Abstract
Perchlorate, a common aquatic contaminant, is well known to disrupt homeostasis of the hypothalamus-pituitary-thyroid axis. This study utilizes the threespine stickleback (Gasterosteus aculeatus) fish to determine if perchlorate exposure during certain windows of development has morphological effects on thyroid and gonads. Fish were moved from untreated water to perchlorate-contaminated water (30 and 100 mg/L) starting at 0, 3, 7, 14, 21, 42, 154 and 305 days post fertilization until approximately one year old. A reciprocal treatment (fish in contaminated water switched to untreated water) was conducted on the same schedule. Perchlorate exposure increased angiogenesis and follicle proliferation in thyroid tissue, delayed gonadal maturity, and skewed sex ratios towards males; effects depended on concentration and timing of exposure. This study demonstrates that perchlorate exposure beginning during the first 42 days of development has profound effects on stickleback reproductive and thyroid tissues, and by implication can impact population dynamics. Long-term exposure studies that assess contaminant effects at various stages of development provide novel information to characterize risk to aquatic organisms, to facilitate management of resources, and to determine sensitive developmental windows for further study of underlying mechanisms.
Keywords: Gasterosteus aculeatus, endocrine disruption, thyroid hormone, ovary, testis, histology
1. Introduction
The perchlorate ion (ClO4−) is a common aquatic contaminant in the United States (U.S.) and has been classified as a contaminant of concern by the U.S. Environmental Protection Agency (EPA), which has committed to setting drinking water limits under the Clean Water Act (USEPA, 2011). The U.S. Department of Defense (DoD) uses a majority of the commercially available perchlorate for ammunition and solid rocket propellant and most contamination originates from military and manufacturing sites (Morrison et al., 2006; Trumpolt et al., 2005; Urbansky, 2002). Naturally produced perchlorate salts persist at low levels in arid regions such as Antarctica, the Atacama Desert in Chile, and the Southwestern U.S. (Kounaves et al., 2010; Rao et al., 2007; Urbansky et al., 2001).
Perchlorate salts are highly water soluble and the ion is kinetically stable, nonreactive and resistant to adsorption in solution (Brown and Gu, 2006; Morrison et al., 2006). These properties make perchlorate highly mobile and readily available to aquatic organisms that are exposed through ambient water (via respiratory and gastrointestinal epithelia, and integument) and/or ingested food (Furin et al., 2013; Huber et al., 2011). The widespread occurrence and persistence of this known endocrine disruptor in water sources is a concern for the health of wildlife and humans. Understanding the adverse effects of exposure throughout an organism’s lifecycle (fertilization to maturation) is crucial to elucidating fitness and population level consequences as well as identifying cohorts and stages of concern.
The toxic effects of perchlorate stem from its ionic similarity to iodide. It competitively inhibits the uptake of iodide via the sodium/iodide symporter (NIS, alias SLC5a5) in the basolateral membrane of thyroid follicle cells (Carr et al., 2005; Wolff, 1998). In sufficient concentration and with chronic exposure, perchlorate effectively inhibits the synthesis of thyroid hormones (TH: thyroxine, T4 and triiodothyronine, T3), which are essential regulators of metabolism, growth, development and metamorphosis in vertebrates (Carr et al., 2005; Choksi et al., 2003; Wolff, 1998). Lack of iodide uptake halts production of T4 and can lead to increased thyroid stimulating hormone due to lack of negative feedback to the hypothalamus and pituitary as well as hypothyroidism and changes in thyroid tissue morphology; these changes include follicle cell hypertrophy, proliferation of follicles and surrounding tissue, increased vasculature, and reduced colloid (Blanton and Specker, 2007; Choksi et al., 2003; Mukhi et al., 2005; Tietge et al., 2010).
Perchlorate interferes with normal development and reproduction in multiple taxa, including amphibians, birds, and mammals (Kendall and Smith, 2006). Studies utilizing zebrafish (Danio rerio) (Mukhi et al., 2005; Patiño and Mukhi, 2007; Patiño et al., 2007; Patiño et al., 2003; Schmidt et al., 2012), eastern mosquitofish (Gambusia holbrooki) (Bradford et al., 2005; Park et al., 2006a), threespine stickleback (Gasterosteus aculeatus; hereafter, stickleback) (Bernhardt and von Hippel, 2008; Bernhardt et al., 2006; Bernhardt et al., 2011; Furin et al., 2015; Petersen et al., 2015), fathead minnows (Pimephales promelas) (Crane et al., 2005; Pickford et al., 2005), and short-finned molly (Poecilia sphenops) (Burcu et al., 2009) have all found detrimental effects on development and/or reproduction from perchlorate exposure. These detrimental effects include: reduced growth rates (Bernhardt et al., 2011; Crane et al., 2005; Liu et al., 2008; Mukhi and Patiño, 2007; Mukhi et al., 2007a; Park et al., 2006b; Schmidt et al., 2012), skeletal abnormalities (Bernhardt et al., 2011; Furin et al., 2015), abnormal thyroid histomorphology (Bradford et al., 2005; Crane et al., 2005; Liu et al., 2006; Liu et al., 2008; Mukhi et al., 2005; Mukhi and Patiño, 2007; Patiño et al., 2003; Petersen et al., 2015; Schmidt et al., 2012), reduced reproductive output (Bernhardt and von Hippel, 2008; Bernhardt et al., 2006; Mukhi and Patiño, 2007; Patiño et al., 2003), skewed sex ratio (Mukhi et al., 2007a), and intersex gonads (Bernhardt et al., 2006).
Increasing evidence shows that thyroid hormones play a critical role in regulating gonadal development in teleosts (Liu et al. 2011, Flood et al. 2013). Thyroid hormone receptors are present on teleost ovarian and testicular cells (Nelson and Habibi 2009) and goitrogens affect gonadal development in fishes including increased numbers of Sertoli and germ cells in tilapia (Oreochromis niloticus) exposed to thiouracil (PTU) (Matta et al., 2002) and impaired testicular and ovarian recrudescence in catfish (Clarias gariepinus) exposed to thiourea (Supriya et al., 2005; Swapna et al., 2006). Perchlorate causes a female bias in the sex ratio of zebrafish (Mukhi et al., 2007a; Sharma and Patiño, 2013), while evidence of masculinization was found in stickleback (Bernhardt et al., 2006). A critical window for sex determination in stickleback was determined to be within the first 14 days post hatch (dph) (Hahlbeck et al., 2004). Lewis et al. (Lewis et al., 2008) found that sex determination in stickleback was accompanied by proliferation of primordial germ cells in genetic females, and no proliferation in genetic males, before 11 days post fertilization (dpf). Perchlorate has the potential to cause perturbations during these sensitive early developmental periods.
To determine the sensitive developmental phases, we studied the morphological effects of perchlorate exposure on stickleback thyroid and gonads using various exposure durations and time points during development. Changes in thyroid tissue histomorphology are a sensitive endpoint for some endocrine disruptors, such as perchlorate, that directly alter iodide disposition (Carr and Patiño, 2011). Due to the importance of TH during development, the masculinizing effects of perchlorate on stickleback and their early sex determination, we hypothesized that perchlorate exposure during early development and metamorphosis (through 21 dpf) would cause significant histological changes to thyroid tissue such as thyrocyte hypertrophy, colloid depletion and follicle hyperplasia, as well as abnormal gonads and male-biased sex ratios.
2. Materials and Methods
2.1. Experimental design
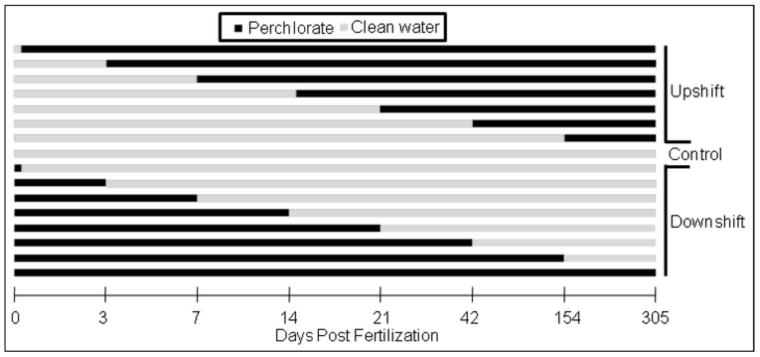
In a chronic static renewal test, fish were either introduced or removed from sodium perchlorate (> 98% purity, Sigma-Aldrich, St. Louis, MO, USA) contaminated (30 mg/L or 100 mg/L) water at varying times during development. This was done to determine critical developmental windows (timing) of exposure. Specifically two exposure regimes were carried out in which fish were either moved from control water to perchlorate-treated water (upshift) or from perchlorate-treated water to control water (downshift) at 0, 3, 7, 14, 21, 42, 154 or 305 dpf (Fig. 1). Once they reached approximately one year old, stickleback were euthanized and processed for histological analysis of thyroid and gonads.
Figure 1.
Perchlorate exposure regime used for both 30 and 100 mg/L exposures with three replicates at each dpf. Zero dpf downshift fish were exposed during fertilization and for 15 minutes post fertilization before their transfer to clean water.
