Abstract
The purpose of this study was to develop an injectable in situ liquid crystal formulation for intra-articular (IA) administration, and in situ forming a viscous liquid crystalline gel with long-term release of sinomenine hydrochloride (SMH) upon water absorption. The pseudo-ternary phase diagram of phytantriol (PT)-ethanol (ET)-water was constructed, and isotropic solutions were chosen for further optimization. The physicochemical properties of isotropic solutions were evaluated, and the phase structures of liquid crystalline gels formed by isotropic solutions in excess water were confirmed by crossed polarized light microscopy (CPLM) and small-angle X-ray scattering (SAXS). In vitro drug release studies were conducted by using a dialysis membrane diffusion method. The optimal in situ cubic liquid crystal (ISV2) (PT/ET/water, 64:16:20, w/w/w) loaded with 6 mg/g of SMH showed a suitable pH, showed to be injectable, and formed a cubic liquid crystalline gel in situ with minimum water absorption within the shortest time. The optimal ISV2 was able to sustain the drug release for 6 days. An in situ hexagonal liquid crystal (ISH2) system was prepared by addition of 5% vitamin E acetate (VitEA) into PT in the optimal ISV2 system to improve the sustained release of SMH. This ISH2 (PT/VitEA/ET/water, 60.8:3.2:16:20, w/w/w/w) was an injectable isotropic solution with a suitable pH range. The developed ISH2 was found to be able to sustain the drug release for more than 10 days and was suitable for IA injection for the treatment of rheumatoid arthritis (RA).
KEY WORDS: in situ cubic liquid crystal, in situ hexagonal liquid crystal, phytantriol, sinomenine hydrochloride, sustained drug release
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammation of the joints, with proliferation of the synovium and progressive erosion of cartilage and bone (1,2). RA may lead to significant disability and consequently a reduction in patients’ quality of life. According to reports (3,4), RA affects about 1% of the worldwide population and is associated with a large expenditure of health care resources.
Sinomenine (7,8-didehydro-4-hydroxy-3,7-dimethoxy-17-methyl-morphinan-6-one) is an alkaloid found in the root of the climbing plant Sinomenium acutum (5). Sinomenine has significant anti-inflammatory, analgesic, and immune-suppressive effects and has been widely used in clinical treatment of RA (6,7). It has been demonstrated that sinomenine is more efficacious and safe than traditional non-steroidal anti-inflammatory medicines (6). In this study, sinomenine hydrochloride (SMH) was chosen as a model drug due to its good water solubility.
Intra-articular (IA) injection of SMH, unlike traditional drug delivery methods such as oral, parenteral (i.v., i.m., s.c.), and transdermal administrations, is able to directly deliver drugs to joint cavity, thus increasing local drug concentration and decreasing drug dose and systemic side effects (8). Unfortunately, it is demonstrated that small molecules are rapidly cleared from joint cavity after IA injection (9). Therefore, there is an urgent need to develop a sustained release formulation of SMH to reduce frequent dose and improve patient compliance.
Inverse bicontinuous cubic liquid crystals (V2) and inverse hexagonal liquid crystals (H2) have recently received much attention due to their highly ordered internal structures, which offers the potential as a slow release matrix for active pharmaceutical ingredients with various size and polarity (10–12). In addition, both vehicles demonstrate the ability to protect unstable drugs from enzymatic and physical degradation (13–16). In the past decade, phytantriol (PT) has been widely used to form V2 and H2 (10,17). PT is a well-known active ingredient used in cosmetic industry (18), which has been widely used as a cubic phase-forming material in excess water at physiological temperature (17,19,20). PT-water system forms the V2 at physiological temperature, with a transition to H2 at a higher temperature. The transition temperature from V2 to H2 may be suppressed at 25°C by addition of 5% (w/w) vitamin E acetate (VitEA) to PT (19). By the addition of 5% VitEA to PT, the release of hydrophilic drugs (e.g., glucose, Allura Red, and FITC-dextrans) from the PT-based H2 was significantly slower than that from V2 at 37°C (10).
