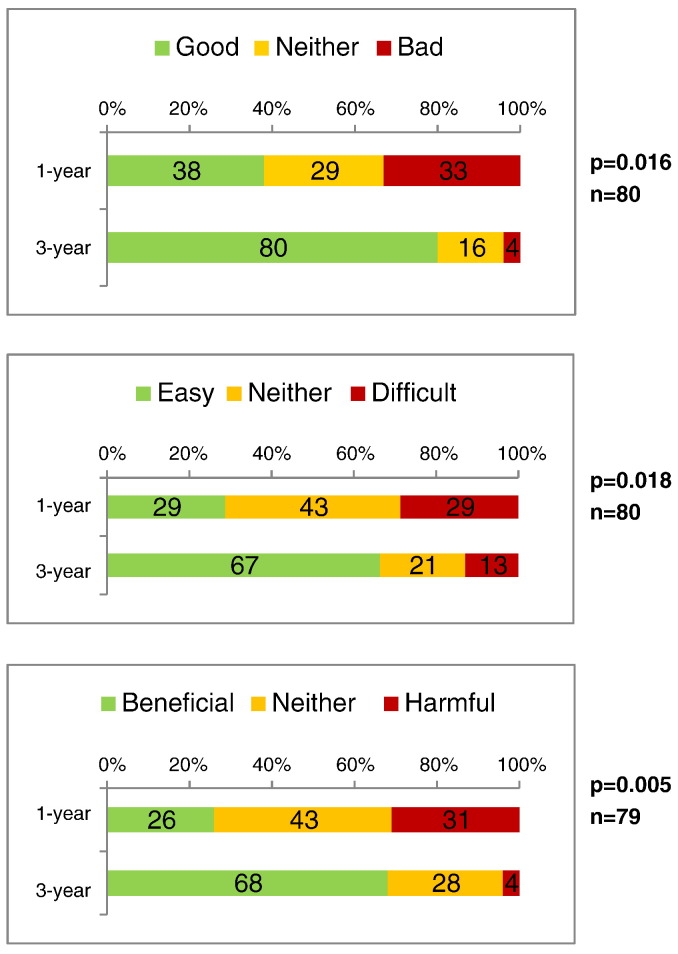
Providers were asked their beliefs about extending screening intervals for a woman aged ≥ 30 years with a normal co-test result. Providers who recommended a 3-year interval after a normal co-test were more likely to report that extending routine screening to 3 years would be good (80%), easy (67%) and beneficial (68%) compared to providers who recommended annual screening after a normal co-test (p < .05) (Fig. 1).
Fig. 1.
Beliefs about extending cervical cancer screening intervals to 3 years after a normal co-test,a according to screening interval recommendations, among 82 providers at Federally Qualified Health Centers, Illinois, 2009–2010.
Significance of associations between these two interval recommendations and beliefs were tested with ordered logistic regression and adjusted for clustered sampling design.
a Co-test (i.e., simultaneous Pap and human papillomavirus test).
Providers were asked to report level of agreement with common concerns about extending the screening interval after a normal co-test, including the patient not visiting annually for other screening tests, increased risk for the patient developing cervical cancer, higher rates of pre-cancer, or the patient losing contact with the medical system. Providers who recommended a 3-year interval were significantly more likely to disagree that an extended interval would put the patient at increased risk for cancer (56%) (p < .05) and would result in higher rates of pre-cancer (68%) (p = .055) (Table 2).
Table 2.
Level of agreement with statements about extending the cervical cancer screening interval to 3 years after a normal co-testa, according to screening interval recommendations, among 82 providers at Federally Qualified Health Centers, Illinois, 2009–2010.
| Recommend 1-year interval (%) (n = 57) | Recommend 3-year interval (%) (n = 25) | p-Value | |||
|---|---|---|---|---|---|
| Extending the screening interval would… | Result in patient not visiting annually for other screening tests (reverse) | Agree | 75 | 80 | 0.555 |
| Neither | 7 | 12 | |||
| Disagree | 18 | 8 | |||
| Put patient at increased risk for cervical cancer | Agree | 40 | 12 | 0.036 | |
| Neither | 23 | 32 | |||
| Disagree | 37 | 56 | |||
| Result in higher rates of cervical pre-cancer | Agree | 35 | 20 | 0.055 | |
| Neither | 23 | 12 | |||
| Disagree | 42 | 68 | |||
| Cause patients to lose contact with medical care system | Agree | 68 | 48 | 0.088 | |
| Neither | 9 | 24 | |||
| Disagree | 23 | 28 | |||
| Do the following entities encourage or discourage you to extend the screening interval? | Patients | Discourage | 18 | 40 | 0.105 |
| Neither | 67 | 48 | |||
| Encourage | 16 | 12 | |||
| Colleagues | Discourage | 28 | 16 | 0.165 | |
| Neither | 47 | 40 | |||
| Encourage | 25 | 44 | |||
| Clinic administration | Discourage | 21 | 20 | 0.126 | |
| Neither | 65 | 56 | |||
| Encourage | 14 | 24 | |||
| Professional journals | Discourage | 16 | 0 | 0.000 | |
| Neither | 36 | 20 | |||
| Encourage | 47 | 80 | |||
| Professional specialty organizations | Discourage | 20 | 0 | 0.000 | |
| Neither | 36 | 20 | |||
| Encourage | 45 | 80 | |||
| National health organizations | Discourage | 16 | 0 | 0.051 | |
| Neither | 31 | 24 | |||
| Encourage | 53 | 76 | |||
Significance of associations between these two interval recommendations and beliefs was tested with ordered logistic regression and adjusted for clustered sampling design. No missing data.