2.2. Fish collection and husbandry
Stickleback were collected from Rabbit Slough (61.534° N, 149.266° W) in the Matanuska-Susitna Valley, Alaska on 4 June 2008. Rabbit Slough fish were chosen to represent the ancestral oceanic ecotype (Cresko, 2000; Hohenlohe et al., 2010). Fish were kept in outdoor pools filled with de-chlorinated city water with 3g/L Instant Ocean© added. A mass cross using eggs stripped from 40 females and sperm collected from 40 males was performed on 10 June, 2008. Embryo medium consisted of reverse osmosis purified water to which Instant Ocean© was added to 4 g/L. Contaminated (treatment) water was produced by adding sodium perchlorate dried at 90° C before weighing, at a nominal 30 and 100 mg/L. Clutches of eggs from the 40 females were combined in one container in order to randomize them before dividing them into 38 Petri dishes (100 X 20mm) with a previously determined water treatment (control: no perchlorate added, 30 or 100 mg/L of perchlorate). The treatments were divided among the 38 Petri dishes as follows: control = eight, 30 mg/L upshift = eight, 100 mg/L upshift = eight, 30 mg/L downshift = seven, and 100 mg/L downshift = seven. The eggs were subsequently fertilized with mixed sperm from the 40 males. Each Petri dish of embryos was then subdivided into three Petri dishes with approximately 100 embryos per dish for three replicates of each treatment (114 total Petri dishes). Embryos were incubated at 20 ± 0.5° C. Water was changed daily and dead embryos were removed for the first 10 dpf.
By 10 dpf, most embryos had hatched and embryos from each Petri dish were transferred to their own 56.8L aquarium (60cm X 31cm X 32cm) with aerated AZOO© multi sponge filters (65mm diameter). All aquaria started with 6L of water because of the small size of the fish. The water level was proportionally increased as the fish grew to maintain fish density at appropriate and consistent conditions (1L water per 1cm fish). Water changes (15% of total volume) were carried out biweekly and as needed. Two to three ml of Bacta-pur© N3000 live bacteria (IET-Aquaresearch Ltd., Quebec, Canada) were added to each tank once a week to limit nitrate concentrations. Reverse osmosis purified water was added weekly to compensate for evaporative loss. A YSI photometer model 9100 (Yellow Springs Instrument Co., Yellow Springs, OH, USA) was used to periodically check salinity (4-5 g/Kg), pH (7.0-8.0), and ammonia (<2 mg/L total nitrogen); no out of range levels were detected. Perchlorate concentrations in aquaria were measured with an Acorn Ion 6 meter (Oakton Instruments, Vernon Hills, USA) with a perchlorate ISE electrode (Cole-Parmer, Vernon Hills, IL, USA). Stickleback fry (<2 months old) were fed live brine shrimp and a mixture of Golden Pearls 100 (a commercial larval food), Artemia food (both from Aquatic Ecosystems, Apopka, FL, USA), and frozen ground brine shrimp (Artemia sp.; Brine Shrimp Direct, Ogden, UT, USA). Juvenile and adult fish were fed frozen brine shrimp daily. Perchlorate intake from food sources was assumed to be negligible. The photoperiod in the aquarium room was adjusted weekly to mimic natural conditions for Anchorage, Alaska. Mean temperature in the aquaria throughout the experiment was maintained at 13.5 ± 0.5°C.
2.3. Histology
At approximately one year old fish were euthanized and collected for histological analysis. A total of 5 fish from each aquarium were collected with the exception of two aquaria in which no fish survived. Fish were euthanized with an overdose of MS-222 anesthetic (Argent Chemical Laboratories, Richmond, WA, USA) and a digital photograph was taken of both sides of each fish. The caudal fin was removed and preserved in 95% undenatured ethanol for DNA analysis. The abdomen was opened with a scalpel before placing the carcass in Dietrich’s solution (Kent et al., 2012). After fixing for 2-5 days, fish were preserved in 70% isopropanol. Fish were then dehydrated in a graded series of ethanol using an auto processor, embedded in TissuePrep-2 Embedding Media (Fisher Scientific, PA, USA), coronally sectioned at 5 μm thickness and stained with hematoxalin and eosin. Stained sections were embedded with Cytoseal XYL (Richard-Allan Scientific, MA, USA) and a coverslip was added. Evidence of thyroid histopathology was examined for 194 adult stickleback. Sections were also used to characterize gonads of 282 adult fish (145 males and 137 females). The coronal sectioning was not always successful in capturing the target tissues and sections missing those tissues were excluded from the analysis (see Results for sample sizes of each treatment). Additionally, due to the number of treatments and fish, samples were collected over six weeks when the fish were 344-386 dpf (median = 365 dpf). Variation in age of stickleback was evenly distributed among the treatments.
2.4. Thyroid hormone extraction and ELISA
A subset of adult fish was collected for TH analysis including fish from control (n = 7), 100 mg/L 154 dpf downshift (n = 4), and 100mg/L 3 dpf upshift (chronic; n = 6) treatments. Fish were collected as above, but not placed in fixative, and were stored frozen whole at −80°C until hormone analysis. T4 and T3 assays were carried out following the procedures in Petersen et al. (2015). Briefly, THs were extracted from whole-body homogenates as outlined in Crane et al. (2004) except that the final extract was stored dry at −80°C until the day of the assay. Each sample was reconstituted with 330μL of EIA buffer (0.1M PBS, 0.15M NaCl, 0.1% BSA, pH 7.4). T4 was assayed in duplicate from each sample using 25μL per well (using the kit: Total T4 EIA, MP Biomedicals, Santa Ana, CA) and T3 was assayed in triplicate using 38μL per well (using the kit: Total T3 EIA, MP Biomedicals). Both EIAs were validated using tests of parallelism and standard addition. Intra-assay variation was 6.9% for T3 and 3.6% for T4. Inter-assay variation was 12.2% and 13.0% for T3 and T4, respectively.
2.5. Imaging and histomorphology measurements
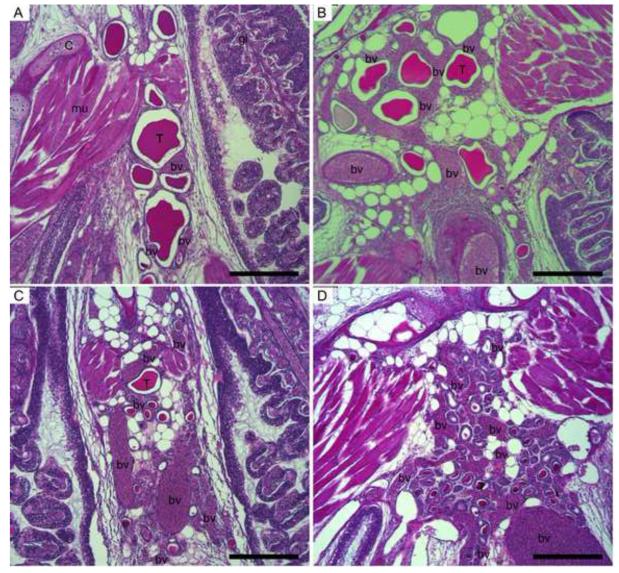
Photomicrographs of the assessed target tissues were generated using a Leica DM4500B microscope, Leica DFC420C microscope camera and Leica Application Suite imaging software (Leica Microsystems, Wetzlar, Germany). Measurements were made on micrographs using ImageJ (Rasband, 1997-2013) to quantify thyroid tissue morphology. All follicles were counted on the section with the greatest amount of thyroid tissue. Subsequently, four follicles per section were randomly chosen (tools provided at www.random.org) and the following measurements made: 1) area of colloid, 2) area of follicle (lumen, not including follicle cells), 3) circularity of follicle , 4) cell height of four follicle cells at approximately the 12, 3, 6 and 9 o’clock positions, and 5) area of follicle with no apparent colloid (follicle area (number 2 above) - colloid area (number 1 above)). Means of these measures were calculated for each fish. In addition, the colloid was characterized qualitatively including the evaluation of depletion (reduction in colloid area and quality), texture and density. The extent of small vessels surrounding thyroid follicles (angiogenesis score) was determined using control (untreated water for the entire duration of the study) histological sections as a baseline and assigning a score to each section as similar to control or as slight, moderate, or severe increase in angiogenesis (Fig. 2).
Figure 2.
Photomicrographs of threespine stickleback thyroid tissue showing typical angiogenesis scores of normal (A), mild (B), moderate (C), or severe (D). bv = blood vessels, T = thyroid follicle, C = cartilage, gi = gill, mu = muscle. Scale bar = 200 μm.