Since V2 and H2 are too viscous for IA injection, low-viscosity precursor systems were prepared which form V2 or H2 upon exposure to excess solution/synovial fluids (10,21). These low-viscosity precursor systems are called in situ liquid crystal delivery systems, such as in situ cubic liquid crystals (ISV2) and in situ hexagonal liquid crystals (ISH2). In situ liquid crystal delivery systems have several advantages, such as simple production, easy administration, dose reduction capability, and local and sustained delivery (22). In recent years, injectable in situ liquid crystal formulations with low viscosity have been extensively studied in sustained parenteral drug delivery (22–26). Han et al. (25) developed an ISV2 system (PT/ethanol (ET)/water, 0.8:1:3.2, w/w/w) as an embolic agent for the treatment of hepatocellular carcinoma. The in vitro dissolution results showed that docetaxel in the formulation sustained release for up to 30 days. In another study, Borgheti-Cardoso et al. (22) developed an ISH2 system (monoglycerides/oleylamine/propylene glycol/tris buffer, 8.16:0.34:76.5:15, w/w/w/w) for local small interfering RNA delivery, which was able to form a complex with the small interfering RNA to avoid its degradation and transform into a hexagonal liquid crystalline gel after subcutaneous injection.
In this study, two types of SMH-loaded in situ liquid crystal systems, i.e., ISV2 and ISH2, were developed for the treatment of RA via IA administration. ISV2 was prepared by PT, ET, and water. The pseudo-ternary phase diagram of PT-ET-water was constructed and the component influence on ISV2 formation was investigated. The prepared ISV2 systems were characterized by crossed polarized light microscopy (CPLM) and small-angle X-ray scattering (SAXS) and optimized by their physicochemical properties and in vitro release behavior. ISH2 system was developed with addition of 5% VitEA into PT in the optimal ISV2 system and characterized by CPLM and SAXS. The in vitro release behavior of ISH2 was also performed and compared to that of ISV2.
MATERIALS AND METHODS
Materials
Phytantriol (3,7,11,15-tetramethyl-1,2,3-hexadecanetriol, PT, GC > 95%) and vitamin E acetate (VitEA, GC >98%) were purchased from Tokyo Chemical Industry Co., Ltd. (Shanghai, China). Ethanol absolute was supplied by Nanjing Chemical Reagent Co., Ltd. (Nanjing, China). Sinomenine hydrochloride was obtained by Chengdu Must Biotechnology Co., Ltd. (Chengdu, China). All reagents were of analytical grade except that methanol and triethylamine were of HPLC grade. Purified water from a Milli-Q system (Millipore, Bedford, USA) was used throughout the experiment.
Preparation of In Situ Liquid Crystal Systems
ISV2 was prepared using PT, ET, and water. PT was gently melted at 60 ± 0.5°C. After that, ET was added into PT to facilitate the flowability of the system. Meanwhile, an aqueous solution of SMH was prepared by dissolving the drug in Milli-Q water. This aqueous solution was pre-warmed at the same temperature as PT and then mixed with PT and ET. The mixture was then vortex-mixed homogeneously for 3 min and centrifuged at 3000 rpm for 30 min. The resulting formulations were finally sterilized by filtration through a 0.22-μm filter and kept in closed vials at room temperature for 48 h for equilibration before any experimental execution. ISH2 was prepared in the same manner except for the addition of 5% VitEA into PT before melting.
Construction of Pseudo-ternary Phase Diagram
A phase diagram was constructed by first mixing PT with ET in the ratios of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1 (w/w). A predetermined amount of each mixture was then transferred into a vial and mixed with water at the ratios of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1 (w/w) to a total weight of 0.2 g. The resulting formulations were maintained 48 h at room temperature to reach equilibrium. After that, each phase was identified by visual observation and CPLM at room temperature, as described below.
