Abstract
Background
Increased low density lipoprotein cholesterol (LDL-C) level and the presence of metabolic syndrome (MetS) are important risk factors for cardiovascular disease (CVD) in type 2 diabetes mellitus (T2DM). Recent studies demonstrated apolipoprotein B (apoB), a protein mainly located in LDL-C, was an independent predictor of the development of CVD especially in patients with T2DM. The aim of this study was to investigate the relationship between apoB and MetS in T2DM patients.
Methods
We analyzed 912 patients with T2DM. Fasting blood samples were taken for glycated hemoglobin, high-sensitivity C-reactive protein, total cholesterol, triglyceride (TG), high density lipoprotein cholesterol, LDL-C, and apoB. MetS was defined by the modified National Cholesterol Education Program Adult Treatment Panel III criteria. We performed a hierarchical regression analysis with apoB as the dependent variable. Age, sex, the number of components of MetS and LDL-C were entered at model 1, the use of lipid-lowering medications at model 2, and the individual components of MetS were added at model 3.
Results
Seventy percent of total subjects had MetS. ApoB level was higher in subjects with than those without MetS (104.5±53.3 mg/dL vs. 87.7±33.7 mg/dL, P<0.01) even after adjusting for LDL-C. ApoB and LDL-C were positively correlated to the number of MetS components. The hierarchical regression analysis showed that the increasing number of MetS components was associated with higher level of apoB at step 1 and step 2 (β=0.120, P<0.001 and β=0.110, P<0.001, respectively). At step 3, TG (β=0.116, P<0.001) and systolic blood pressure (β=0.099, P<0.05) were found to significantly contribute to apoB.
Conclusion
In patients with T2DM, apoB is significantly related to MetS independently of LDL-C level. Of the components of MetS, TG, and systolic blood pressure appeared to be determinants of apoB.
Keywords: Diabetes mellitus, type 2; Apolipoproetin B; Metabolic syndrome; Cardiovascular diseases
INTRODUCTION
Dyslipidemia is a major risk factor of atherosclerosis and an increase of low density lipoprotein cholesterol (LDL-C) showed a close relationship with cardiovascular disease (CVD) [1,2]. A number of large clinical trials demonstrated the prevention of CVD with statin treatment that targeted the reduction of LDL-C [3,4,5,6]. However, recently there are epidemiological data reporting that apolipoprotein is more useful as a risk factor of CVD than LDL-C or high density lipoprotein cholesterol (HDL-C) [7,8]. LDL consists of colonies of various particles of different sizes, densities and chemical composition [9], and small dense LDL (sdLDL) is known to have a high risk of atherosclerosis due to its physiochemical properties [10,11,12]. Especially in patients with type 2 diabetes mellitus (T2DM), dyslipidemias with low HDL-C and high triglyceride (TG) occurs due to insulin resistance, and these patients are known to have increased sdLDL despite their normal range of LDL-C [13]. Apolipoprotein B (ApoB) is a marker that represents the total number of atherosclerotic particles and is considered more useful in predicting the risk of CVD than LDL-C in T2DM with increased sdLDL since it reflects the amount of LDL.
Metabolic syndrome (MetS) is defined by the group of risk factors that increase CVD risks such as abdominal obesity, hypertriglyceridemia, low HDL-C, hyperglycemia, and hypertension [14]. The incidence and risk of CVD is known to be five-fold higher when MetS is accompanied with diabetes [15]. Insulin resistance has been reported to raise the level of apoB due to an increase of very low density lipoprotein (VLDL) in the liver [16]. Thus, MetS where insulin resistance plays an important role would be considered to have a correlation with apoB. Previous studies have reported such correlations between the increase of apoB and MetS in the general population [17]; however, there are not many studies conducted among the patients with diabetes. The present study was aimsed to determine the relationship between MetS and apoB in patients with T2DM.
