Abstract
Background and Purpose
This study evaluates the potential efficacy and robustness of functional bone marrow sparing (BMS) using intensity-modulated proton therapy (IMPT) for cervical cancer, with the goal of reducing hematologic toxicity.
Material and Methods
IMPT plans with prescription dose of 45 Gy were generated for ten patients who have received BMS intensity-modulated x-ray therapy (IMRT). Functional bone marrow was identified by 18F-flourothymidine positron emission tomography. IMPT plans were designed to minimize the volume of functional bone marrow receiving 5–40 Gy while maintaining similar target coverage and healthy organ sparing as IMRT. IMPT robustness was analyzed with ±3% range uncertainty errors and/or ±3mm translational setup errors in all three principal dimensions.
Results
In the static scenario, the median dose volume reductions for functional bone marrow by IMPT were: 32% for V5GY, 47% for V10Gy, 54% for V20Gy, and 57% for V40Gy, all with p<0.01 compared to IMRT. With assumed errors, even the worst-case reductions by IMPT were: 23% for V5Gy, 37% for V10Gy, 41% for V20Gy, and 39% for V40Gy, all with p<0.01.
Conclusions
The potential sparing of functional bone marrow by IMPT for cervical cancer is significant and robust under realistic systematic range uncertainties and clinically relevant setup errors.
Keywords: bone marrow sparing, cervical cancer, proton, robustness
Introduction
Chemoradiation improves clinical outcome for cervical cancer patients, but is associated with significant hematologic toxicity [1, 2]. This is because a large percentage of bone marrow lies in the pelvic region [3], and receives significant radiation dose using standard radiotherapy approaches for pelvic treatment. Often, chemotherapy is put on hold or the dose is reduced when hematologic toxicity occurs. Data shows that patients receiving reduced chemotherapy cycles have worse progression-free and overall survival [4]. Reduction of hematologic toxicity from radiation is therefore of significant clinical interest.
Studies demonstrate that the volume of pelvic bone marrow receiving a low dose of 10 Gy to 20 Gy as the primary factor associated with hematologic toxicity [5–8]. The RTOG0418 suggested that bone marrow receiving 40 Gy is also correlated with increased Grade 2 hematologic toxicity[9]. Researchers therefore have focused on reducing radiation dose to pelvic bones using intensity-modulated radiation therapy (IMRT) [10, 11]. More recently, studies have investigated identifying functionally active bone marrow in the pelvic region as a more accurate organ-at-risk (OAR) to spare in treatment planning. Liang et al [11] used 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) to identify active regions of the bone marrow in the pelvic region. McGuire et al [12, 13] utilized 18F-flourothymidine (FLT) PET. FLT is incorporated into DNA during replication and thus a good marker of cell proliferation [14, 15]; the detailed process of relating functional bone marrow to FLT uptake has been described previously [13]. Identifying functional bone marrow within the pelvic bones reduces avoidance volumes incorporated into bone marrow sparing radiation therapy designs, which may allow more targeted and effective reduction of hematologic toxicity.
Proton therapy is a promising modality that may improve bone marrow sparing due to its ability to reduce integral dose. Its ability to achieve satisfactory target dose distributions using only a few beams enables bone marrow sparing. Song et al [16] showed that passively scattered proton therapy can reduce the bone marrow volume receiving low dose radiation more effectively than IMRT. Intensity-modulated proton therapy (IMPT) with spot scanning is able to produce further improvement in conformity of target coverage and better sparing of normal tissue. The superior plan quality of IMPT is still subject to range uncertainty and setup errors [17]. Range uncertainty as a systematic error is usually between 3% to 3.5% [17]. Daily patient setup errors, by themselves or when combined with systematic range errors, may significantly impact proton dose distributions because the radiological path length of proton beams may be different from the intended length when setup error is considered. The robustness of an IMPT plan, in terms of both target coverage and normal tissue sparing, is therefore an important factor in the evaluation of plan quality [18, 19]. The present study aims to answer whether 1) IMPT may better achieve functional bone marrow sparing than IMRT and 2) Whether bone-marrow sparing and target coverage in IMPT are maintained under clinically relevant range uncertainty and setup error conditions.
Methods and Materials
Patients and Imaging Data
Ten patients diagnosed with Stage I or II cervical carcinoma and received concurrent chemoradiation therapy were enrolled in Institutional Review Board-approved treatment planning studies NCT01075412 or NCT01717391 (ClinicalTrials.gov identifier, United States National Institutes of Health). Each patient received a simulation CT scan, one or more MR scans of different sequences, an FDG-PET scan, and an FLT-PET scan. Each patient received FLT-PET scans during therapy for evaluation of their bone marrow response [13], though these images are not used in this study. The ten patients all received BMS IMRT treatment using 8 beams with functional bone marrow identified by FLT-PET. A summary of patient characteristics and clinical treatment is available in Supplemental Materials. All patients received BMS IMRT to the pelvis of 45 Gy in 25 fractions, followed by boosts using external photon beam and/or brachytherapy consistent with standard care.