Visual Observation and CPLM
The samples were characterized by visual observation and CPLM (CK-500, Caikon, China) at room temperature. Visual observation was used to inspect sample homogeneity and phase separation. CPLM was used to identify sample phase structures. In addition, CPLM was also used to confirm the phase structures of liquid crystalline gels formed by ISV2 and ISH2 in excess water.
SAXS Measurements
The phase structures of liquid crystalline gels formed by ISV2 and ISH2 in excess water were further confirmed by SAXS at 48 h after formation. The measurements were performed on an SAXSess mc2 SAXS (Anton Paar, Austria) with X-ray source of wavelength λ = 0.154 nm, and operated at 40 kV and 50 mA. The optics and sample chamber were under vacuum to minimize air scatter. Measurements were performed at 37°C, and samples were equilibrated for 10 min prior to collection of scattering pattern for 10 min. Scattering files were background-subtracted, and scattering intensities were plotted versus q value, which enabled the identification of peak positions.
Physicochemical Characterization
Evaluation of Syringeability
Syringeability is considered as the ability of a preparation to be successfully administered by syringe with an appropriate needle (27). In this study, 1-mL plastic syringes with 25 G needles (Shifeng®, Shanghai, China) were used to evaluate the syringeability of the chosen formulations. And, the syringeability was manually assessed at room temperature and was considered as an all-or-nothing process in evaluating the ability of the selected formulation to be injected (27).
Determination of pH Value
For parenteral formulations, the general acceptable pH range is 4–9 (28). The pH values of chosen formulations were determined by a SevenMuti type multi-tester (Mettler Toledo, Shanghai, China).
Determination of the Minimum Volume of Water for Gelation (Vmin)
After IA injection, ISV2 needs to absorb water to form a cubic liquid crystalline gel for sustained drug release. The Vmin of the chosen formulations was determined using the magnetic stirring method (29). First, 0.1 g of the sample was aliquoted into a 5-mL vial, and at the same time, a magnetic bar (10 mm × 6 mm) was added into the vial. The vial was then incubated in a water bath at 37.0 ± 0.5°C for 5 min with the magnetic stirring speed of 30 rpm. After that, 10 μL of water was added into the vial every 1 min until the magnetic bar completely stopped moving due to gelation. The total volume of the water added into the vial was determined to be the Vmin of the sample.
Determination of Gelation Time (Tg)
The Tg of the chosen formulations was also determined using the magnetic stirring method. First, 0.1 g of the sample was aliquoted into a 5-mL vial, and at the same time, a magnetic bar (10 mm × 6 mm) was added into the vial. The vial was then incubated in a water bath at 37.0 ± 0.5°C for 5 min with the magnetic stirring speed of 30 rpm. After that, excess water (0.3 mL) was added into the vial, and the time when the magnetic bar completely stopped moving due to gelation was determined to be the Tg of the sample.
In Vitro Drug Release
In vitro release study was conducted by using a dialysis membrane diffusion method (21). The formulations (0.5 g) in triplicate were placed separately in 6-cm dialysis bags (14,000 Da MWCO). The dialysis bags were then closed and immersed into 6 mL of pH 7.4 PBS in glass bottles, which were further placed in a horizontal shaker (37.0 ± 0.5°C, 60 rpm). Sodium azide (0.02%, w/v) was added to the release medium to prevent bacterial contamination. Sink conditions were provided throughout the experiments. At predetermined time points of 0.5, 1, 2, 4, 6, 12, 24, 48, 72, 96, 120, 144, 168, 192, 216, and 240 h, all of the release medium was withdrawn and replaced with fresh medium. The release medium samples were filtered through 0.45-μm millipore filters and analyzed using reverse phase high-performance liquid chromatography (RP-HPLC).
The amount of SMH released was analyzed using a validated RP-HPLC method. The RP-HPLC system was equipped with a binary pump (Waters 1525), an UV detector (Waters 2489) set at 263 nm, a 1500 series column heater set at 30°C, and a Breeze chromatographic working station. Chromatographic separation was performed using a Phenomenex Gemini C18 column (250 mm × 4.6 mm, 5 μm). The isocratic mobile phase was composed of 18% methanol and 82% water containing 0.075% phosphoric acid (v/v) and 0.125% triethylamine (v/v). The flow rate was set at 1 mL/min and the injection volume was set at 20 μL.