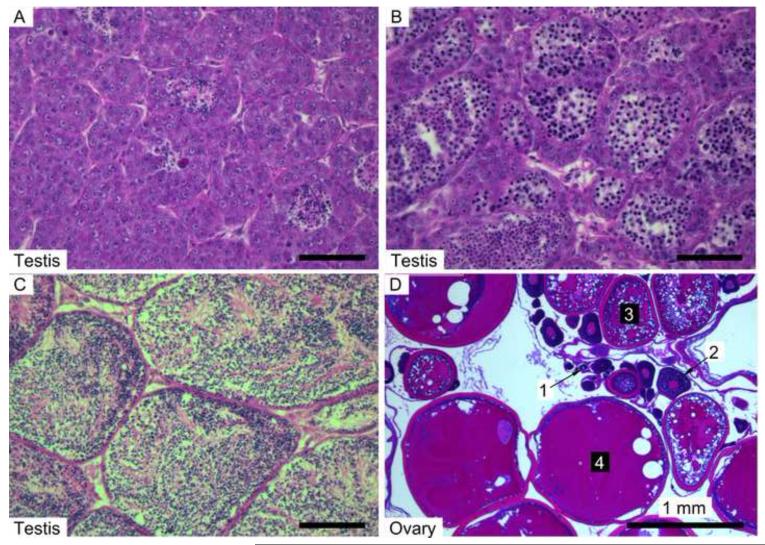
The stage of maturity was determined for gonads of 4-6 fish from each treatment. Stages (1-4) of all oocytes visible on a slide were counted. Stages identified in this study were drawn from Sokołowska and Kulczykowska (2006): early (Stages 1 and 2), mid (3 and 4), late (5 and 6), and mature (7 and 8) (Fig. 3 & Suppl. Table 1). Testicular lobules tended to be homogenous in their maturity state and were given one score. Stages for testicular lobules were also modified from Sokołowska and Kulczykowska: early (Stages 6 and 7), intermediate (1 and 2), late (3 and 4), and sexual phase with little to no spermatozoa present in tubules (5) (Fig. 3 & Suppl. Table 1).
Figure 3.
Photomicrographs of threespine stickleback gonads. Testes were scored for maturity stage as: early (A), intermediate (B), or late (C). Ovaries contained oocytes at various stages (D) and were scored using four stages: early (1), mid (2), late (3) and mature (4).
2.6. Determination of sex via genotyping
Genomic PCR was used to determine the genotypic sex following the methods of Griffiths et al. (2000). We genotyped the sex of 146 individual stickleback (control, n = 63; 30mg/L, n = 43; 100mg/L, n = 40) for which the phenotypic sex was determined from visual inspection of gonads in histology slides. Genomic DNA was extracted from caudal fins using a DNeasy Blood and Tissue Kit (Qiagen Inc., Valencia, CA) following manufacturer’s instructions for purification of total DNA from animal tissues. The PCR reactions were carried out in 25-μl volumes containing 10X PCR buffer (100mM Tris-HCL, 500 mM KCl), 0.5 μM of each primer (Ga1F: CTTCTTTCCTCTCACCATACTCA and Ga1R: AGATGACGGGTTGATAAACAG), 50 μM of each deoxyribonucleotide triphosphate, 100 to 150 ng of target DNA, 1.25 U of TaqDNA polymerase (TaKaRa Taq, Fisher Scientific), and 1.5 mM MgCl2. The thermocycler conditions were 94°C for 3 min, followed by 36 cycles of 94°C for 45 sec, 44°C for 45 sec, and 72°C for 45 sec, followed by a final extension at 72°C for 10 min. The primers produce fragments of two sizes, approximately 370 and 600 bp, with characteristic male and female XY (both bands) and XX (only the 600-bp band) genotypes, respectively. Genotypes were visualized by electrophoresis on a 1.5% agarose gel and genotypic sex was determined by gel banding pattern.
2.7. Data analysis
Normality of dependent variables was determined using the Shapiro-Wilk test. Area measurements and number of follicles were positively skewed and were log transformed (log(1+x)) to achieve normality. Pearson’s product-moment correlation was used to determine the relationships between colloid area, follicle area and no colloid area. A multivariate analysis of variance test (MANOVA) was used to evaluate interactions and significance between two dependent variables (number of follicles and area of colloid) and four categorical independent variables (concentration, dpf, upshift/downshift, and tank). The proportion of all fish examined for gonad maturity with the most mature stage (stage 3 in males and stage 4 in females) present was used as an indicator of reproductive maturity due to insufficient sample sizes to detect differences in timing of exposure. Results were combined for fish in the 0, 3, 7, and 14 dpf treatment groups in the upshift exposure regime to increase sample size and examine those fish exposed for the longest durations and during early development. Differences in thyroid morphology between treatments (dpf) within each exposure regime and concentration were analyzed using the Kruskal-Wallis test. Pair-wise contrasts of treatment and control fish were achieved with a non-parametric Tukey multiple contrast test with the nparcomp package v2.0 (Konietschke et al., 2014). The pooled mean of all control fish was used for these contrasts (n=48). Contingency tables were evaluated with a Fisher Exact Test or Chi-square test as appropriate. When the assumptions for parametric tests were violated, the Kruskal-Wallis test was used. All analyses were considered significant when p < 0.05 and were performed using R (version 3.0.1, (RCoreTeam, 2013)).
3. Results
3.1. Thyroid hormone levels
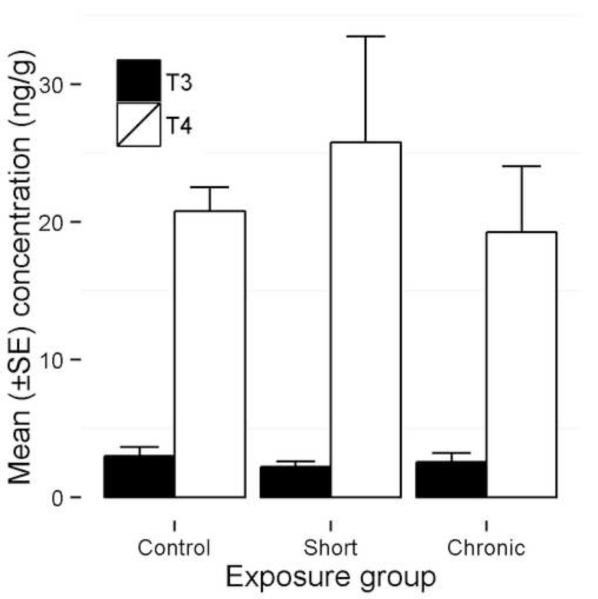
Controls showed no significant differences with either 100 mg/L 154 dpf downshift or 100 mg/L 3 dpf upshift treatment groups for whole body homogenate T4 and T3 (Kruskal-Wallis: T4, Χ2 = 1.98, p = 0.37; T3, Χ2 = 0.49, p = 0.78; Fig. 4). As expected, T4 levels were consistently higher than T3 levels (mean = 2.99 ± 2.2ng/g and 0.88 ± 0.32ng/g, respectively; t-test: t = −8.27, df = 17, p <0.0001).
Figure 4.
Whole body homogenate tissue concentration of thyroxine (T4) and triiodothyronine (T3) in threespine stickleback exposed to 100 mg/L perchlorate either chronically beginning at 3 dpf (chronic) or beginning at fertilization and rescued at 154 dpf (short), as compared to controls. Fish were collected at one year of age.
3.2. Thyroid histomorphology
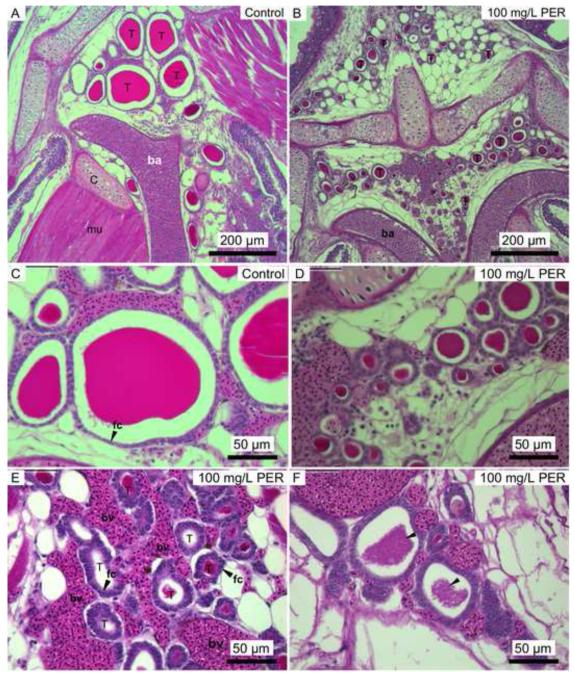
Thyroid tissue from fish maintained in untreated water had large, colloid filled follicles surrounded by a layer of squamous or cuboidal epithelial cells (Fig. 5A & C). Stickleback chronically exposed to perchlorate-treated water exhibited a proliferation of small follicles (hyperplasia), hypertrophy of follicle cells, and colloid depletion (Fig. 5B, D, E, & F). An increased abundance of small blood vessels (increased angiogenesis) was evident in many of the chronically-treated fish compared to controls (Fig. 5E).
Figure 5.
Photomicrographs of threespine stickleback thyroid tissue from control water (A & C) and 100 mg/L perchlorate exposures (B, D, E & F). T = thyroid follicle, ba = branchial artery, bv = blood vessels, fc = follicular cell. Some perchlorate-exposed fish exhibited thyrocyte (fc) hypertrophy (arrows in E) compared to control thyrocytes (arrow in C) and grainy colloid (arrows in F). Note the proliferation of small follicles in exposed fish (B) compared to control fish (A).
Area measurements of the follicle and colloid were highly correlated (Pearson’s product-moment correlations, n = 194; follicle area and colloid area, r = 0.97, p < 0.0001; follicle area and no colloid area, r = 0.98, p < 0.0001; and colloid area and no colloid area, r = 0.92, p < 0.0001). To avoid redundancy and because other measurements characterize the follicle, area of colloid was chosen to represent these measurements in further statistical analyses. All independent variables except tank were significant in the comprehensive MANOVA: concentration of perchlorate (number of follicles, p = 0.0032; colloid area, p < 0.0001), upshift or downshift exposure regime (number of follicles, p < 0.0001; colloid area, p < 0.0001), and dpf of upshift or downshift (number of follicles, p = 0.0229; colloid area, p < 0.0001). The upshift/downshift regime interacted significantly with both dpf and concentration (p < 0.0001 and p = 0.0057, respectively), which was anticipated due to the experimental design.