Treatment Planning
The dose volume objectives in BMS IMPT were identical to those of the clinical BMS IMRT plans, delivering 45 Gy to 97% of PTV in 25 fractions, and keeping dose to the bladder, bowel, and rectum similar or below that in the corresponding IMRT plan. In general, healthy tissue OAR objectives followed the RTOG 0418 protocol with the exception of bone marrow. Regions with the body weight-normalized Standarized Uptake Value (SUV) of ≥ 4 on the FLT-PET images were considered functional bone marrow; the determination of SUV was based on the whole body without choosing a region of interest or excluding any non-uptake region. The detail of this process was previously described by McGuire et al [13]. As a primary objective, the percentage volume of FLT-PET SUV4 receiving 5 Gy to 40 Gy was to be reduced as much as possible when objectives to PTV and other OAR were met.
The same planning CT images and patient contours used for IMRT planning were used for IMPT planning. The radiation oncologist contoured the gross tumor volume (GTV) and clinical tumor volume (CTV) based on CT, FDG-PET, and/or magnetic resonance imaging (MRI). CTV to PTV expansion for primary tumor or tumor bed was 7 mm in all directions. Nodal CTV to PTV expansion was 5 mm toward bone and 7 mm in other directions. Bladder, bowel, rectum, and the femoral heads were also contoured. Functional bone marrow was auto-segmented using a threshold SUV ≥ 4 on an FLT-PET image co-registered to its attenuation correction CT which was registered bone to bone with the simulation CT. The functional bone marrow is the organ-at-risk that was primarily spared during the optimization process.
Three non-coplanar beams, very similar to those used by Song et al [16], were used in the BMS IMPT plan: one beam came from the anterior inferior direction with a couch kick, typically at gantry angle 30 degrees and couch angle 90 degrees; two posterior oblique beams at typical gantry angles of 150 degrees and 210 degrees. Figure 1a uses arrows to indicate the typical beam angles. This beam arrangement allowed maximum sparing of bone marrow as well as anatomic stability. Multi-field optimization (MFO) was used in the proton treatment planning process.
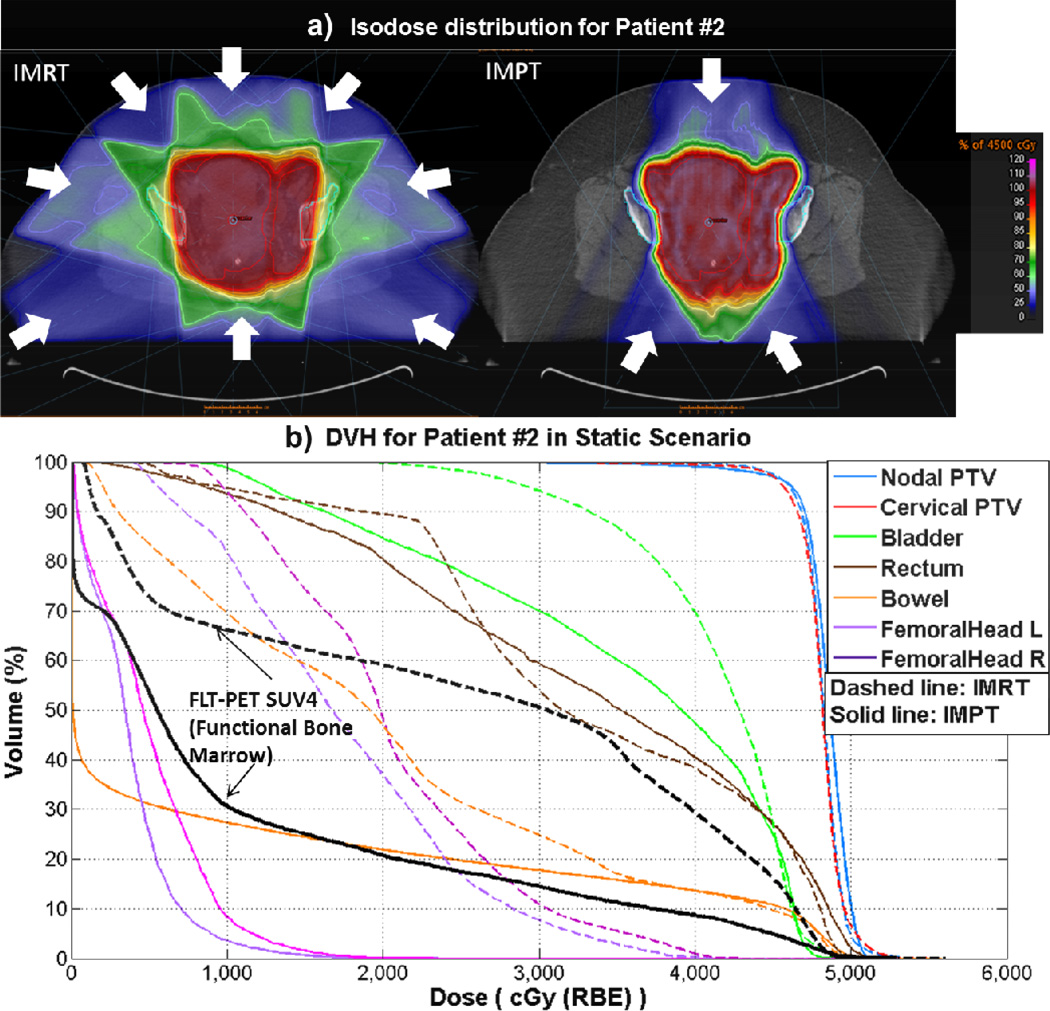
Figure 1.