Statistical Analysis
The similarity factor f2 was used for release profile comparison (30) and calculated using the following equation:
| 1 |
where f2 is the similarity factor, and Rt and Tt are the percent drug released at each time point for the two compared samples, respectively (31). As indicated by Shah et al. (30), the f2 value has to be higher than 50 to assess two release profiles as being similar.
RESULTS AND DISCUSSION
Phase Diagram
PT and V2 are too viscous to be injected directly (21,25). In order to obtain an injectable ISV2 system which will transform into cubic liquid crystalline gel after contact with synovial fluids, a co-solvent of ET was added into the PT-water system (21). The ternary phase diagram of PT-ET-water was constructed to characterize different phase areas and identify the compositions of the isotropic solution phases (Fig. 1). In the phase diagram, each phase was identified by visual observation and CPLM at room temperature (22,25). The solution samples that showed a dark background under CPLM were characterized as isotropic solution phase. Samples visualized as emulsion with phase separation after 48 h were characterized as milky phase. Samples visualized as gel that showed a dark background under CPLM were characterized as cubic phase. Samples that showed Maltese crosses under CPLM were classified as lamellar phase. Samples that showed a birefringence fan-like structure under CPLM were regarded as hexagonal phase.
Fig. 1.
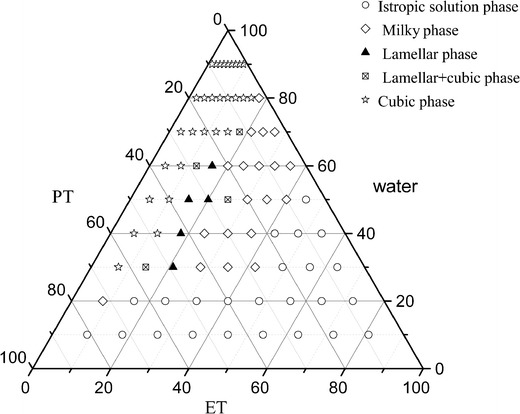
Phase diagram of the PT-ET-water system at 25.0 ± 0.5°C
As shown in Fig. 1, the isotropic solution phase formed at a water content of less than 50%. A viscous liquid crystalline phase (lamellar, mixture of lamellar and cubic phase, or cubic phase) was observed when the water content was higher than 30% and ET content less than 30%. An isotropic solution phase and two liquid crystalline phases were shown in the constructed phase diagram, which was consistent with the results generated by Wadsten-Hindrichsen et al. (32).
According to the diagram, the isotropic solution phase was mainly formed in lower water content areas (less than 20%) whereas the cubic phase was mainly obtained in higher water content areas (higher than 70%). This behavior can be explained by the critical packing parameter (P), which is used to predict the phase structure of liquid crystal formed by amphiphile molecules. The formula is P = v/al, where P is critical packing parameter, v is the hydrophobic chain volume, a is the cross-sectional area of the polar headgroup and l is the hydrophobic chain length (33). The P values are commonly 1 and >1 for lamellar and cubic phases (34). When water content increases, the polar head of PT will move more freely. This results in a disorder of the hydrophobic chain, increasing v. a and l tends to be constant. Therefore, the P value will increase which favors the transformation of isotropic solution phase into cubic phase.
Since the isotropic solution phases showed liquid-like viscosity, the formulations from this region might be suitable for IA injection. The cubic phase was formed when the ET content was less than 20%, and as the ET content in PT-water mixtures increased, the amount of water required to form the cubic phase increased (Fig. 1). Therefore, isotropic solution phases with lower ET content were chosen as ISV2 for further study.