METHODS
Study subjects
This study included 912 patients with T2DM who were admitted to Jeju University Hospital from September 2005 to July 2013. Patients who were suspected to have a type 1 diabetes were excluded (serum C-peptide less than 0.6 ng/mL, increase of anti-glutamic acid decarboxylase titer, multiple doses of insulin injection from the time of the diagnosis, a history of diabetic ketoacidosis), also other diabetic patients from the secondary causes (drug induced, chronic pancreatitis, and pancreatectomy) were also excluded.
Study methods
Physical measurements, blood pressure (BP), and biochemical laboratory tests of all participating patients were conducted. Waist circumference was measured at the narrowest part between the chest and iliac crest parallel to the ground while maintaining normal breathing. The BP was recorded as the average of two BP measurements after having a stable condition for more than 10 minutes. Additional measurements included glycated hemoglobin (HbA1c), high-sensitivity C-reactive protein, total cholesterol, HDL-C, LDL-C, and apoB. Age, the presence or absence of antihypertensive and lipid-lowering medication, and duration of T2DM were collected based upon their interview. MetS was defined based on National Cholesterol Education Program Adult Treatment Panel III criteria [18] and the obesity standard in Korea [19] as follows: (1) waist circumference: more than 90 cm in male and more than 85 cm in female; (2) BP greater than 130/85 mm Hg or administration of antihypertensive medication; (3) fasting glucose greater than 100 mg/dL; (4) TG greater than 150 mg/dL; and (5) HDL-C less than 40 mg/dL in male, less than 50 mg/dL in female. Since this study was conducted among the patients with T2DM, all of their fasting glucose levels satisfied one of the criteria for MetS; thus, MetS was diagnosed when more than two of the other four criteria except for the fasting glucose level were satisfied.
Statistical analysis
The results were presented in mean±SD and the categorical variables were presented in percentage. The participating patients were divided into a MetS and non-MetS group, and for the continuous variables, the mean values were compared by using the Student t test. The chi-square test was also used for the categorical variables. Also, the analysis of variance (ANOVA) with the linear trend test was performed after dividing the participating patients into five groups according to the number of MetS factors in order to investigate the correlation between apoB and MetS. ApoB was the primary apolipoprotein of LDL-C and more than 90% of apoB is usually found in LDL [20], and thus the analysis of covariance (ANCOVA) of apoB was conducted to exclude LDL-C as a confounding factor between MetS and non-MetS group and LDL-C was covariance. Hierarchical regression analysis was used to analyze the effect of the various factors on apoB. In hierarchical regression analysis, model 1 included age, gender, LDL-C, and the number of MetS components. Model 2 corrected the use of lipid-lowering medication that could affect lipid measures; and MetS components were added in model 3. All the analysis used PASW version 18.0 (IBM Co., Armonk, NY, USA) and P<0.05 was defined to be statistically significant. This study was approved by an Institutional Review Board of Jeju National University Hospital (2010-33). Informed consents were waived due to the retrospective nature of this study.
RESULTS
This study enrolled 912 participants (male: female, 516 [56%]:396 [44%]), and their mean age was 58.4±12.9 years. Their mean BMI was 25.2±4 kg/m2, which indicated most of the patients were obese. The waist circumferences in males and females were 90.4±0.4 and 89.2±0.5 cm, respectively, which indicated both many of the male and female had abdominal obesity. The mean duration of diabetes was 9.8±0.3 years with mean HbA1c of 9.3%±2.5%, which indicated many of the patients had poor control of blood glucose. The mean LDL-C was 109.4±39.8 mg/dL, apoB was 99.5±48.9 mg/dL, and apoAI was 136.8±35.9 mg/dL (Table 1).
Table 1. Comparison of Clinical and Laboratory Variables between MetS and Non-MetS Groups.