Co-test (simultaneous Pap and human papillomavirus test).
Providers were asked about their perceived support for extending the screening interval after a normal co-test from patients, clinic administration, colleagues, professional specialty organizations, national health organizations, and professional journals. Providers who recommended a 3-year interval were significantly more likely to perceive support for that practice from professional specialty organizations and journals (80%) (p < .05) and national health organizations (76%) (p = .051) (Table 2).
Discussion
Despite guidelines recommending longer intervals between cervical cancer screenings, women continue to be screened annually. These data identify specific beliefs associated with providers guideline-consistent screening interval recommendations, including beliefs about the goodness, ease, and benefit of extending screening intervals with a normal co-test result, and that extending the interval will not put the patient at increased risk of developing cancer or pre-cancer. Disseminating the positive beliefs found to be associated with 3-year intervals through professional specialty organizations and professional journals could be effective for promoting guideline-consistent interval recommendations among this population, as was found in a survey of Indiana primary care providers King et al., 2014. Notably, nonmodifiable provider characteristics such as specialty, age, and gender were not associated with interval recommendations in this analysis.
Additional methods found to be effective for changing provider's cervical cancer screening knowledge, behaviors and attitudes include electronic medical record-based tools (White & Kenton, 2013) provider assessment and feedback, Sabatino et al., 2012 and academic detailing (Sheinfeld et al., 2000), patient driven inquiry, and management guidelines (King et al., 2014). Interventions and messages about cervical cancer screening should prevent the harms and risks of routine screening, in addition to the benefits. CDC has produced materials to educate providers and patients on the appropriate use of the co-test that may be adapted to reflect the needs of the target population and the latest science (Benard et al., 2014).
To date, no studies have examined provider and patient acceptance of co-testing with longer screening intervals in a medically underserved population, and therefore these are novel data. Collecting information on beliefs associated with FQHC provider screening interval recommendations is essential because as the science evolves, so will screening guidelines and screening modalities, and targeting provider beliefs and attitudes will be critical to promote uptake of new evidence-based recommendations. Results from this study will assist CDC in providing technical assistance to cancer screening programs regarding use of the co-test. Because of the small convenience sample, these findings may not be generalizable to other FQHCs or providers in Illinois. Other limitations include the data were self-reported, incentives were provided to complete the survey, and additional details on provider training and demographics are unknown.
Conclusion
Cervical cancer screening in the United States can be improved by reducing the frequency of screening intervals for over-screened women to be consistent with guidelines, and directing realized savings toward increasing screening among rarely or never-screened women (Kim et al., 2013). We found that screening interval recommendations were dictated not by provider demographics but by positive beliefs regarding longer screening intervals. While this is not surprising, these findings do indicate avenues for dissemination of such beliefs that could be promoted and disseminated to counter views about the risks of longer screening intervals. Future research regarding how to communicate relative risk and harms of over-screening is critical. Provider's perceived risks such as being held accountable for a missed diagnosis (Roland et al., 2013), as well as losing the patient to follow-up (Roland et al., 2013) and her risk of developing cancer are genuine. The lack of widespread and equitable screening participation, particularly among under- and over-screened women, is a call to action for public health programs (Plescia et al., 2012). Reducing unnecessary clinical intervention is essential when community-based primary care settings with finite resources, such as FQHCs, are expected to provide optimal preventive care services to an underserved patient population.
Conflict of interest statement
There are no conflicts of interest to report, or financial disclosures. This manuscript was written in the course of employment by the United States Government and it is not subject to copyright in the United States.
Acknowledgment
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The authors gratefully acknowledge the medical directors, administrators and providers from the FQHCs who participated in the study, and the Illinois Breast and Cervical Cancer Early Detection Program.
Footnotes
Source of funding: Support to A. Greek was provided by Centers for Disease Control and Prevention, contract 200-2002-00573, Task Order No. 0006 to Battelle.