a).Isodose distribution from one example patient (Patient #2) with white arrows indicating beam angles for BMS IMRT and BMS IMPT, respectively. b). BMS IMPT and BMS IMRT DVH for this example patient.
Pinnacle (Philips Health Systems, Fitchburg, WI) was used for all clinical IMRT plans. RayStation v2.4 (RaySearch Laboratories, Stockholm, Sweden) was used for IMPT planning. The proton beams used were from a pre-commissioned machine based on the IBA Dedicated Nozzle with energy layer spacing of 1cm and spot spacing of 0.75cm.
The dose unit of Gray (Gy) used throughout this study refers to values weighted by the relative biological effectiveness (RBE). A generic RBE value of 1.1 was used for proton dose [14, 15]. RBE = 1.0 was used for photon dose.
Plan Evaluation and Robustness Analysis
A radiation oncologist reviewed all BMS IMPT plans in the static scenario and approved them as clinically suitable based on dosimetric distributions and dose volume histograms. The BMS IMPT plans were first compared to the BMS IMRT plan in the static scenario, i.e, no range uncertainty or setup errors were considered.
Robustness analysis was performed on the BMS IMPT plan by calculating delivered dose distributions in the “perturbed scenario”, i.e., with range uncertainty and/or setup errors. The perturbed scenarios included three systematic range uncertainty errors of +3%, −3%, and 0% (no error) by scaling the density of each voxel on the planning CT, and nine translational setup errors by shifting the planning CT +3mm or −3mm in all three principal dimensions relative to proton beam isocenter, as well as no error. The systematic range error value of ±3% was based on the errors in the conversion of CT number to proton stopping power, reported by Lomax [19], and also falls within the range used by a number of institutions [17].
The nine individual setup errors were combined with the three systematic range errors, creating twenty-seven individually “perturbed doses”, including one static dose (no error). Each individual perturbed dose was generated and plotted together as a DVH band [20]. For each particular target or OAR dose-volume objective evaluated, the worst value (“IMPT Worst” in Table 2) was extracted from the DVH band of twenty-seven individually perturbed doses; i.e., the lowest value on tumor target DVH points and the highest value on OAR DVH points were reported as “IMPT Worst” for each objective respectively, and they are not necessarily from the same perturbed scenario. Three average dose distributions were created under the assumption that the three systematic range uncertainty errors, with the nine individual setup errors were considered to occur with equal probability.
Table 2.
Selected dose-volume statistics for other organ-at-risk in IMPT in the static and 1 error scenarios, as well as the values in IMRT plan. IMPT dose-volume values were either statistically significantly smaller than IMRT value, or not significantly different from IMRT values, from paired Wilcoxon signed-rank tests.
| IMRT | IMPT Static |
IMPT Worst |
IMPT Average +3% |
IMPT Average −3% |
IMPT Average 0% |
||
|---|---|---|---|---|---|---|---|
| Bowel V40Gy (%) |
Median (Min- Max) |
20.5 (12.2–29.7) |
18.4 (10.1–27.1) |
22.5 (12.8–29.9) |
16.2 (8.9–23.8) |
20.1 (10.6–28.5) |
18.0 (9.8–26.2) |
| p-value | Reference | 0.01 | 0.77 | <.01 | 0.01 | <.01 | |
| Bowel DMean (Gy) |
Median (Min- Max) |
23.9 (20.3–30.8) |
14.8 (10.4–20.4) |
16.9 (11.9–21.9) |
14.2 (10.1–19.5) |
15.7 (10.7–21.5) |
14.9 (10.4–20.5) |
| p-value | Reference | <.01 | <.01 | <.01 | <.01 | <.01 | |