| Variable | MetS group (n=641) | Non-MetS group (n=271) | P value | Total |
|---|---|---|---|---|
| Age, yr | 58.6±12.8 | 58.1±13.4 | 0.59 | 58.4±0.4 |
| Male sex, % | 51.8 | 67.9 | <0.001 | 56.6 |
| Duration of diabetes, yr | 9.6±8.6 | 9.1±7.8 | 0.43 | 9.8±0.3 |
| Glycated hemoglobin, % | 9.2±2.3 | 9.4±2.8 | 0.342 | 9.3±0.1 |
| C-peptide, ng/mL | 2.9±0.1 | 1.9±1.1 | <0.001 | 2.6±0.1 |
| Body mass index, kg/m2 | 26.2±3.9 | 22.9±3.1 | <0.001 | 25.2±0.1 |
| Waist circumference, cm | 92.7±10.0 | 83.4±8.6 | <0.001 | 89.9±0.3 |
| Systolic blood pressure, mm Hg | 135.0±17.4 | 123.4±14.8 | <0.001 | 131.5±0.6 |
| Diastolic blood pressure, mm Hg | 81.4±10.7 | 76.3±9.1 | <0.001 | 79.9±0.3 |
| Triglyceride, mg/dL | 197.7±161.6 | 97.3±47.2 | <0.001 | 167.9±4.8 |
| HDL-C, mg/dL | 43.2±20.1 | 51.4±12.5 | <0.001 | 45.6±0.6 |
| LDL-C, mg/dL | 112.2±40.6 | 102.9±37.3 | 0.001 | 109.4±1.3 |
| Apolipoprotein B, mg/dL | 104.5±53.3 | 87.7±33.7 | <0.001 | 99.5±1.6 |
| hsCRP, mg/dL | 0.5±1.9 | 0.4±1.6 | 0.58 | 0.5±0.1 |
| Use of antilipidemic agents, % | 49.6 | 36.2 | <0.001 | 45 |
Values are expressed as mean±SD.
MetS, metabolic syndrome; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; hsCRP, high sensitivity C-reactive protein.
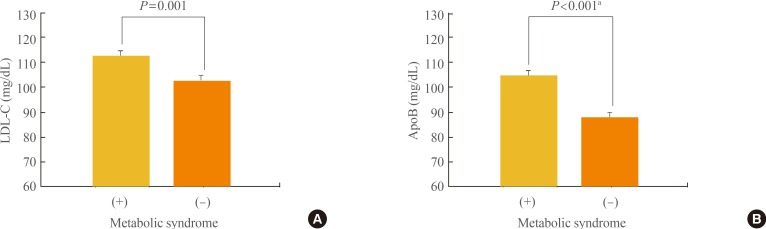
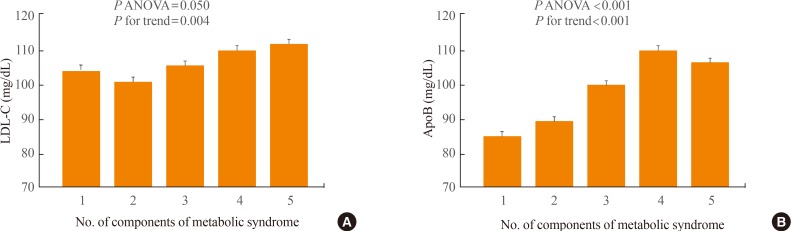
Seventy percent of the study subjects were diagnosed as having MetS, and their mean LDL-C and apoB levels were higher in the MetS group than in the non-MetS group (112.2±40.6 mg/dL vs. 102.9±37.3 mg/dL, P=0.001; 104.5±53.3 mg/dL vs. 87.7±33.7 mg/dL, P<0.001, respectively). Using the ANCOVA for the correction of LDL-C, apoB was significantly higher in the MetS group than in the non-MetS group (Fig. 1). Also, each of the MetS components showed significant differences between MetS and non-MetS (Table 1). Both LDL-C and apoB showed a significant relationship to the number of MetS components when ANOVA with the linear trend test was conducted in the five groups that were divided according to the number of MetS components, and apoB showed a higher relationship to the number of MetS components than LDL-C (Fig. 2).
Fig. 1. (A) Differences in low density lipoprotein cholesterol (LDL-C) and (B) apolipoprotein B (apoB) according to the presence of metabolic syndrome. Values are expressed as mean±SE. aAdjusted for LDL-C by analysis of covariance.