Sex was a significant factor in cell height, with males having significantly greater hypertrophy of thyrocytes than females regardless of treatment (t = −6.11, df = 144, p < 0.0001). All other thyroid metrics were not significantly different between sexes.
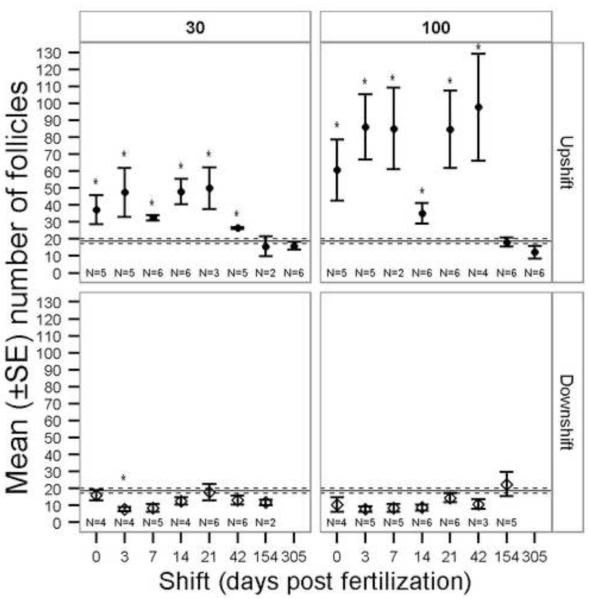
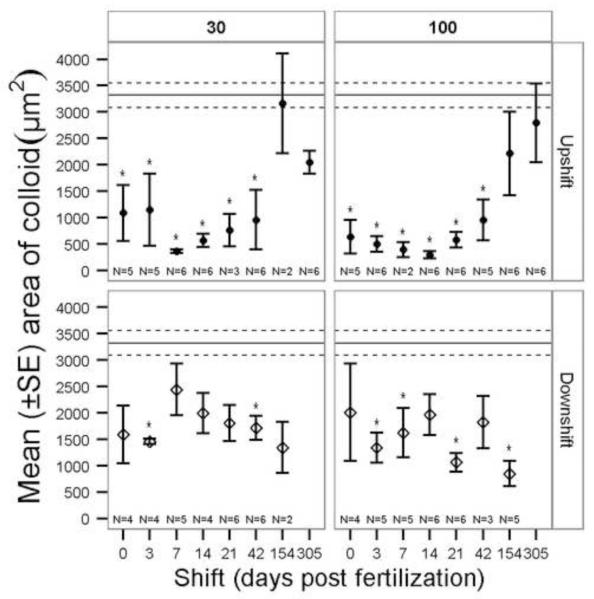
The number of follicles was significantly greater in the 100 mg/L and 30 mg/L upshift regimes than in the control treatment when exposures were initiated ≤ 42 dpf (Fig. 6). The Kruskal-Wallis for the 100 mg/L downshift treatments indicated significant differences present (Table 1), but there were no definitive patterns and no post-hoc significant differences. The area of colloid was significantly reduced in both upshift and downshift exposure regimes (Table 1, Fig. 7). Colloid area was reduced in the upshift 100 mg/L and 30 mg/L when exposure began ≤42 dpf. The upshift and downshift regimes had opposing trends as would be expected due to total duration effects of perchlorate exposure (Fig. 7).
Figure 6.
Mean (±SE) number of thyroid follicles in slides from perchlorate-exposed threespine stickleback. 30 = 30 mg/L perchlorate, 100 = 100 mg/L perchlorate. Exposure regime: Downshift = fish began in contaminated water and were moved to untreated water on the given day post fertilization (dpf), Upshift = fish began in untreated water and were moved to contaminated water on the given dpf. The Kruskal-Wallis test was used to compare treatments within each panel (concentration and exposure regime). The solid horizontal line is the control group mean and dashed lines are the SE of the control group. Asterisks indicate a significant difference from control (α = 0.05). Note that the x axis is not a linear scale.
Table 1.
Kruskal-Wallis tests (p value, H statistic) of each thyroid measurements within each exposure regime and concentration. Concentrations: 30 = 30 mg/L perchlorate, 100 = 100 mg/L perchlorate. Exposure regime: Downshift = fish began in contaminated water and were moved to clean water on the given day post fertilization (dpf), Upshift = fish began in clean water and were moved to contaminated water on the given dpf.
| 30 upshift | 30 downshift | 100 upshift | 100 downshift | |
|---|---|---|---|---|
| Area of colloid | <0.0001 39.72 |
0.001 24.35 |
<0.0001 56.72 |
<0.0001 32.08 |
| Area of follicles | <0.0001 37.38 |
0.002 22.41 |
<0.0001 55.24 |
<0.0001 30.85 |
| Total number of follicles |
0.0005 28.08 |
NS | <0.0001 44.88 |
0.032 15.28 |
| Thyrocyte cell height |
NS | 0.046 14.31 |
0.019 18.29 |
NS |
| Angiogenesis score |
<0.0001 60.26 |
<0.0001 52.59 |
<0.0001 70.73 |
<0.0001 33.40 |
| Circularity | NS | NS | 0.002 24.18 |
0.034 15.19 |
| Area without colloid |
<0.0001 34.38 |
0.005 20.48 |
<0.0001 51.48 |
<0.0001 25.69 |
Figure 7.
Mean (±SE) area of colloid in four follicles for perchlorate-exposed threespine stickleback. 30 = 30 mg/L perchlorate, 100 = 100 mg/L perchlorate. Exposure regime: Downshift = fish began in contaminated water and were moved to untreated water on the given day post fertilization (dpf), Upshift = fish began in untreated water and were moved to contaminated water on the given dpf. The Kruskal-Wallis test was used to compare treatments within each panel (concentration and exposure regime). The solid horizontal line is the control group mean and dashed lines are the SE of the control group. Asterisks indicate a significant difference from control (α = 0.05). Note that the × axis is not a linear scale.
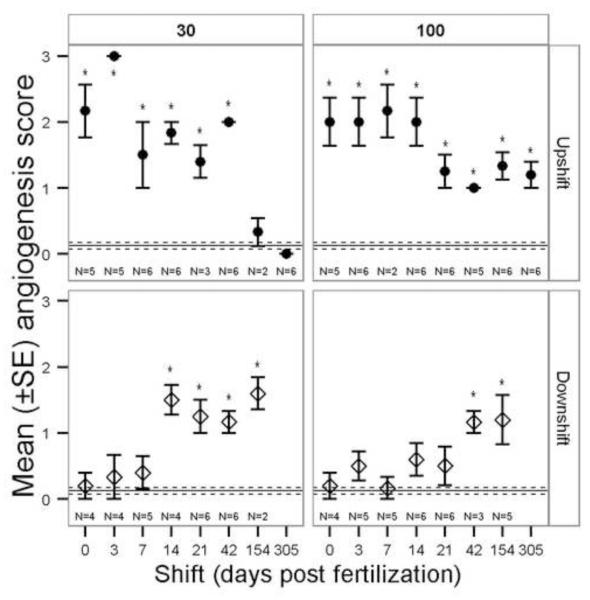
The angiogenesis score significantly changed among exposure regimes (Table 1, Fig. 8). In 30 mg/L upshift treatments all but 154 and 305 dpf had significantly greater number of blood vessels than controls while in 100 mg/L upshift, all treatments were significantly greater than controls. Upshift and downshift exposure regimes had opposite trends (Fig. 8). Downshift beginning at 14 dpf and later resulted in more angiogenesis in 30 mg/L exposures while downshift at 42 dpf and later resulted in more angiogenesis in 100 mg/L exposures (Fig. 8).
Figure 8.
Mean (±SE) angiogenesis score for perchlorate-exposed threespine stickleback. 30 = 30 mg/L perchlorate, 100 = 100 mg/L perchlorate. Exposure regime: Downshift = fish began in contaminated water and were moved to untreated water on the given day post fertilization (dpf), Upshift = fish began in untreated water and were moved to contaminated water on the given dpf. The Kruskal-Wallis test was used to compare treatments within each panel (concentration and exposure regime). The solid horizontal line is the control group mean and dashed lines are the SE of the control group. Asterisks indicate a significant difference from control (α = 0.05). Note that the × axis is not a linear scale.
Height of follicle cells was affected by perchlorate exposure (Table 1). In the 100 mg/L upshift exposure regime, the Kruskal-Wallis was significant, but only the 0 dpf sample had significantly greater cell height compared to controls and there was a negative trend with cell height decreasing as the day of exposure initiation increased. The 30 mg/L downshift exposure regime had significantly greater cell height in 7 and 42 dpf treatments, but overall showed no clear statistically significant trend. Circularity ranged from 0.68 to 0.96 with 1.0 defined as a perfect circle. The control group mean was 0.83 (n = 48). The 100 mg/L upshift exposure regime had significantly more circular follicles in the 14, 21 and 42 dpf treatments while only the 42 dpf treatment in 100 mg/L downshift had significantly more circular follicles. Neither timing of exposure nor concentration of perchlorate conclusively affected follicle circularity.