References
- Abrams M.K., Doty M.M., Ryan J., Hall D., Riley P. The Commonwealth Fund. 2014. Ready or not? How community health centers view their preparedness to care for newly insured patients. [Google Scholar]
- Benard V.B., Johnson C.J., Thompson T.D. Examining the association between socioeconomic status and potential human papillomavirus-associated cancers. Cancer. 2008;113(10 Suppl.):2910–2918. doi: 10.1002/cncr.23742. [DOI] [PubMed] [Google Scholar]
- Benard V.B., Saraiya M., Greek A. Overview of the CDC Cervical Cancer (Cx3) Study: an educational intervention of HPV testing for cervical cancer screening. J. Womens Health (Larchmt) 2014;23(3):197–203. doi: 10.1089/jwh.2013.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbelli J., Borrero S., Bonnema R. Differences among primary care physicians' adherence to 2009 ACOG guidelines for cervical cancer screening. J. Womens Health (Larchmt) 2014;23(5):397–403. doi: 10.1089/jwh.2013.4475. [DOI] [PubMed] [Google Scholar]
- Fedewa S.A., Cokkinides V., Virgo K.S., Bandi P., Saslow D., Ward E.M. Association of insurance status and age with cervical cancer stage at diagnosis: National Cancer Database, 2000–2007. Am. J. Public Health. 2012;102(9):1782–1790. doi: 10.2105/AJPH.2011.300532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habbema D., De Kok I.M., Brown M.L. Cervical cancer screening in the United States and the Netherlands: a tale of two countries. Milbank Q. 2012;90(1):5–37. doi: 10.1111/j.1468-0009.2011.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins N.A., Benard V.B., Greek A., Roland K.B., Manninen D., Saraiya M. Patient knowledge and beliefs as barriers to extending cervical cancer screening intervals in Federally Qualified Health Centers. Prev. Med. 2013;57(5):641–645. doi: 10.1016/j.ypmed.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.J., Sharma M., Ortendahl J. Optimal interval for routine cytologic screening in the United States. JAMA Intern. Med. 2013;173(3):241–242. doi: 10.1001/2013.jamainternmed.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N.R., Kasper K.M., Daggy J.K., Tucker E.B. Current practice patterns in cervical cancer screening in Indiana. Am. J. Obstet. Gynecol. 2014;210(3):265–268. doi: 10.1016/j.ajog.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Perkins R.B., Anderson B.L., Gorin S.S., Schulkin J.A. Challenges in cervical cancer prevention: a survey of U.S. obstetrician–gynecologists. Am. J. Prev. Med. 2013;45(2):175–181. doi: 10.1016/j.amepre.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Plescia M., Richardson L.C., Joseph D. New roles for public health in cancer screening. CA Cancer J. Clin. 2012;62(4):217–219. doi: 10.3322/caac.21147. [DOI] [PubMed] [Google Scholar]
- Roland K.B., Soman A., Benard V.B., Saraiya M. Human papillomavirus and Papanicolaou tests screening interval recommendations in the United States. Am. J. Obstet. Gynecol. 2011;205(5):447–448. doi: 10.1016/j.ajog.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Roland K.B., Benard V.B., Greek A., Hawkins N.A., Manninen D., Saraiya M. Primary care provider practices and beliefs related to cervical cancer screening with the HPV test in Federally Qualified Health Centers. Prev. Med. 2013;57(5):419–425. doi: 10.1016/j.ypmed.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino S.A., Lawrence B., Elder R. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am. J. Prev. Med. 2012;43(1):97–118. doi: 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Sheinfeld G.S., Gemson D., Ashford A. Cancer education among primary care physicians in an underserved community. Am. J. Prev. Med. 2000;19(1):53–58. doi: 10.1016/s0749-3797(00)00153-7. [DOI] [PubMed] [Google Scholar]
- Spence A.R., Goggin P., Franco E.L. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev. Med. 2007;45(2-3):93–106. doi: 10.1016/j.ypmed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Teoh D.G., Marriott A.E., Isaksson V.R. Adherence to the 2012 national cervical cancer screening guidelines: a pilot study. Am. J. Obstet. Gynecol. 2015;212(1):62–69. doi: 10.1016/j.ajog.2014.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrilli L., Winer R.L., Mao C. Adherence to cervical cancer screening guidelines by gynecologists in the Pacific Northwest. J. Low. Genit. Tract Dis. 2014;18(3):228–234. doi: 10.1097/LGT.0000000000000008. [DOI] [PubMed] [Google Scholar]
- White P., Kenton K. Use of electronic medical record-based tools to improve compliance with cervical cancer screening guidelines: effect of an educational intervention on physicians' practice patterns. J. Low. Genit. Tract Dis. 2013;17(2):175–181. doi: 10.1097/LGT.0b013e3182607137. [DOI] [PubMed] [Google Scholar]
- Yabroff K.R., Saraiya M., Meissner H.I. Specialty differences in primary care physician reports of Papanicolaou test screening practices: a national survey, 2006 to 2007. Ann. Intern. Med. 2009;151(9):602–611. doi: 10.7326/0003-4819-151-9-200911030-00005. [DOI] [PubMed] [Google Scholar]