| Rectum V30Gy (%) |
Median (Min- Max) |
80.6 (49.4–99.3) |
62.9 (49.3–94.1) |
77.7 (66.7–99.0) |
62.1 (49.0–94.7) |
69.5 (54.7–96.5) |
65.3 (51.3–95.1) |
| p-value | Reference | 0.06 | 0.43 | 0.05 | 0.32 | 0.13 | |
| Rectum DMean (Gy) |
Median (Min- Max) |
40.9 (29.8–47.1) |
34.1 (29.1–45.1) |
40.5 (33.8–47.9) |
32.5 (28.8–43.9) |
36.3 (29.6–46.3) |
34.3 (29.2–45.1) |
| p-value | Reference | <.01 | 0.38 | <.01 | 0.05 | 0.01 | |
| Bladder V45Gy (%) |
Median (Min- Max) |
44.0 (22.0–79.2) |
31.9 (22.6–55.7) |
52.5 (29.9–73.1) |
22.2 (14.0–48.4) |
43.4 (27.4–63.7) |
32.6 (20.6–56.0) |
| p-value | Reference | 0.04 | 0.16 | <.01 | 0.85 | 0.04 | |
| Bladder DMean (Gy) |
Median (Min- Max) |
42.5 (32.2–46.6) |
37.5 (29.2–39.8) |
40.6 (32.3–45.7) |
36.2 (27.6–38.7) |
39.0 (31.3–41.4) |
37.5 (29.3–39.8) |
| p-value | Reference | <.01 | 0.02 | <.01 | <.01 | <.01 | |
| Rt Femoral Head V30Gy(%) |
Median (Min- Max) |
12.3 (10.4–13) |
2.8 (0–23.9) |
10.8 (2.6–37.9) |
2.3 (0–22.7) |
2.8 (0–25.1) |
2.7 (0–23.9) |
| p-value | Reference | 0.06 | 0.43 | 0.05 | 0.32 | 0.13 | |
| Lt Femoral Head V30Gy(%) |
Median (Min- Max) |
12.7 (7.6–13.6) |
5.3 (0–25.0) |
16.0 (2.5–41.0) |
4.7 (0–23.7) |
5.8 (0–25.8) |
5.1 (0–24.4) |
| p-value | Reference | 0.13 | 0.19 | 0.05 | 0.13 | 0.11 |
Because PTV was used to account for uncertainties during the delivery that could affect the intended CTV dose, in all three types of robustness analysis the CTV was used as the evaluation target volume.
Statistics
For the evaluation of CTV coverage in each scenario, the median value of the doses to 99% of CTV volume (D99%) in this scenario for the 10 patients were compared to 95% of the prescription dose (Rx, 42.8 Gy) using one-sample Wilcoxon signed-rank tests. For the evaluation of OAR and functional bone marrow sparing by IMPT in each scenario, the median values of various OAR dose-volume metrics in this scenario for the 10 patients were compared to the IMRT values using paired, Wilcoxon signed-rank tests. The relative change of various OAR dose-volume metrics was calculated for each patient in the following manner: ([IMPT Scenario]-IMRT)/IMRT, and Wilcoxon signed-rank tests were utilized to assess the significance of the relative change between each IMPT scenario and IMRT. Median, range (minimum, maximum) and two-tailed p-values are reported where applicable. All statistical testing was assessed for significance at the 5% level using SAS v9.4 (SAS Institute, Cary, NC).
Results
In the static scenario, BMS IMPT plans had similar coverage for nodal CTV and cervical CTV, and had similar or better dose for all OARs compared to BMS IMRT (Table 1 and Table 2, “IMPT Static” column), with statistical significance. IMPT achieved significant lower dose-volume values for functional bone marrow receiving doses of 5 Gy to 40 Gy (Table 1, “IMPT Static” column). The relative median reductions for bone marrow dose volume were: 32% for V5Gy, 47% for V10Gy, 51% for V15Gy, 54% for V20Gy, 60% for V35Gy, and 57% for V40Gy, all with p < 0.01. An example of an isodose distribution and the dose-volume-histogram (DVH) is shown in Figure 1.
Table 1.
Statistics for target coverage and functional bone marrow (SUV4 region in FLT-1 PET) sparing in IMPT in static and error scenarios. For target coverage, dose to 99% of CTV was compared to 95% of prescription dose (Rx) 42.8 Gy in one-sample Wilcoxon signed-rank test; target coverage was maintained if D99% ≥ 95%Rx. For sparing of bone marrow (represented by SUV4 in FLT-PET), dose volume was compared to IMRT plans using paired Wilcoxon signed-rank tests.