Fig. 2. (A) Differences in low density lipoprotein cholesterol (LDL-C) and (B) apolipoprotein B (apoB) according to the number of components of metabolic syndrome. Values are expressed as mean±SE. ANOVA, analysis of variance; P for trend, P value by the test for linear trend.
Hierarchical regression analysis was conducted to investigate the correlation between the number of MetS components and apoB even after the correction of LDL-C, age, and gender, and to determine the major MetS components which affects apoB. The number of MetS components was the most significant factor that determines apoB when compared with gender and LDL-C in model 1, which was corrected for age, gender and LDL-C, and the number of MetS components remained the independent predictor for apoB, even after the addition of the use of lipid lowering medications in model 2. In model 3, which was to find out the MetS component for the determination of apoB, systolic BP, and TG were determinant factors for apoB as well as LDL-C (Table 2).
Table 2. Hierarchical Regression Analysis between Apolipoprotein B and Clinical Variables.
| Variable | Model 1 (R2 =0.270) | Model 2 (R2 =0.273) | Model 3 (R2 =0.287) | |||
|---|---|---|---|---|---|---|
| βa | P value | βa | P value | βa | P value | |
| Age | -0.019 | 0.510 | -0.021 | 0.468 | -0.013 | 0.678 |
| Male | 0.070 | 0.017 | 0.072 | 0.014 | 0.055 | 0.071 |
| LDL-C | 0.490 | <0.001 | 0.506 | <0.001 | 0.494 | <0.001 |
| No. of MetS components | 0.120 | <0.001 | 0.110 | <0.001 | 0.036 | 0.401 |
| Use of antilipidemic agents | - | - | 0.064 | 0.031 | 0.055 | 0.064 |
| Waist circumference | - | - | - | - | -0.006 | 0.863 |
| Systolic blood pressure | - | - | - | - | 0.099 | 0.018 |
| Diastolic blood pressure | - | - | - | - | -0.040 | 0.314 |
| Triglyceride | - | - | - | - | 0.116 | <0.001 |
| HDL-C | - | - | - | - | -0.014 | 0.660 |
Apolipoprotein B level was the dependent variable.
R2, adjusted R-squared; LDL-C, low density lipoprotein cholesterol; MetS, metabolic syndrome; HDL-C, high density lipoprotein cholesterol.
aPartial regression coefficient.
DISCUSSION
This study was conducted to determine the relationship between MetS and apoB in patients with T2DM. ApoB was significantly higher in diabetic patients with MetS than those without MetS, and this difference was significant even after correcting with LDL-C. Also apoB and LDL-C, and the number of MetS factors showed a significant positive correlation. As the results of hierarchical regression analysis indicated, the number of MetS components was the an independent predictive factor for apoB, even after the correction with LDL-C and use of lipid-lowering medications; and in each MetS component, systolic BP and TG were the predictive factors for apoB.
ApoB is one of the components found in chylomicron, VLDL, intermediate density lipoprotein, and LDL. One molecule of apoB is present on each lipoprotein particle, and more than 90% of apoB is usually found in LDL [20], reflecting the close relation between two factors. However, the concentration of LDL-C represents only the amount of lipid per LDL particle, and thus does not reflect the various size of LDL particles. On the other hand, apoB reflects the number of lipoproteins that can cause atherosclerosis and also an increase of sdLDL. Therefore, we may consider apoB is significantly related to the risk of CVD independent of LDL-C levels.
In 1996, a prospective cohort study in 2,155 Canadian males reported that there was a strong association between apoB and ischemic heart disease [8], and it was the first prospective study that demonstrated that apoB is a more useful factor to predict CVD risk than LDL-C or other lipid measures. Also Walldius et al. [21] conducted a 5.5-year follow-up study of 175,553 patients in Sweden to compare the mortality rates due to acute myocardial infarction (AMI) and lipid parameters, and reported that apoB had a closer relationship to the AMI occurrence than LDL-C. Especially, apoB is considered to be a valuable predictor of the CVD in patients with T2DM having elevated levels of sdLDL [22], but only apoB had a significant relationship to coronary artery calcification in the study that evaluated the relation between apoB, LDL-C and coronary artery calcification [23]. Results of these large-scale studies are consistent with our finding that apoB was higher in patients in MetS group than those in non-MetS group regardless of their LDL-C level.