Most treatments exhibited greater qualitative colloid descriptors (depletion, grainy texture and decreased density) than controls regardless of concentration or timing of exposure. The percentage of individuals with colloid depletion for control, 30 and 100 mg/L exposed stickleback was 12.5, 31.3, and 50.0%, respectively, and there was a significant reduction of colloid in fish exposed to 100 mg/L beginning in the first 14 dpf compared to controls (Fisher exact test, p<0.05).
3.3. Gonad maturity and sex ratio
The maturity score was equivalent among the four earliest dpf treatments, in which all stages were observed in both males and females (Kruskal-Wallis test, df = 3: Females; 30 upshift, H = 5.97, p = 0.113, 100 upshift, H = 1.80, p = 0.616, Males; 30 upshift, H = 1.97, p = 0.579, 100 upshift, H = 1.30, p = 0.729). The frequency of individuals with late stage testes and oocytes significantly decreased with chronic exposure to perchlorate in both exposure concentrations for males and in 30 mg/L for females (Table 2). Additionally, sex ratio (identified by gonadal inspection) significantly shifted toward males in a concentration dependent manner (Table 2). Control stickleback had a sex ratio of 0.62:1 males:females (38% males), while the stickleback in 100 mg/L had a sex ratio of 2.08:1 (68% males), or 2 males for each female. Genotypic sex matched phenotypic sex in all but three individuals; one control fish was phenotypically male but genetically female, while two fish exposed to 100 mg/L perchlorate were phenotypically opposite of their genetic sex (one male and one female).
Table 2.
Percentage of late stage testes (stage 3) and oocytes (stage 4), and sex ratio in threespine stickleback exposed to perchlorate continuously beginning on 0, 3, 7, and 14 days post fertilization as combined data (upshift). Parentheses contain actual counts and asterisk indicates significant difference from control (Fishers Exact Test: p < 0.05)
| Male | Female | Sex ratio (M/F) |
|
|---|---|---|---|
| Control | 42.0 (10/24) | 41.0 (16/39) | 0.62 |
| 30 mg/L | 11.1 (2/18)* | 8.0 (2/25)* | 0.72 |
| 100 mg/L | 3.7 (1/27)* | 23.1 (3/13) | 2.08* |
4. Discussion
The present study utilized strategic timing of exposure during the stickleback lifecycle and varying durations of exposure to two perchlorate-treated water concentrations (compared to control, untreated water) over a one-year period. We did this to determine the sensitive developmental windows during which perchlorate exposure has morphological effects on stickleback thyroid and gonad tissues. One potentially confounding effect on sensitive developmental windows in this study is a difference in duration of exposure between the “shift” treatments (i.e., not all fish were exposed to perchlorate for the same duration). Given that this study took place over a year with longer exposure periods than most fish studies and in light of the results obtained, the differences in duration of exposure likely did not have a significant confounding effect in identifying the sensitive developmental windows. The novelty of the experimental design with both upshift and downshift exposure regimes that target specific points in development, not durations, provide evidence that sensitive developmental windows exist.
The concentrations of perchlorate in this study are within the environmentally relevant range (Carr and Patiño, 2011; Smith et al., 2001; Theodorakis et al., 2006a; Theodorakis et al., 2006b) and similar responses of thyroid histomorphology have been described at concentrations lower than those used in the present study for zebrafish (Liu et al., 2006; Schmidt et al., 2012) and mosquitofish (Bradford et al., 2005). Thyroid tissue, sex ratios and gonad maturity were sensitive to chronic exposure that evoked observable changes during early stages of development. The upshift/downshift experimental design illuminated critical windows during which perchlorate exposure caused histomorphological changes to thyroid tissue. In contrast, whole body T4 and T3 levels did not vary between control and treated fish (Fig. 4). This result is consistent with other studies using zebrafish (Mukhi et al., 2005), mosquitofish (Bradford et al., 2005), fathead minnow (Crane et al., 2005) and stickleback (Petersen et al., 2015). Thyroid tissue morphology may be more sensitive than whole body TH levels to perchlorate exposure due to direct effects on follicle cells, the complexity of HPT-axis feedback mechanisms, and TH reservoirs (Blanton and Specker, 2007; Carr and Patiño, 2011).
Hyperplasia of follicles has previously been described as a useful biomarker for perchlorate exposure in fish (Patiño et al., 2003; Schmidt et al., 2012) and our study confirmed its utility for stickleback. Follicle hyperplasia was the most obvious histomorphological response of the thyroid. The total number of follicles increased in fish exposed chronically to perchlorate beginning in the first 42 dpf (Fig. 6). Later initiation of exposures resulted in no detectable hyperplasia indicating a sensitivity to perchlorate only during early development. Fish in downshift treatments did not differ significantly from controls, demonstrating that follicle hyperplasia is not permanent (within the confines of one year old fish) and complete recovery is possible. The degree of hyperplasia was concentration-dependent (Fig. 6).
Area of colloid (highly correlated with area of follicle) was significantly reduced in fish exposed chronically to perchlorate beginning in the first 42 dpf (Fig. 7). The area of colloid in downshift treatments tended to be reduced, but showed no significant temporal trend. Recovery from reduced area of colloid occurred for some treatments, but not as readily as recovery from hyperplasia (compare downshift in Figs. 6 & 7). The response of colloid area was independent of perchlorate concentration (Fig. 7). In perchlorate-exposed stickleback, colloid area per follicle was reduced, but overall colloid area might have been maintained due to the increased number of follicles, which presumably compensated for reduced iodide uptake.
The circularity of follicles was variable but effects did not correlate with temporal differences in exposure to perchlorate. A concentration-dependent relationship was not detected. Conclusions from gross inspection of follicles include consistently circular small follicles in perchlorate-exposed fish and elongated or circular large follicles in control fish (compare Fig. 5A & B), but a difference in shape was not statistically significant. Schmidt et al. (2012) detected semi-quantitative changes in follicle shape (in- and out-foldings) in zebrafish exposed to 500 and 5000 μg/L perchlorate, which are lower concentrations that those used in the current study.
Mean hypertrophy of thyrocytes increased in some treatments, particularly in 100 mg/L perchlorate, but showed no statistically supported trends. The extended duration of exposure may have allowed for recovery from hypertrophy. Increased cell height has routinely been found in other fish studies with perchlorate exposure periods of 90 days or less (zebrafish (Liu et al., 2006; Liu et al., 2008; Mukhi et al., 2005; Mukhi and Patiño, 2007; Mukhi et al., 2007a; Patiño et al., 2003; Schmidt et al., 2012), mosquitofish (Bradford et al., 2005; Park et al., 2006b), fathead minnow (Crane et al., 2005)). In zebrafish, thyrocyte hypertrophy is less sensitive to perchlorate exposure than follicle number (Schmidt et al., 2012) and colloid depletion (Mukhi and Patiño, 2007; Patiño et al., 2003; Schmidt et al., 2012), but the opposite is true for African-clawed frogs (Xenopus laevis) (Goleman et al., 2002). In zebrafish, complete recovery from thyrocyte hypertrophy was documented in less than 12 weeks after a 12 week exposure period (Mukhi et al., 2005) and less than 15 days after a 37 day exposure period (Liu et al., 2008). Hypertrophy of follicle cells may be the result of stimulation from thyroid stimulating hormone (TSH) (Carr and Patiño, 2011; Choksi et al., 2003). TSH was not measured in the current study.
Colloid condition (texture, density) varied by individual and was not reliable for detecting temporal effects of exposure to perchlorate. Colloid appears to recover from perchlorate perturbations relatively quickly in fish (e.g., within four weeks in zebrafish (Mukhi et al., 2005)). Combining results from stickleback that were chronically exposed to perchlorate beginning in the first 14 dpf (upshift) demonstrated a concentration-dependent response in the percent occurrence of colloid depletion. Chronically exposed stickleback had reduced colloid per follicle with perchlorate exposure, consistent with previous studies (Liu et al., 2008; Mukhi et al., 2007a; Patiño et al., 2003; Schmidt et al., 2012).
Of the metrics employed in this study, angiogenesis had the most distinctive critical window with exposure between 7 and 154 dpf and 21 and 305 dpf for 30 and 100 mg/L, respectively (Fig. 8). Stickleback exposed to perchlorate anytime within those developmental windows expressed significantly increased angiogenesis. The precise boundaries of these developmental windows could not be determined from our experimental design because, for example, we conducted no exposure shifts between 42 and 154 dpf. The 100 mg/L downshift exposed fish recovered when removed from perchlorate up to 21 dpf while paradoxically, 30 mg/L exposed fish recovered only when removed from perchlorate up to 7 dpf (Fig. 8). Lack of observed increased angiogenesis in fish downshifted early may be due to the presence of maternal TH and lack of functioning thyroid follicles. Once angiogenesis occurred in stickleback, it was permanent within the timeframe of this study. In the upshift exposure regime, no matter when fish were placed in 100 mg/L perchlorate, they developed increased angiogenesis. Increased angiogenesis can occur quickly as demonstrated in zebrafish, which displayed increased angiogenesis after only two weeks of exposure to 90, 1131 and 11,480 μg/L perchlorate (Mukhi et al., 2005).