| IMRT | IMPT Static |
IMPT Worst |
IMPT Average +3% |
IMPT Average −3% |
IMPT Average 0% |
||
|---|---|---|---|---|---|---|---|
| CTV Cervix D99% (Gy) |
Median (Min-Max) |
45.7 (45.2–46.6) |
45.0 (44.5–45.3) |
41.1 (37.5–44.9) |
44.6 (42.0–45.1) |
45.4 (44.8–46.1) |
45.5 (45.0–45.9) |
| p-value | <.01 | <.01 | 0.16 | 0.01 | <.01 | <.01 | |
| CTV Cervix DMean (Gy) |
Median (Min-Max) |
48.2 (47.3–49) |
47.2 (46.9–48.3) |
46.8 (45.9–47.0) |
47.0 (46.6–47.6) |
47.4 (47.0–49.1) |
47.2 (47.0–48.3) |
| p-value | <.01 | <.01 | <.01 | <.01 | <.01 | <.01 | |
| CTV Nodes D99% (Gy) |
Median (Min-Max) |
45.7 (45.0–46.9) |
44.8 (43.4–45.7) |
41.6 (37.3–44.6) |
43.9 (42.8–46.3) |
45.0 (44.3–45.5) |
45.5 (43.9–46.0) |
| p-value | <.01 | <.01 | 0.28 | <.01 | <.01 | <.01 | |
| CTV Nodes DMean (Gy) |
Median (Min-Max) |
48.4 (47.1–48.6) |
47.3 (46.9–48.1) |
46.9 (46.3–47.4) |
47.2 (46.6–47.8) |
47.7 (47.1–48.6) |
47.4 (47.0–48.2) |
| p-value | <.01 | <.01 | <.01 | <.01 | <.01 | <.01 | |
| SUV4 V5Gy (%) |
Median (Min-Max) |
78.8 (64.6–83.6) |
50.8 (47.3–76.5) |
56.7 (54.7–78.0) |
52.2 (48.1–76.9) |
56.1 (54.3–77.7) |
53.8 (50.5–77.3) |
| p-value | Reference | <.01 | <.01 | <.01 | <.01 | <.01 | |
| SUV4 V10Gy (%) |
Median (Min-Max) |
72.5 (58.4–77.8) |
39.5 (30.5–61.1) |
45.9 (34.7–63.8) |
39.3 (30.4–61.2) |
45.5 (34.0–63.6) |
42.5 (31.8–62.1) |
| p-value | Reference | <.01 | <.01 | <.01 | <.01 | <.01 | |
| SUV4 V15Gy (%) |
Median (Min-Max) |
70.3 (54.5–74.0) |
32.2 (25.8–54.5) |
39.6 (29.1–57.7) |
31.2 (23.8–54.0) |
38.2 (28.6–57.3) |
34.1 (26.6–55.4) |
| p-value | Reference | <.01 | <.01 | <.01 | <.01 | <.01 | |
| SUV4 V20Gy (%) |
Median (Min-Max) |
60.7 (50.4–69.5) |
27.4 (20.6–48.0) |
34.5 (25.8–52.8) |
25.8 (17.8–46.8) |
32.1 (25.2–51.7) |
28.4 (22.5–49.2) |
| p-value | Reference | <.01 | <.01 | <.01 | <.01 | <.01 | |
| SUV4 V35Gy (%) |
Median (Min-Max) |
46.0 (33.3–55.1) |
15.9 (9.3–27.7) |
23.5 (17.9–34.1) |
13.9 (5.8–24.5) |
18.8 (13.8–31.0) |
15.5 (9.0–27.6) |
| p-value | Reference | <.01 | <.01 | <.01 | <.01 | <.01 | |
| SUV4 V40Gy (%) |
Median (Min-Max) |
37.5 (22.6–44.6) |
12.3 (6.0–20.3) |
19.7 (13.6–26.1) |
8.3 (2.9–16.4) |
14.6 (9.5–22.2) |
11.4 (5.5–19.3) |
| p-value | Reference | <.01 | <.01 | <.01 | <.01 | <.01 |
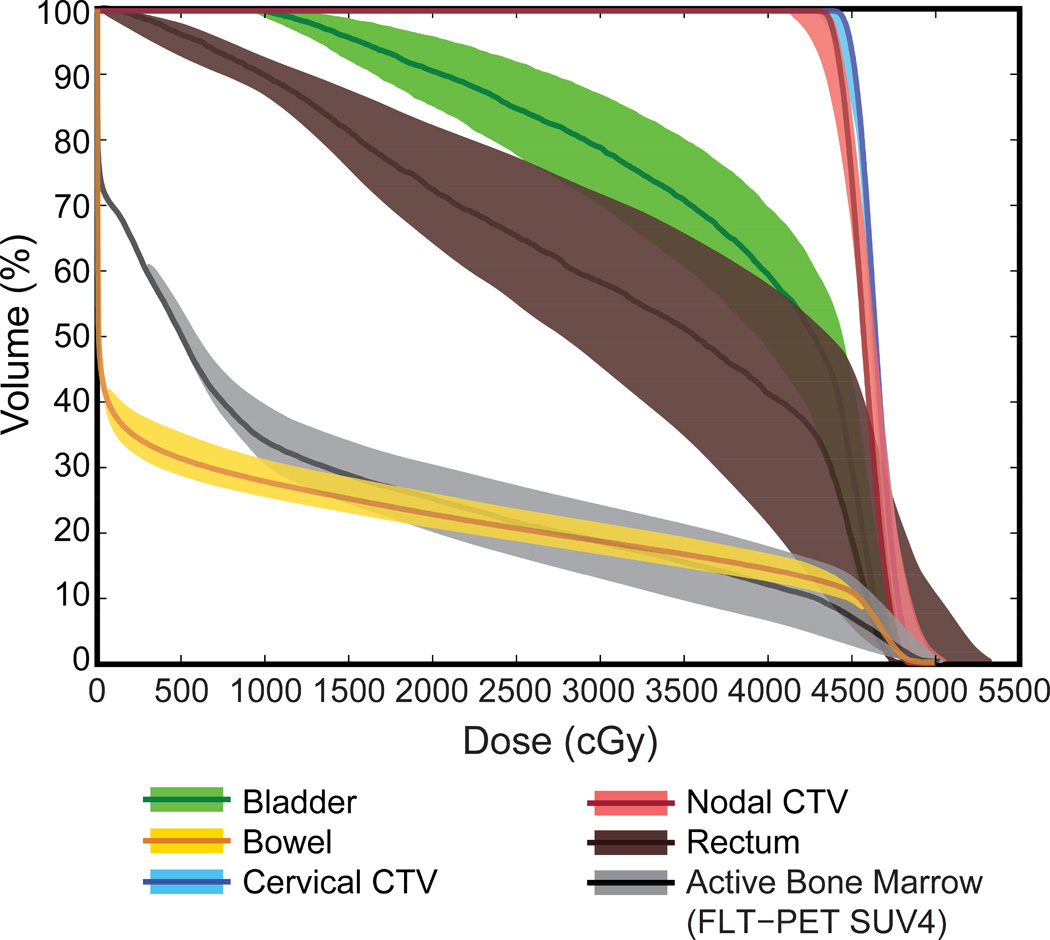
In all twenty-seven individual error scenarios, the worst values for cervical and nodal CTV D99% were statistically not different from 95% Rx (Table 1, “IMPT Worst” column). The median worst value of CTV cervical and nodes D99% was 41.1 Gy and 41.6 Gy, below the 95% Rx value of 42.8 Gy (p = 0.28 and p = 0.16, respectively). All the IMPT worst case values for OAR were better than or statistically not different from IMRT values (Table 2, “IMPT Worst” column). Importantly, the highest volumes of functional bone marrow (FLT-PET SUV4) at dose levels from 5 Gy to 40 Gy were still significantly below IMRT values. The worst-case relative median reductions of functional bone marrow by IMPT were: 23% for V5Gy, 37% for V10Gy, 43% for V15Gy, 41% for V20Gy, 45% for V35Gy, and 39% for V40Gy, all with p < 0.01 compared to IMRT. DVH bands for all individual error scenarios were plotted for the example patient in Figure 2. Figure 2 exhibits deterioration of CTV coverage under the error scenarios and the deviation of OAR DVH from the planned values.