Recently Ryoo et al. [17] conducted a cohort study which followed up 25,193 healthy Korean males without MetS for 5 years, and reported that apoB was a predictive factor for MetS. During 5 years of follow-up, 5,407 (21.5%) were diagnosed with MetS, and there was a significant positive correlation between the occurrence of MetS and apoB levels. These results are fairly consistent with our finding that found apoB was higher in the MetS group than the non-MetS group and it proportionally increases with the number of MetS factors. However, Ryoo et al. [17] conducted the study in healthy subjects and LDL-C was not corrected in the analysis of the relationship between apoB and MetS occurrence. On the other hand, the present study was a cross-sectional study with T2DM patients and factors that may affect apoB such as LDL-C and the use of lipid-lowering medications were corrected. It is necessary to conduct a large, prospective study of patients with T2DM in order to clarify the relationship between apoB and MetS.
Hypertriglyceridemia is one of the well-known risk factors for CVD [24,25,26,27], but it is not clear whether the treatment helps in the prevention of CVD [28]. The serum TG is produced in the liver in fasting state and transported in VLDL and absorbed in the small intestine after a meal and transported by chylomicron [29,30]. In the past, it was the fasting hypertriglyceridemia that showed the relationship with CVD, but recently, the effect of postprandial hypertriglyceridemia on the occurrence of CVD is being observed [31]. However the diagnosis of MetS uses the TG level measured in the fasting state while apoB is not affected by food intake, which makes apoB much simpler to be used when compared with other lipid parameters.
MetS and T2DM are well-known causes of secondary hypertriglyceridemia, and it is known to be related to the following: increase of free fatty acids influx into the liver, increased production of VLDL, decreased activity of lipoprotein lipase, and increased activity of cholesteryl ester transfer protein [32]. TG was found to be an association factor for apoB from the multiple regression analysis in this study, which may be due to insulin resistance. Insulin resistance increases the production of VLDL in the liver, and thus TG and apoB consisting of VLDL are also expected to increase at the same time in the presence of insulin resistance [33]. Also systolic BP showed an independent relationship with apoB, and the other studies showed similar results [34,35]. Ryomoto et al. [34] conducted a study in twenty three hypertensive patients without glucose intolerance and obesity and found out that apoB and sdLDL were higher in hypertensive patients than in a healthy control group. Okosun et al. [35] conducted a study of 1,844 abdominal obese female patients and reported that there was an association between apoB and systolic and diastolic BP. Apart from dyslipidemias, hypertension is also related to an increase of insulin resistance [36,37]. Therefore, the significant correlation between systolic BP and apoB is probably due to the co-etiology of insulin resistance, and a further study to find out the detailed mechanism is warranted. This study has a few limitations. First, this study was retrospective and there was a considerable difference between the number of subjects in the MetS group and the non-MetS group. Therefore the authors performed more precise analyses by investigating each factor of MetS and their correlation with apoB apart from the group comparison. Second, we did not directly analyze the occurrence of CVD and apoB. Due to the limitation of the cross-sectional study, the occurrence of CVD events could not be investigated, and instead, the correlations of MetS, apoB, and LDL-C, which have a close relationship to CVD in T2DM patients, was analyzed. The incidence of CVD and its risk factors such as apoB should be studied in T2DM patients with MetS.
In conclusion, apoB showed a significant relationship with MetS in T2DM regardless of LDL-C. TG and systolic BP were identified as determinant factors of apoB among the risk factors for MetS in regression analysis. The level of apoB can be measured regardless of food intake, it may be applied more conveniently in the process of evaluating the CVD risk in the clinical situation. Therefore, apoB is suggested to be one of effective risk factors for predicting CVD in patients with T2DM, and a guideline including apoB as the treatment goal should be developed.