Physicochemical Characterization
Composition of the selected ISV2 formulations is shown in Table I. A suitable IA injected ISV2 formulation requires specific properties, such as suitable syringeability, appropriate pH, and the ability to form a cubic liquid crystalline gel in situ rapidly with minimum water absorption. The physicochemical properties of the formulations F1–F5, such as syringeability, pH value, Vmin, and Tg, are characterized and summarized in Table II.
Table I.
Composition of Investigated ISV2 Formulations (All Quantities Are Given as Percentage by Weight)
| Formulations | PT (%) | ET (%) | Water (%) | SMH (mg/g) |
|---|---|---|---|---|
| F1 | 64 | 16 | 20 | 6 |
| F2 | 56 | 24 | 20 | 6 |
| F3 | 48 | 32 | 20 | 6 |
| F4 | 68 | 17 | 15 | 6 |
| F5 | 72 | 18 | 10 | 6 |
PT phytantriol, ET ethanol, SMH sinomenine hydrochloride
Table II.
Physicochemical Properties of Investigated Formulations (Mean ± SD, n = 3)
| Formulations | Syringeability | pH | V min (μL) | T g (s) |
|---|---|---|---|---|
| F1 | Injectable | 5.20 ± 0.06 | 63.33 ± 5.77 | 4.21 ± 0.40 |
| F2 | Injectable | 5.14 ± 0.05 | 143.33 ± 5.77 | 33.84 ± 3.12 |
| F3 | Injectable | 5.08 ± 0.04 | 206.67 ± 11.55 | 90.77 ± 7.96 |
| F4 | Injectable | 5.23 ± 0.07 | 66.67 ± 11.55 | 7.31 ± 0.27 |
| F5 | Injectable | 5.26 ± 0.04 | 80.00 ± 5.77 | 9.20 ± 0.33 |
As reported in Table II, all formulations were injectable when evaluated by 1-mL plastic syringes with 25 G needles. It should be noted that in the case of the IA injection to the knee, narrow needles from 20 to 25 G are currently used (27,35,36). In this study, the narrowest needles of 25 G were used to minimize the invasion. In addition, the pH values of all formulations were slightly acidic (pH 5.08–5.26) (28).
The effects of different compositions in formulation to form cubic liquid crystalline gels in situ were also evaluated by Vmin and Tg (Table II). Formulations F1, F2, and F3 had the same water content (20%) but with different ratios of PT/ET (8:2, 7:3, and 6:4). Formulations F1, F4, and F5 had the same ratio of PT/ET (8:2) but with different water contents (20, 15, and 10%). When the water content kept constant (formulations F1, F2, and F3), Vmin and Tg increased with decreased ratio of PT/ET (Table II). When the ratio of PT/ET was kept at 8:2 (formulations F1, F4, and F5), water content decrease resulted in the increase of Vmin and Tg (Table II). As shown in Table II, formulation F1 had minimum values of Vmin and Tg compared to other four formulations. This indicated that formulation F1 formed a cubic liquid crystalline gel in situ with minimum water absorption within the shortest time.
In order to confirm whether the ISV2 formulations transformed into the cubic liquid crystalline gels in excess water, CPLM was used to characterize the structures of the formed gels. CPLM is the easiest technique to qualitatively identify different phases by their textures. All investigated gels showed a dark background under CPLM and were characterized as cubic liquid crystals. In addition to CPLM, SAXS is particularly useful in identifying liquid crystalline phases (37). Therefore, the phase structure of the gel formed from formulation F1 in excess water was further confirmed by SAXS, with a formulation without SMH as a control. The SAXS scattering profiles are illustrated in Fig. 2. Three Bragg peaks with relative positions at ratios of √2:√3:√4 were marked in the figure. The SAXS data of the samples showed the presence of Pn3m cubic phase structure, which was consistent with the previous studies on PT (17). Moreover, the loaded model drug of SMH in the formulation did not affect the phase behavior (Fig. 2).
Fig. 2.