Figure 2.
BMS IMPT DVH for Patient #2 under range and setup errors. The color band represents the range of DVH under the twenty-seven individual error scenarios, while the solid line represents the planned value.
In the three average scenarios, cervical and nodal CTV D99% were significantly higher than 95% Rx (42.8 Gy), and all OAR dose volume metrics were significantly better than or not different from IMRT values (Table 1 and 2, last three columns). Specifically, the volumes of functional bone marrow (FLT-PET SUV4) at dose levels from 5 Gy to 40 Gy were significantly below IMRT values.
Relative changes in dose-volume metrics for functional bone marrow and other OAR are available in the Supplemental Materials.
Discussion
Robustness of IMPT for the pelvic region is of significant interest because of the potentially large range uncertainty and setup variation [21–24]. This study investigates the topic of IMPT robustness for pelvic treatment with a new focus on the bone-marrow sparing efficacy and robustness.
A review by Kutcher et al [25] found the average inter-fractional displacements in any dimension were below 3mm, and the average total displacements were below 1 cm, using portal imaging. Errors under cone-beam CT guidance are even smaller [26]. While the newest proton therapy facilities have begun to utilize cone-beam CT or CT on-rails for daily treatment imaging, many others are still using orthogonal x-ray images for setup. Setup errors of ±3mm in all three dimensions, with a total magnitude of 5.2 mm, was chosen in this study and are considered clinically relevant and not optimistic to investigate the safety and robustness of BMS IMPT.
The worst scenarios of target coverage for the ten patients all occurred with the range error of +3%, that is, when the delivered beams under-shoot compared to the planned range. This underlines the importance of reducing range uncertainty in future technological development.
The construction of PTV for proton therapy is a topic of debate and further investigation. The geometric concept of PTV may not fully account for the CTV dose distribution uncertainty in proton therapy [27, 28]. Many researchers have proposed alternative approaches, for example, proton beam specific individual PTVs [29], or robust optimization [30]. However, there has not been a single best approach identified, and the current clinical practice is to use the conventional PTV in IMPT planning [18, 31, 32], as adopted by the present study,
Robustness evaluation using a fixed-shape CTV may be over-optimistic. Internal anatomic variations, for example, bladder filling, can change the shape and location of cervical CTV from day to day [33]. Also, daily anatomic changes in the patient may affect the proton beam range. Variations in bladder, bowel and rectum filling may be reduced to a minimum by controlling the time and amount of water and food intake before treatment; consistent and rigid immobilization could reduce patient positional variation. Without reliable volumetric imaging throughout the treatment course, the effect of anatomic changes was not assessed in this study. A follow-up study on this issue is being conducted at the authors’ institution based on weekly CT data.