ACKNOWLEDGMENTS
This research was supported by a grant for a regional branch from the Korean Endocrine Society in 2014. The biospecimen and data used in this study were provided by the Biobank of Jeju National University Hospital, a member of Korea Biobank Network.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA. 1988;260:1917–1921. [PubMed] [Google Scholar]
- 2.Koba S, Hirano T, Kondo T, Shibata M, Suzuki H, Murakami M, Geshi E, Katagiri T. Significance of small dense low-density lipoproteins and other risk factors in patients with various types of coronary heart disease. Am Heart J. 2002;144:1026–1035. doi: 10.1067/mhj.2002.126119. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y; Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 4.Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, Ward K, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM J, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 6.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 7.Walldius G, Jungner I. The apoB/apoA-I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy: a review of the evidence. J Intern Med. 2006;259:493–519. doi: 10.1111/j.1365-2796.2006.01643.x. [DOI] [PubMed] [Google Scholar]
- 8.Lamarche B, Moorjani S, Lupien PJ, Cantin B, Bernard PM, Dagenais GR, Despres JP. Apolipoprotein A-I and B levels and the risk of ischemic heart disease during a five-year follow-up of men in the Quebec cardiovascular study. Circulation. 1996;94:273–278. doi: 10.1161/01.cir.94.3.273. [DOI] [PubMed] [Google Scholar]
- 9.Packard CJ, Shepherd J. Lipoprotein heterogeneity and apolipoprotein B metabolism. Arterioscler Thromb Vasc Biol. 1997;17:3542–3556. doi: 10.1161/01.atv.17.12.3542. [DOI] [PubMed] [Google Scholar]
- 10.Anber V, Millar JS, McConnell M, Shepherd J, Packard CJ. Interaction of very-low-density, intermediate-density, and low-density lipoproteins with human arterial wall proteoglycans. Arterioscler Thromb Vasc Biol. 1997;17:2507–2514. doi: 10.1161/01.atv.17.11.2507. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen LB. Transfer of low density lipoprotein into the arterial wall and risk of atherosclerosis. Atherosclerosis. 1996;123:1–15. doi: 10.1016/0021-9150(96)05802-9. [DOI] [PubMed] [Google Scholar]
- 12.Krauss RM. Atherogenic lipoprotein phenotype and diet-gene interactions. J Nutr. 2001;131:340S–343S. doi: 10.1093/jn/131.2.340S. [DOI] [PubMed] [Google Scholar]
- 13.Watson KE, Horowitz BN, Matson G. Lipid abnormalities in insulin resistant states. Rev Cardiovasc Med. 2003;4:228–236. [PubMed] [Google Scholar]
- 14.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.Bonora E, Targher G, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A, Gemma L, Santi L, Bonadonna RC, Muggeo M. The metabolic syndrome is an independent predictor of cardiovascular disease in type 2 diabetic subjects. Prospective data from the Verona Diabetes Complications Study. Diabet Med. 2004;21:52–58. doi: 10.1046/j.1464-5491.2003.01068.x. [DOI] [PubMed] [Google Scholar]
- 16.Ginsberg HN, Zhang YL, Hernandez-Ono A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res. 2005;36:232–240. doi: 10.1016/j.arcmed.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Ryoo JH, Park SK. Association of apolipoprotein B and incidence of metabolic syndrome in Korean men: a 5-years' follow-up study. Atherosclerosis. 2013;226:496–501. doi: 10.1016/j.atherosclerosis.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 19.Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, Kim DY, Kwon HS, Kim SR, Lee CB, Oh SJ, Park CY, Yoo HJ. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Sniderman AD, Marcovina SM. Apolipoprotein A1 and B. Clin Lab Med. 2006;26:733–750. doi: 10.1016/j.cll.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–2033. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 22.Bruno G, Merletti F, Biggeri A, Bargero G, Prina-Cerai S, Pagano G, Cavallo-Perin P. Effect of age on the association of non-high-density-lipoprotein cholesterol and apolipoprotein B with cardiovascular mortality in a Mediterranean population with type 2 diabetes: the Casale Monferrato study. Diabetologia. 2006;49:937–944. doi: 10.1007/s00125-006-0195-6. [DOI] [PubMed] [Google Scholar]