The timing of perchlorate exposure, especially in the upshift regime, appeared to contribute more than perchlorate concentration to inducing histomorphological changes to the thyroid. Due to the relatively high, but relevant, concentrations used in this study, further testing with lower perchlorate concentrations is needed to determine if dose-response relationships exist at fixed time points. The stark differences between upshift treatments initiated at ≤ 42 dpf vs. ≥ 154 dpf in area of colloid (30 and 100 mg/L; Fig. 7), number of follicles (30 and 100 mg/L; Fig. 6) and angiogenesis (30 mg/L; Fig. 8) provide convincing evidence that early developmental exposure to perchlorate alters the HPT-axis in stickleback.
Most important developmental milestones in stickleback occur by 42 dpf (Swarup, 1958). Thyroid tissue in stickleback appears between 8-11 dpf (unpublished data). At some point between 42 and 154 dpf, the critical window “closes” and most measured thyroid tissue is not permanently altered by exposure to perchlorate. Because adult stickleback thyroid tissue in exposures that began after 42 dpf is indistinguishable from thyroid tissue of fish in untreated water (for metrics that will recover with removal from perchlorate), it appears that adult thyroid tissue is able to compensate for the disruption caused by the perchlorate ion. This finding could be due to: 1) a decreased demand for TH post embryogenesis and metamorphosis, 2) altered TSH levels due to other stimuli (neural, hormonal, external, etc.), 3) physiological adjustments to the HPT-axis to provide adequate TH, or 4) increased sensitivity to TH so that low levels in treated fish evoke the same response as high levels in control fish. Greater sensitivity to perchlorate due to increased demand for TH in larvae and fry (Carr and Patiño, 2011) may contribute to the shift after 42 dpf.
It is likely that phenotypic differences in stickleback exposed to different perchlorate concentrations were caused by greater competitive inhibition of iodide uptake in the higher concentration, which in turn would result in less T4 synthesis and less negative feedback at the hypothalamus and/or pituitary. This feedback loop would stimulate TSH release, which is known to stimulate thyroid tissue hyperplasia and follicle cell hypertrophy (Carr and Patiño, 2011; Zoeller et al., 2007). In fathead minnows exposed to methimazole (a thyrotoxin that inhibits thyroperoxidase, an enzyme whose job is to oxidize iodide to iodine, and thus blocks TH synthesis) the level of the mRNA for the thyrotropin β-subunit was elevated in pituitary tissue (Lema et al., 2009).
The less compact thyroid tissue in freshwater fish is more dynamic than mammalian glandular thyroid and has been hypothesized to go through a histophysiological cycle (Blanton and Specker, 2007; Eales, 1979). Acceleration of this cycle due to perchlorate exposure could explain the reduction in colloid area per follicle (fewer large colloid filled follicles) and proliferation of small follicles more suited to concentrate iodide because of increased thyrocyte surface area (Eales, 1979). Additionally, small follicles may have different sensitivities to colloid depletion than large follicles, which could explain the lack of consistent results for colloid depletion in this study. Age and species of experimental animals and duration of exposure may affect the sensitivity of endpoints measured due to complexities of feedback pathways of the HPT-axis (Blanton and Specker, 2007; Carr and Patiño, 2011).
The frequency of late stage testes decreased in a concentration dependent manner in male stickleback chronically exposed to perchlorate (Table 2). In females, the frequency of late stage oocytes responded more strongly to 30 mg/L than 100 mg/L perchlorate, but was reduced in both concentrations (Table 2). This finding is consistent with a separate study in which we found that 100 mg/L perchlorate exposure led to females with predominantly early stage ovarian follicles (Petersen et al. 2015). Perchlorate may alter the rate of gametogenesis in both sexes. Sharma and Patiño (2013) described a greater proportion of late stage testes in 60 dpf male zebrafish along with a greater proportion of early stage oocytes in female zebrafish exposed to 100 mg/L sodium perchlorate. The temporal differences of exposure between the present study, Petersen et al. (2015), and Sharma and Patiño (2013) make comparisons difficult, but together these studies provide convincing evidence that perchlorate can alter gamete maturation in fish.
Gonad development changed despite the finding that whole body thyroid hormone levels in adults were not altered by perchlorate exposure, suggesting that perchlorate may act on gonads by extra-thyroidal pathways (see also Petersen et al. (2015)). Further investigation is needed to determine if perchlorate affects gonad maturation through direct or indirect pathways, perhaps through interactions between thyroidal and reproductive endocrine systems (Blanton and Specker, 2007; Cyr and Eales, 1996; Duarte-Guterman et al., 2014; Habibi et al., 2012).
The sex ratio was skewed toward males in fish exposed continuously to 100 mg/L perchlorate beginning in the first 14 dpf (Table 2). This result is in agreement with Lewis et al.’s (2008) finding that the likely window of stickleback sex determination is within approximately 11 dpf. Additionally, Bernhardt et al. (2006) concluded that perchlorate has a masculinizing effect on stickleback and observed hypertrophy of testes in males and ovotestes in some genotypic females chronically exposed to 100 mg/L perchlorate. None of the individuals in the present study had intersex gonads and genotypic sexing ruled out complete sex reversal as an explanation for the biased sex ratio. Greater mortality of females in the 100 mg/L treatments is likely the major cause of the observed altered sex ratio. Future experiments should test the hypothesis that males are more tolerant of perchlorate than females. The bias towards males in this study is in contrast to a female-biased sex ratio in zebrafish exposed to perchlorate (Mukhi et al., 2007a; Sharma and Patiño, 2013). Despite the sex-specific differential mortality due to perchlorate, our present data (Table 2) and previous studies on gonad development ((Bernhardt et al., 2006; Petersen et al., 2015)) still clearly demonstrate an effect of perchlorate on gonad differentiation in genotypic males and females; results are consistent with a masculinizing effect of perchlorate in stickleback.
The growing literature on effects of perchlorate on gonadal development in stickleback (Bernhardt et al., 2006; Petersen et al., 2015) and other taxa (Baldridge et al., 2004; Mukhi et al., 2007b; Sharma and Patiño, 2013) suggests a role for perchlorate in disrupting sex determination in vertebrates. Sex determination in teleosts varies by species and both genetic and environmental factors can influence the outcome (Mank and Avise, 2006). The direction of sex bias due to perchlorate may be related to species-specific sex determination mechanisms; further investigation of the underlying mechanisms could help to identify specific tissue targets and temporal sensitivities to perchlorate on a species-specific basis. Mechanistic studies would also inform potential risks of perchlorate exposure to human sexual development.
The results from this study demonstrate the scientific and management importance and implications of timing of water-based perchlorate exposure. Short term exposure studies can detect acute responses of thyroid tissue (e.g., (Bradford et al., 2005; Liu et al., 2008; Patiño et al., 2007; Tietge et al., 2010)), and determine thyroid status during developmentally critical stages, but do not necessarily translate into long term effects and consequences to fitness of the individual or to the population (such as changes in sex ratios) (Guillette et al., 1995). Chronic exposures can evaluate fitness endpoints and multigenerational effects (e.g., (Bernhardt and von Hippel, 2008; Bernhardt et al., 2006; Bernhardt et al., 2011; Petersen et al., 2015)), but cannot detect temporal sensitivities. The plasticity and variation between species of the HPT-axis system necessitates investigation of disparate taxa using different developmental exposures with the goal of synthesizing mechanisms of toxicity.
Knowledge of temporal sensitivities to contaminants over key lifecycle stages is invaluable. For example, management of waterways and wastewater discharges can be maximized to protect sensitive species during critical developmental windows. Exposure experiments examining the critical windows over all potentially sensitive life stages (e.g., organogenesis, metamorphosis, and gonadal recrudescence) can provide enlightening and critical knowledge about the effects of contaminants.
5. Conclusions
The experimental design of this study successfully detected critical windows of sensitivity to perchlorate exposure for thyroid histological biomarkers. Hyperplasia, colloid area and angiogenesis were sensitive during development. Recovery occurred readily for hyperplasia (Fig. 6), somewhat for colloid area (Fig. 7), and rarely for angiogenesis (Fig. 8). The proportion of late stage testes and oocytes decreased and sex ratio was significantly biased toward males in fish chronically exposed to perchlorate (Table 2). Long-term exposure studies that assess contaminant effects at various stages of development provide novel information to characterize risk to aquatic organisms and management of resources. Future work should focus on the specific time points and targets of perchlorate toxicity, including molecular mechanisms of action. Specifically, the first 42 dpf are critical in stickleback and perturbations during that time could have fitness and population consequences. Additionally, lower concentrations of perchlorate should be evaluated for effects on thyroid histomorphology and influence on sex ratio and gonad maturity at the sensitive windows identified in this study.
Supplementary Material
Highlights.