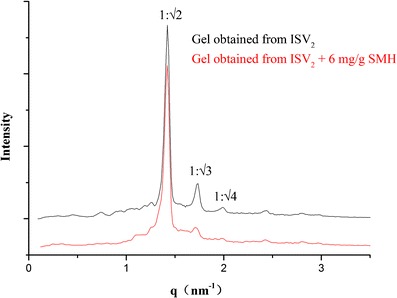
SAXS profiles of the gels obtained from ISV2 formulations (PT/ET/water, 64:16:20, w/w/w) with and without 6 mg/g SMH in excess water. The graphics show the ratio between the interplanar distances
In Vitro Drug Release Studies
The Influence of Compositions of ISV2Formulations
In vitro release studies were conducted to investigate the influence of the initial compositions of ISV2 formulations on release behavior for a model hydrophilic drug SMH. The formulations in Table I were chosen for drug release studies, and the release profiles are illustrated in Figs. 3 and 4.
Fig. 3.
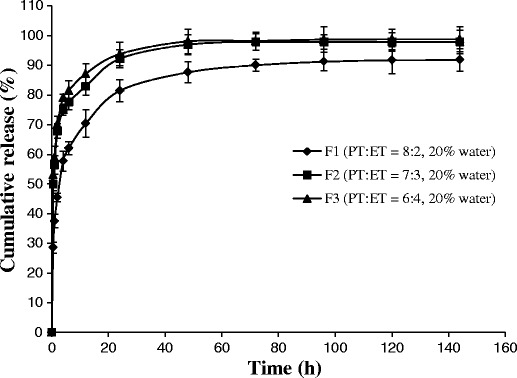
Effect of PT/ET ratio on the SMH (6 mg/g) release from ISV2 formulations (n = 3, mean ± SD)
Fig. 4.
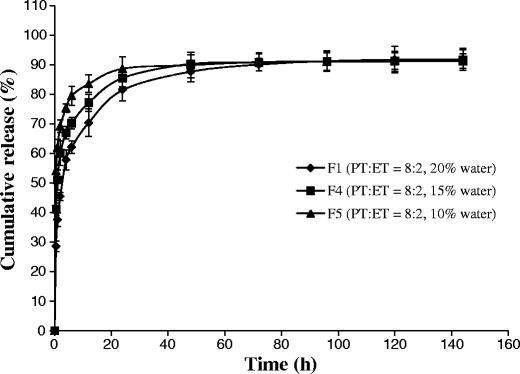
Effect of water content on the SMH (6 mg/g) release from ISV2 formulations (n = 3, mean ± SD)
The release of SMH from formulations F1, F2, and F3 with the same water content (20%) but different ratios of PT/ET (8:2, 7:3, and 6:4) is illustrated in Fig. 3. The release profile of SMH obtained from formulation F1 was significantly slower than that from formulation F2 (f2 = 38.9) and formulation F3 (f2 = 35.5). However, no significant difference was observed between F2 and F3 (f2 = 74.7). The SMH released from formulations F1, F4, and F5 with the same ratio of PT/ET (8:2) but different water contents (20, 15, and 10%) is illustrated in Fig. 4. When the water content of the formulation decreased from 20 to 15% (F1 vs. F4), the release of SMH was slightly faster, but not significant (f2 = 50.3). If the water content of the formulation further decreased from 20 to 10% (F1 vs. F5), SMH release was significantly faster (f2 = 36.9).
From above results, it was indicated that the drug release decreased with the ratio of PT/ET increased (Fig. 3) or the water content increased (Fig. 4), which was consistent with the results obtained by Ahmed et al. (26). As reported by Ahmed et al. (26) in their study, increasing the ratio of PT/ET or water content increased the viscosity of the formulation, thus resulting to the decreased drug release. Additionally, increasing the ratio of PT/ET also increased the matrix thickness of the fully swollen cubic phase, which also contributed to the decreased drug release (26,38).