It is unknown which SUV threshold in FLT-PET imaging is most representative of active bone marrow. For the purpose of treatment planning, however, we have found that setting dose objectives using SUV2, SUV3, or SUV4 in IMPT optimization did not lead to different plan qualities, and the DVH using relative volume for SUV2, SUV3, and SUV4 were very close to each other. Therefore, the SUV4 region was chosen for treatment planning and evaluation of OAR.
This study has shown that IMPT can spare functional bone marrow relative to IMRT in a wide range of dose levels, from 5 Gy to 40 Gy, even with range and setup uncertainties. While the limiting dose level for functional bone marrow is still subject to further investigation, the results of this study indicate that IMPT may bring clinical benefits regardless of the critical dose levels.
Conclusion
This study demonstrates the potential clinical benefits of IMPT in reducing pelvic bone marrow toxicity in the treatment of cervical cancer. A significant reduction of functional bone marrow volume receiving 5 Gy to 40 Gy can be achieved by IMPT compared to IMRT, even when ±3% range uncertainties and ±3mm translational setup errors in all three principal dimensions are considered. This indicates the potential of IMPT to further reduce hematologic toxicity when compared to IMRT, which may lead to improved outcomes for cervical cancer patients by improving their tolerance to concomitant standard chemotherapy.
Supplementary Material
Acknowledgement
Eric Dinges and Nicole Felderman were supported by the Iowa Center for Research by Undergraduates. The authors thank RaySearch Laboratories for providing RayStation treatment planning system. The authors acknowledge and thank the clinical trials research team responsible for this study, including K. Bodeker, J. Hershberger, S. Vollstedt, and J. Koeppel; the authors thank Gareth Smith for assistance in preparing the figures. FLT PET imaging in this work was in part funded by the National Cancer Institute (NIH 3P30CA086862 and NIH 1R01CA169336-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: none.
References
- 1.Brixey CJ, Roeske JC, Lujan AE, et al. Impact of intensity-modulated radiotherapy on acute hematologic toxicity in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;54:1388–1396. doi: 10.1016/s0360-3016(02)03801-4. [DOI] [PubMed] [Google Scholar]
- 2.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. New Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 3.Mell LK, Kochanski JD, Roeske JC, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:1356–1365. doi: 10.1016/j.ijrobp.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Nugent EK, Case AS, Hoff JT, et al. Chemoradiation in locally advanced cervical carcinoma: an analysis of cisplatin dosing and other clinical prognostic factors. Gynecol Oncol. 2010;116:438–441. doi: 10.1016/j.ygyno.2009.09.045. [DOI] [PubMed] [Google Scholar]
- 5.Albuquerque K, Giangreco D, Morrison C, et al. Radiation-Related Predictors of Hematologic Toxicity After Concurrent Chemoradiation for Cervical Cancer and Implications for Bone Marrow–Sparing Pelvic IMRT. Int J Radiat Oncol Biol Phys. 2011;79:1043–1047. doi: 10.1016/j.ijrobp.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 6.Mell LK, Schomas DA, Salama JK, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:1431–1437. doi: 10.1016/j.ijrobp.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 7.Platta CS, Bayliss A, McHaffie D, et al. A dosimetric analysis of tomotherapy based intensity modulated radiation therapy with and without bone marrow sparing in gynecologic malignancies. Technol Cancer Res T. 2013;12:19–29. doi: 10.7785/tcrt.2012.500300. [DOI] [PubMed] [Google Scholar]
- 8.Rose BS, Aydogan B, Liang Y, et al. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:800–807. doi: 10.1016/j.ijrobp.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klopp AH, Moughan J, Portelance L, et al. Hematologic toxicity in RTOG 0418: a phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys. 2013;86:83–90. doi: 10.1016/j.ijrobp.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mell LK, Tiryaki H, Ahn K-H, et al. Dosimetric comparison of bone marrow-sparing intensity-modulated radiotherapy versus conventional techniques for treatment of cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:1504–1510. doi: 10.1016/j.ijrobp.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 11.Liang Y, Bydder M, Yashar CM, et al. Prospective study of functional bone marrow-sparing intensity modulated radiation therapy with concurrent chemotherapy for pelvic malignancies. Int J Radiat Oncol Biol Phys. 2013;85:406–414. doi: 10.1016/j.ijrobp.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 12.McGuire SM, Menda Y, Boles Ponto LL, et al. 3’-deoxy-3’-[18F] fluorothymidine PET Quantification of Bone Marrow Response to Radiation Dose. Int J Radiat Oncol Biol Phys. 2011;81:888–893. doi: 10.1016/j.ijrobp.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGuire SM, Menda Y, Boles Ponto LL, et al. A methodology for incorporating functional bone marrow sparing in IMRT planning for pelvic radiation therapy. Radiother Oncol. 2011;99:49–54. doi: 10.1016/j.radonc.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Agool A, Schot BW, Jager PL, Vellenga E. 18F-FLT PET in hematologic disorders: a novel technique to analyse the bone marrow compartment. J Nucl Med. 2006;47:1592–1598. [PubMed] [Google Scholar]