Perchlorate increases thyroid follicle numbers and reduces colloid area
Perchlorate decreases the proportion of late stage testes and oocytes
Perchlorate causes a male biased sex ratio in stickleback
Acknowledgments
The authors thank D. Dillon, M. Sherbick, L. Smayda, E. Kittel, and L. Matthews for laboratory support. The authors would also like to thank the staff at the UAF Biological Research and Diagnostics Facility and the UO Institute of Neuroscience Histology lab for providing histology support. Thanks to R. Bernhardt, A. Gardell, A. Petersen, and J. Willacker for advice and discussion. Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM103395. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding was also provided by NIH grant number 1RO1ES017039. Fish were collected under Alaska Department of Fish and Game permit SF-2008-019, and all research protocols were approved by the UAA Institutional Animal Care and Use Committee; IACUC # 2007vonhi1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldridge MG, Stahl RL, Gerstenberger SL, Tripoli V, Hutz RJ. In utero and lactational exposure of Long-Evans rats to ammonium perchlorate (AP) disrupts ovarian follicle maturation. Reproductive Toxicology. 2004;19:155–161. doi: 10.1016/j.reprotox.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Bernhardt RR, von Hippel FA. Chronic perchlorate exposure impairs stickleback reproductive behaviour and swimming performance. Behaviour. 2008;145:527–559. doi: 10.1163/156853908792451511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt RR, von Hippel FA, Cresko WA. Perchlorate induces hermaphroditism in threespine sticklebacks. Environmental Toxicology & Chemistry. 2006;25:2087–2096. doi: 10.1897/05-454r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt RR, von Hippel FA, O’Hara TM. Chronic perchlorate exposure causes morphological abnormalities in developing stickleback. Environmental Toxicology and Chemistry. 2011;30:1468–1478. doi: 10.1002/etc.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton ML, Specker JL. The hypothalamic-pituitary-thyroid (HPT) axis in fish and its role in fish development and reproduction. Critical Reviews in Toxicology. 2007;37:97. doi: 10.1080/10408440601123529. [DOI] [PubMed] [Google Scholar]
- Bradford CM, Rinchard J, Carr JA, Theodorakis C. Perchlorate affects thyroid function in eastern mosquitofish (Gambusia holbrooki) at environmentally relevant concentrations. Environmental Science & Technology. 2005;39:5190–5195. doi: 10.1021/es0484505. [DOI] [PubMed] [Google Scholar]
- Brown GM, Gu B. The chemistry of perchlorate in the environment. In: Gu B, Coates JD, editors. Perchlorate: Environmental Occurance, Interactions and Treatment. Springer; New York, New York: 2006. pp. 17–47. [Google Scholar]
- Burcu KT, Sema IU, Ozlem O. The effects of sodium perchlorate on the liver of molly fish (Poecilia sphenops, Cyprinidae, Teleostei) African Journal of Biotechnology. 2009;8:2640–2644. [Google Scholar]
- Carr JA, McNabb FMA, Smith EE. Thyroid function and mechanisms of perchlorate action in vertebrates. In: Kendall R, Smith PN, editors. Perchlorate Ecotoxicology. SETAC Press; Pensacola, FL, USA: 2005. pp. 45–77. [Google Scholar]
- Carr JA, Patiño R. The hypothalamus-pituitary-thyroid axis in teleosts and amphibians: endocrine disruption and its consequences to natural populations. Gen. Comp. Endocrinol. 2011;170:299–312. doi: 10.1016/j.ygcen.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Choksi NY, Jahnke GD, St Hilaire C, Shelby M. Role of thyroid hormones in human and laboratory animal reproductive health. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2003;68:479–491. doi: 10.1002/bdrb.10045. [DOI] [PubMed] [Google Scholar]
- Crane HM, Pickford DB, Hutchinson TH, Brown JA. Developmental changes of thyroid hormones in the fathead minnow, Pimephales promelas. Gen. Comp. Endocrinol. 2004;139:55. doi: 10.1016/j.ygcen.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Crane HM, Pickford DB, Hutchinson TH, Brown JA. Effects of ammonium perchlorate on thyroid function in developing fathead minnows, Pimephales promelas. Environ. Health Perspect. 2005;113:396–401. doi: 10.1289/ehp.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresko WA. The Ecology and Geography of Speciation: A Case Study Using an Adaptive Radiation of Threespine Stickleback in Alaska. Clark University; Worchester: 2000. [Google Scholar]
- Cyr DG, Eales JG. Interrelationships between thyroidal and reproductive endocrine systems in fish. Reviews in Fish Biology and Fisheries. 1996;6:165–200. [Google Scholar]
- Duarte-Guterman P, Navarro-Martín L, Trudeau VL. Mechanisms of crosstalk between endocrine systems: Regulation of sex steroid hormone synthesis and action by thyroid hormones. General and Comparative Endocrinology. 2014;203:69–85. doi: 10.1016/j.ygcen.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Eales JG. Thyroid Functions in Cyclostomes and Fishes. In: Barrington EJW, editor. Hormones and Evolution. Acedemic Press INC.; New York, New York: 1979. pp. 341–436. [Google Scholar]
- Furin CG, von Hippel FA, Hagedorn B, O’Hara TM. Perchlorate trophic transfer increases tissue concentrations above ambient water exposure alone in a predatory fish. Journal of Toxicology and Environmental Health, Part A. 2013;76:1072–1084. doi: 10.1080/15287394.2013.836693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furin CG, von Hippel FA, Postlethwait JH, Buck CL, Cresko WA, O’Hara TM. Developmental timing of perchlorate exposure alters threespine stickleback dermal bone. General and Comparative Endocrinology. 2015 doi: 10.1016/j.ygcen.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goleman WL, Carr JA, Anderson TA. Environmentally relevant concentrations of ammonium perchlorate inhibit thyroid function and alter sex ratios in developing Xenopus laevis. Environmental Toxicology and Chemistry. 2002;21:590–597. [PubMed] [Google Scholar]
- Griffiths R, Orr K, Adam A, Barber I. DNA sex identification in the three-spined stickleback. Journal of Fish Biology. 2000;57:1331–1334. [Google Scholar]
- Guillette LJ, Jr., Crain DA, Rooney AA, Pickford DB. Organization versus activation: The role of endocrine-disrupting contaminants (EDCs) during embryonic development in wildlife. Environmental Health Perspectives. 1995;103:157–164. doi: 10.1289/ehp.95103s7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi HR, Nelson ER, Allan ERO. New insights into thyroid hormone function and modulation of reproduction in goldfish. General and Comparative Endocrinology. 2012;175:19–26. doi: 10.1016/j.ygcen.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Hahlbeck E, Griffiths R, Bengtsson BE. The juvenile three-spined stickleback (Gasterosteus aculeatus L.) as a model organism for endocrine disruption I. Sexual differentiation. Aquatic Toxicology. 2004;70:287–310. doi: 10.1016/j.aquatox.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Hohenlohe PA, Bassham S, Etter PD, Stiffler N, Johnson EA, Cresko WA. Population genomics of parallel adaptation in threespine stickleback using sequenced RAD tags. PLoS Genet. 2010;6:e1000862. doi: 10.1371/journal.pgen.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber DR, Blount BC, Mage DT, Letkiewicz FJ, Kumar A, Allen RH. Estimating perchlorate exposure from food and tap water based on US biomonitoring and occurrence data. Journal of Exposure Science and Environmental Epidemiology. 2011;21:395–407. doi: 10.1038/jes.2010.31. [DOI] [PubMed] [Google Scholar]
- Kendall RJ, Smith PN. Perchlorate Ecotoxicology. SETAC Press; Pensocola, FL, USA: 2006. [Google Scholar]
- Kent ML, Spitsbergen JM, Matthews JM, Fournie JW, Murray KN, Westerfield M. Appendix – recipes and protocols, Diseases of Zebrafish in Research Facilities. Zebrafish International Resource Center; 2012. [Google Scholar]
- Konietschke F, Placzek M, Schaarschmidt F, Hothorn LA. nparcomp: An R Software Package for Nonparametric Multiple Comparisons and Simultaneous Confidence Intervals. Journal of Statistical Software. 2014;61:1–17. [Google Scholar]
- Kounaves SP, Stroble ST, Anderson RM, Moore Q, Catling DC, Douglas S, McKay CP, Ming DW, Smith PH, Tamppari LK, Zent AP. Discovery of natural perchlorate in the antarctic dry valleys and Its global implications. Environmental Science & Technology. 2010;44:2360–2364. doi: 10.1021/es9033606. [DOI] [PubMed] [Google Scholar]
- Lema SC, Dickey JT, Schultz IR, Swanson P. Thyroid hormone regulation of mRNAs encoding thyrotropin beta-subunit, glycoprotein alpha-subunit, and thyroid hormone receptors alpha and beta in brain, pituitary gland, liver, and gonads of an adult teleost, Pimephales promelas. Journal of Endocrinology. 2009;202:43–54. doi: 10.1677/JOE-08-0472. [DOI] [PubMed] [Google Scholar]