The Influence of Drug Loading
It was widely reported that the drug release from V2 is independent of initial drug loading (39,40). However, only few research studies investigated the effect of the drug loading on the release behavior of ISV2. In this study, ISV2 formulations (PT/ET/water, 64:16:20, w/w/w) loaded with 6, 8, and 12 mg/g of SMH, respectively, were chosen for in vitro release studies. As shown in Fig. 5, a higher drug loading led to a faster release of SMH. The release profile of formulation loaded with 12 mg/g of SMH was significantly faster than the formulation loaded with 6 mg/g (f2 = 35.1) and 8 mg/g (f2 = 48.9) of SMH. Similar in vitro release results were found for propranolol hydrochloride (21), but the exact mechanism is not clear and needs further investigation. In addition, it should be noted that the drug was not completely released from the lower drug content formulations even after 144 h. This incomplete release was probably attributed to the binding of the drug to the PT as described in a previous research (25).
Fig. 5.
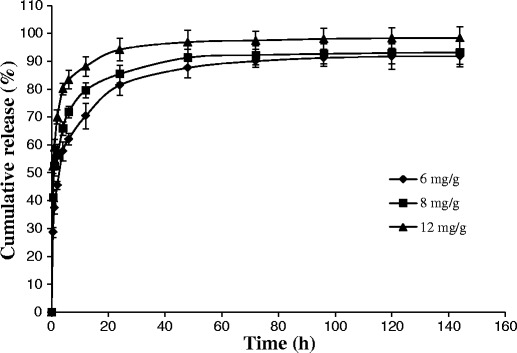
Effect of drug loading on the SMH release from ISV2 formulations with a ratio of PT/ET/water of 64:16:20 (n = 3, mean ± SD)
Preparation and Characterization of ISH2
According to the results from the previous sections, the optimal ISV2 (PT/ET/water, 64:16:20, w/w/w) loaded with 6 mg/g of SMH was able to sustain the drug release for 6 days. However, it was suggested that the selected formulation could be even optimized to further improve the sustained release of SMH. For instance, Lee et al. (10) reported that the liquid crystalline nanostructure was utilized to control the release rate of hydrophilic drugs (e.g., glucose, Allura Red, and FITC-dextrans). When V2 and H2 were prepared using PT with and without 5% VitEA, respectively, the in vitro release rate of each hydrophilic drug from H2 was significantly slower than that from V2. Based on their results, an ISH2 system, which is able to transform into an H2 in excess water, was prepared by addition of 5% VitEA into PT in the optimal ISV2 system. The ISH2 composition was 60.8% of PT, 3.2% of VitEA, 16% of ET, and 20% of water by weight.
Physicochemical properties of the ISH2 were characterized. CPLM study demonstrated that the ISH2 was an isotropic solution and was injectable when evaluated with 1-mL plastic syringes using 25 G needles. The pH value of the ISH2 was 5.51.
CPLM and SAXS were used to identify the phase structure of the liquid crystalline gel formed from ISH2 in excess water. As shown in Fig. 6a, the control gel which was formed from ISV2 in excess water was characterized as a cubic phase (dark background under CPLM). The gel formed from ISH2 in excess water that showed a fan-like texture was characterized as a hexagonal phase (Fig. 6b). The SAXS scattering profiles of the gels obtained from ISH2 with and without 6 mg/g of SMH in excess water are illustrated in Fig. 7. The SAXS profiles of samples exhibited multiple peaks with relative positions at ratios of 1:√3:√4, which indicated a hexagonal phase (22). Moreover, the presence of 6 mg/g model drug SMH in the ISH2 did not affect the phase behavior (Fig. 7).
Fig. 6.
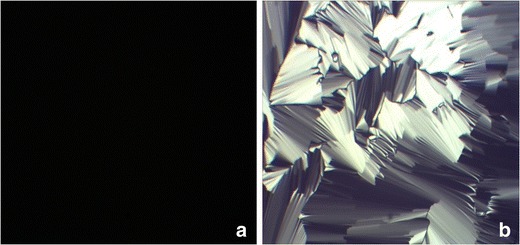
Images of samples under CPLM (magnification ×100). a Cubic liquid crystalline gel formed from ISV2 (PT/ET/water, 64:16:20, w/w/w) in excess water. b Hexagonal liquid crystalline gel formed from ISH2 (PT/VitEA/ET/water, 60.8:3.2:16:20, w/w/w/w) in excess water
Fig. 7.