- 15.Hayman JA, Callahan JW, Herschtal A, et al. Distribution of proliferating bone marrow in adult cancer patients determined using FLT-PET imaging. Int J Radiat Oncol Biol Phys. 2011;79:847–852. doi: 10.1016/j.ijrobp.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 16.Song WY, Huh SN, Liang Y, et al. Dosimetric comparison study between intensity modulated radiation therapy and three-dimensional conformal proton therapy for pelvic bone marrow sparing in the treatment of cervical cancer. J Appl Clin Med Phys. 2010;11:3255. doi: 10.1120/jacmp.v11i4.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paganetti H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys Med Biol. 2012;57:R99. doi: 10.1088/0031-9155/57/11/R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 2: the potential effects of inter-fraction and inter-field motions. Phys Med Biol. 2008;53:1043–1056. doi: 10.1088/0031-9155/53/4/015. [DOI] [PubMed] [Google Scholar]
- 19.Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 1: the potential effects of calculational uncertainties. Phys Med Biol. 2008;53:1027–1042. doi: 10.1088/0031-9155/53/4/014. [DOI] [PubMed] [Google Scholar]
- 20.Trofimov A, Unkelbach J, DeLaney TF, Bortfeld T. Visualization of a variety of possible dosimetric outcomes in radiation therapy using dose-volume histogram bands. Pract Radiat Oncol. 2012;2:164–171. doi: 10.1016/j.prro.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trofimov A, Nguyen PL, Efstathiou JA, et al. Interfractional Variations in the Setup of Pelvic Bony Anatomy and Soft Tissue, and Their Implications on the Delivery of Proton Therapy for Localized Prostate Cancer. Int J Radiat Oncol Biol Phys. 2011;80:928–937. doi: 10.1016/j.ijrobp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qamhiyeh S, Geismar D, Pöttgen C, Stuschke M, Farr J. The effects of motion on the dose distribution of proton radiotherapy for prostate cancer. J Appl Clin Med Phys. 2012:13. doi: 10.1120/jacmp.v13i3.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thörnqvist S, Muren LP, Bentzen L, et al. Degradation of target coverage due to inter-fraction motion during intensity-modulated proton therapy of prostate and elective targets. Acta Oncol. 2013;52:521–527. doi: 10.3109/0284186X.2012.752860. [DOI] [PubMed] [Google Scholar]
- 24.Albertini F, Bolsi A, Lomax AJ, et al. Sensitivity of intensity modulated proton therapy plans to changes in patient weight. Radiother Oncol. 2008;86:187–194. doi: 10.1016/j.radonc.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 25.Kutcher GJ, Mageras GS, Leibel SA. Control, correction, and modeling of setup errors and organ motion. Semin Radiat Oncol. 1995;5:134–145. doi: 10.1054/SRAO00500134. [DOI] [PubMed] [Google Scholar]
- 26.Guckenberger M, Meyer J, Vordermark D, et al. Magnitude and clinical relevance of translational and rotational patient setup errors: a cone-beam CT study. Seminars in Radiat Oncol. 2006;65:934–942. doi: 10.1016/j.ijrobp.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 27.International Commission on Radiation Units and Measurements. Prescribing, Recording, and Report Proton-Beam Therapy (ICRU Report 78) J ICRU. 2007;7:83–94. [Google Scholar]
- 28.Park PC, Cheung JP, Zhu XR, et al. Statistical Assessment of Proton Treatment Plans Under Setup and Range Uncertainties. Int J Radiat Oncol Biol Phys. 2013;86:1007–1013. doi: 10.1016/j.ijrobp.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park PC, Zhu XR, Lee AK, et al. A beam-specific planning target volume (PTV) design for proton therapy to account for setup and range uncertainties. Int J Radiat Oncol Biol Phys. 2012;82:e329–e336. doi: 10.1016/j.ijrobp.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fredriksson A, Forsgren A, Hårdemark B. Minimax optimization for handling range and setup uncertainties in proton therapy. Med Phys. 2011;38:1672–1684. doi: 10.1118/1.3556559. [DOI] [PubMed] [Google Scholar]
- 31.Liu W, Zhang X, Li Y, Mohan R. Robust optimization of intensity modulated proton therapy. Med Phys. 2012;39:1079–1091. doi: 10.1118/1.3679340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer J, Bluett J, Amos R, et al. Spot scanning proton beam therapy for prostate cancer: treatment planning technique and analysis of consequences of rotational and translational alignment errors. Int J Radiat Oncol Biol Phys. 2010;78:428–434. doi: 10.1016/j.ijrobp.2009.07.1696. [DOI] [PubMed] [Google Scholar]
- 33.Lim K, Small W, Jr, Portelance L, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int J Radiat Oncol Biol Phys. 2011;79:348–355. doi: 10.1016/j.ijrobp.2009.10.075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.