- Lewis ZR, McClellan MC, Postlethwait JH, Cresko WA, Kaplan RH. Female-specific increase in primordial germ cells marks sex differentiation in threespine stickleback (Gasterosteus aculeatus) Journal of Morphology. 2008;269:909–921. doi: 10.1002/jmor.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F-J, Wang J-S, Theodorakis CW. Thyrotoxicity of sodium arsenate, sodium perchlorate, and their mixture in zebrafish Danio rerio. Environmental Science & Technology. 2006;40:3429–3436. doi: 10.1021/es052538g. [DOI] [PubMed] [Google Scholar]
- Liu F, Gentles A, Theodorakis CW. Arsenate and perchlorate toxicity, growth effects, and thyroid histopathology in hypothyroid zebrafish Danio rerio. Chemosphere. 2008;71:1369–1376. doi: 10.1016/j.chemosphere.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Mank JE, Avise JC. The evolution of reproductive and genomic diversity in ray finned fishes: insights from phylogeny and comparative analysis. Journal of Fish Biology. 2006;69:1–27. [Google Scholar]
- Matta SLP, Vilela DAR, Godinho HP, França LR. The goitrogen 6-n-Propyl-2-Thiouracil (PTU) given during testisdDevelopment increases sertoli and germ cell numbers per cyst in fish: The tilapia (Oreochromis niloticus) model. Endocrinology. 2002;143:970–978. doi: 10.1210/endo.143.3.8666. [DOI] [PubMed] [Google Scholar]
- Morrison RD, Vavricka EA, Duncan PB. Perchlorate. In: Morrison RD, Murphy BL, editors. Environmental Forensics: Contaminant Specific Guide. Elsevier Inc.; Burlington, MA, USA: 2006. pp. 167–185. [Google Scholar]
- Mukhi S, Carr JA, Anderson TA, Patiño R. Novel biomarkers of perchlorate exposure in zebrafish. Environmental Toxicology and Chemistry. 2005;24:1107–1115. doi: 10.1897/04-270r.1. [DOI] [PubMed] [Google Scholar]
- Mukhi S, Patiño R. Effects of prolonged exposure to perchlorate on thyroid and reproductive function in zebrafish. Toxicological Sciences. 2007;96:246–254. doi: 10.1093/toxsci/kfm001. [DOI] [PubMed] [Google Scholar]
- Mukhi S, Torres L, Patiño R. Effects of larval-juvenile treatment with perchlorate and co-treatment with thyroxine on zebrafish sex ratios. Gen. Comp. Endocrinol. 2007a;150:486–494. doi: 10.1016/j.ygcen.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Mukhi S, Torres L, Patiño R. Effects of larval-juvenile treatment with perchlorate and co-treatment with thyroxine on zebrafish sex ratios. General and Comparative Endocrinology. 2007b;150:486–494. doi: 10.1016/j.ygcen.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Park JW, Rinchard J, Liu F, Anderson TA, Kendall RJ, Theodorakis CW. The thyroid endocrine disruptor perchlorate affects reproduction, growth, and survival of mosquitofish. Ecotoxicology and Environmental Safety. 2006a;63:343–352. doi: 10.1016/j.ecoenv.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Park JW, Rinchard J, Liu FJ, Anderson TA, Kendall RJ, Theodorakis CW. The thyroid endocrine disruptor perchlorate affects reproduction, growth, and survival of mosquitofish. Ecotoxicology and Environmental Safety. 2006b;63:343–352. doi: 10.1016/j.ecoenv.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Patiño R, Mukhi S. Effects of prolonged exposure to perchlorate on thyroid and reproductive function in zebrafish. Toxicological Sciences. 2007;96:246–254. doi: 10.1093/toxsci/kfm001. [DOI] [PubMed] [Google Scholar]
- Patiño R, Mukhi S, Torres L. Effects of larval-juvenile treatment with perchlorate and co-treatment with thyroxine on zebrafish sex ratios. General and Comparative Endocrinology. 2007;150:486–494. doi: 10.1016/j.ygcen.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Patiño R, Wainscott MR, Cruz-Li EI, Balakrishnan S, McMurry C, Blazer VS, Anderson TA. Effects of ammonium perchlorate on the reproductive performance and thyroid follicle histology of zebrafish. Environmental Toxicology & Chemistry. 2003;22:1115–1121. [PubMed] [Google Scholar]
- Petersen AM, Dillon D, Bernhardt RA, Torunsky R, Postlethwait JH, von Hippel FA, Buck CL, Cresko WA. Perchlorate disrupts embryonic androgen synthesis and reproductive development in threespine stickleback without changing whole-body levels of thyroid hormone. General and Comparative Endocrinology. 2015;210:130–144. doi: 10.1016/j.ygcen.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickford DB, Crane HM, Hutchinson TH, Brown JA. Effects of ammonium perchlorate on thyroid function in developing fathead minnows, Pimephales promelas. Environmental Health Perspectives. 2005;113:396–401. doi: 10.1289/ehp.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao B, Anderson TA, Orris GJ, Rainwater KA, Rajagopalan S, Sandvig RM, Scanlon BR, Stonestrom DA, Walvoord MA, Jackson WA. Widespread natural perchlorate in unsaturated zones of the southwest United States. Environmental Science & Technology. 2007;41:4522–4528. doi: 10.1021/es062853i. [DOI] [PubMed] [Google Scholar]
- Rasband WS. Image J. U. S. National Institutes of Health. Bethesda, Maryland, USA; 1997-2013. http://imagej.nih.gov/ij/ [Google Scholar]
- RCoreTeam . In: R Core Team, R: A language and environment for statistical computing. 3.0.1 ed R Foundation for Statistical Computing, editor. R Core Team; Vienna, Austria: 2013. [Google Scholar]
- Schmidt F, Schnurr S, Wolf R, Braunbeck T. Effects of the anti-thyroidal compound potassium-perchlorate on the thyroid system of the zebrafish. Aquatic Toxicology. 2012;109:47–58. doi: 10.1016/j.aquatox.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Sharma P, Patiño R. Regulation of gonadal sex ratios and pubertal development by the thyroid endocrine system in zebrafish (Danio rerio) General and Comparative Endocrinology. 2013;184:111–119. doi: 10.1016/j.ygcen.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Smith PN, Theodorakis CW, Anderson TA, Kendall RJ. Preliminary assessment of perchlorate in ecological receptors at the Longhorn Army Ammunition Plant (LHAAP), Karnack, Texas. Ecotoxicology. 2001;10:305–313. doi: 10.1023/a:1016715502717. [DOI] [PubMed] [Google Scholar]
- Sokolowska E, Kulczykowska E. Annual reproductive cycle in two free living populations of three-spined stickleback (Gasterosteus aculeatus L.): patterns of ovarian and testicular development. Oceanologia. 2006;48:103–124. [Google Scholar]
- Supriya A, Raghuveer K, Swapna I, Rasheeda MK, Kobayashi T, Nagahama Y, Gupta A, Majumdar KC, Senthilkumaran B. Thyroid hormone modulation of ovarian recrudescence of air-breathing catfish Clarias gariepinus. Fish Physiology and Biochemistry. 2005;31:267–270. doi: 10.1007/s10695-006-0034-1. [DOI] [PubMed] [Google Scholar]
- Swapna I, Rajasekhar M, Supriya A, Raghuveer K, Sreenivasulu G, Rasheeda MK, Majumdar KC, Kagawa H, Tanaka H, Dutta-Gupta A, Senthilkumaran B. Thiourea-induced thyroid hormone depletion impairs testicular recrudescence in the air-breathing catfish, Clarias gariepinus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2006;144:1–10. doi: 10.1016/j.cbpa.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Swarup H. Stages in the development of the stickleback Gasterosteus aculeatus (L.) Journal of Embryology and Experimental Morphology. 1958;6:373–383. [PubMed] [Google Scholar]
- Theodorakis C, Rinchard J, Anderson T, Liu F, Park JW, Costa F, McDaniel L, Kendall R, Waters A. Perchlorate in fish from a contaminated site in east-central Texas. Environmental Pollution. 2006a;139:59–69. doi: 10.1016/j.envpol.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Theodorakis CW, Rinchard J, Carr JA, Park JW, McDaniel L, Liu FJ, Wages M. Thyroid endocrine disruption in stonerollers and cricket frogs from perchlorate-contaminated streams in east-central Texas. Ecotoxicology. 2006b;15:31–50. doi: 10.1007/s10646-005-0040-6. [DOI] [PubMed] [Google Scholar]
- Tietge JE, Butterworth BC, Haselman JT, Holcombe GW, Hornung MW, Korte JJ, Kosian PA, Wolfe M, Degitz SJ. Early temporal effects of three thyroid hormone synthesis inhibitors in Xenopus laevis. Aquatic Toxicology. 2010;98:44–50. doi: 10.1016/j.aquatox.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Trumpolt CW, Crain M, Cullison GD, Flanagan SJP, Siegel L, Lathrop S. Perchlorate: Sources, uses, and occurrences in the environment. Remediation Journal. 2005;16:65–89. [Google Scholar]
- Urbansky ET. Perchlorate as an environmental contaminant. Environmental Science and Pollution Research. 2002;9:187–192. doi: 10.1007/BF02987487. [DOI] [PubMed] [Google Scholar]
- Urbansky ET, Brown SK, Magnuson ML, Kelty CA. Perchlorate levels in samples of sodium nitrate fertilizer derived from Chilean caliche. Environmental Pollution. 2001;112:299–302. doi: 10.1016/s0269-7491(00)00132-9. [DOI] [PubMed] [Google Scholar]
- USEPA Drinking water: Regulatory determination on perchlorate. Federal Register. 2011;76:7762–7767. [Google Scholar]
- Wolff J. Perchlorate and the thyroid gland. Pharmacological Reviews. 1998;50:89–105. [PubMed] [Google Scholar]
- Zoeller RT, Tan SW, Tyl RW. General Background on the Hypothalamic-Pituitary-Thyroid (HPT) Axis. Critical Reviews in Toxicology. 2007;37:11. doi: 10.1080/10408440601123446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.