- 23.Martin SS, Qasim AN, Mehta NN, Wolfe M, Terembula K, Schwartz S, Iqbal N, Schutta M, Bagheri R, Reilly MP. Apolipoprotein B but not LDL cholesterol is associated with coronary artery calcification in type 2 diabetic whites. Diabetes. 2009;58:1887–1892. doi: 10.2337/db08-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Triglyceride concentration and ischemic heart disease: an eight-year follow-up in the Copenhagen Male Study. Circulation. 1998;97:1029–1036. doi: 10.1161/01.cir.97.11.1029. [DOI] [PubMed] [Google Scholar]
- 25.Castelli WP. The triglyceride issue: a view from Framingham. Am Heart J. 1986;112:432–437. doi: 10.1016/0002-8703(86)90296-6. [DOI] [PubMed] [Google Scholar]
- 26.Assmann G, Cullen P, Schulte H. The Munster Heart Study (PROCAM). Results of follow-up at 8 years. Eur Heart J. 1998;19(Suppl A):A2–11. [PubMed] [Google Scholar]
- 27.Manninen V, Tenkanen L, Koskinen P, Huttunen JK, Manttari M, Heinonen OP, Frick MH. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation. 1992;85:37–45. doi: 10.1161/01.cir.85.1.37. [DOI] [PubMed] [Google Scholar]
- 28.AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen S, Karpe F. Determinants of VLDL-triglycerides production. Curr Opin Lipidol. 2012;23:321–326. doi: 10.1097/MOL.0b013e3283544956. [DOI] [PubMed] [Google Scholar]
- 30.Meyers CD, Tremblay K, Amer A, Chen J, Jiang L, Gaudet D. Effect of the DGAT1 inhibitor pradigastat on triglyceride and apoB48 levels in patients with familial chylomicronemia syndrome. Lipids Health Dis. 2015;14:8. doi: 10.1186/s12944-015-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolovou GD, Mikhailidis DP, Kovar J, Lairon D, Nordestgaard BG, Ooi TC, Perez-Martinez P, Bilianou H, Anagnostopoulou K, Panotopoulos G. Assessment and clinical relevance of non-fasting and postprandial triglycerides: an expert panel statement. Curr Vasc Pharmacol. 2011;9:258–270. doi: 10.2174/157016111795495549. [DOI] [PubMed] [Google Scholar]
- 32.Yuan G, Al-Shali KZ, Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. CMAJ. 2007;176:1113–1120. doi: 10.1503/cmaj.060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2012;32:2104–2112. doi: 10.1161/ATVBAHA.111.241463. [DOI] [PubMed] [Google Scholar]
- 34.Ryomoto KI, Suzuki M, Kanazawa A, Hasegawa M, Kimura Y, Yamamura T, Harano Y. Hyperapobetalipoproteinemia with compositional abnormality of LDL and IDL, a characteristic lipoprotein alteration in essential hypertension. Am J Hypertens. 2000;13(6 Pt 1):617–624. doi: 10.1016/s0895-7061(99)00256-3. [DOI] [PubMed] [Google Scholar]
- 35.Okosun IS, Choi S, Hash R, Dever GE. Apolipoprotein B, ratio of total cholesterol to HDL-C, and blood pressure in abdominally obese white and black American women. J Hum Hypertens. 2001;15:299–305. doi: 10.1038/sj.jhh.1001181. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki M, Hirose J, Asakura Y, Sato A, Kageyama A, Harano Y, Omae T. Insulin insensitivity in nonobese, nondiabetic essential hypertension and its improvement by an alpha 1-blocker (bunazosin) Am J Hypertens. 1992;5(12 Pt 1):869–874. doi: 10.1093/ajh/5.12.869. [DOI] [PubMed] [Google Scholar]
- 37.Yokota C, Ikebuchi M, Suzuki M, Norioka M, Ikeda K, Shinozaki K, Harano Y. Insulin resistance rather than hyperinsulinemia more closely associated with essential hypertension. Clin Exp Hypertens. 1995;17:523–536. doi: 10.3109/10641969509037422. [DOI] [PubMed] [Google Scholar]