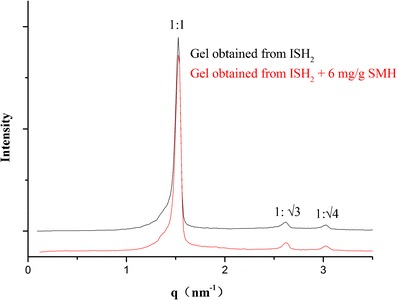
SAXS profiles of the gels obtained from ISH2 (PT/VitEA/ET/water, 60.8:3.2:16:20, w/w/w/w) with and without 6 mg/g of SMH in excess water. The graphics show the ratio between the interplanar distances
Comparison of the In Vitro Drug Release Behavior of ISV2 and ISH2
In vitro release studies were conducted to investigate the difference of release behavior between ISV2 and ISH2. Figure 8 illustrates the in vitro release profiles of 6 mg/g SMH from both ISV2 and ISH2.
Fig. 8.
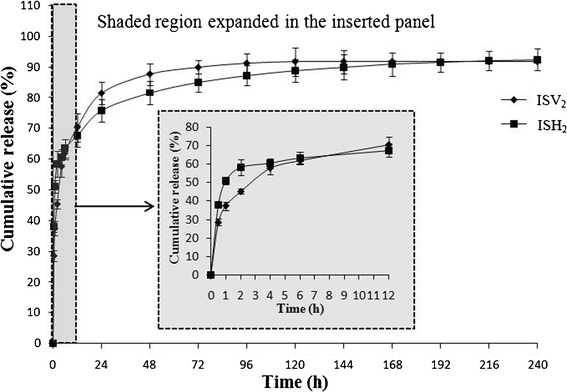
In vitro release profiles of 6 mg/g SMH from ISV2 (PT/ET/water, 64:16:20, w/w/w) and ISH2 (PT/VitEA/ET/water, 60.8:3.2:16:20, w/w/w/w). Inserted panel shows the in vitro release profiles in the first 12 h
As shown in Fig. 8, ISH2 sustained the drug release for more than 10 days, while the drug level in the release medium was undetectable after 6 days for ISV2 group. In addition, it should be noted that the inserted panel (from 0–12 h) indicated that the drug release rate of ISH2 was faster than that of ISV2 in the first 1 h. This could be probably due to the addition of the hydrophobic VitEA to the system, which hindered the process of water absorption to transform into a hexagonal liquid crystalline gel. As time was going on, ISV2 and ISH2 transformed into cubic and hexagonal liquid crystalline gels, respectively, and the release rate of SMH from the matrixes was mainly dependent on the diameter and tortuosity of water channels, as well as whether they are open channels or closed (41). It is well known that the release rate of hydrophilic drugs from H2 with rod-like closed water channels is much slower than that from V2 with larger open water channels, which explained the slower release rate of ISH2 group after 1 h (10,41,42). The results of this study further demonstrated that the phase structure of liquid crystals formed from their precursor systems (ISV2 and ISH2) also had an influence on the release behavior of ISV2 and ISH2.
CONCLUSION
In this study, ISV2 and ISH2 systems with long-term release of SMH for IA injection were successfully formulated using the PT-ET-water system, with and/or without VitEA. The preliminary in vitro studies showed that both ISV2 and ISH2 were injectable and within acceptable pH range. ISV2 and ISH2 systems were able to convert in situ into viscous cubic and hexagonal liquid crystalline gel, respectively, after contact with excess water. In vitro release studies showed that the optimal ISV2 sustained the drug release for 6 days, while the ISH2 was able to sustain the drug release for more than 10 days. This ISH2 composed of 60.8% PT, 3.2% VitEA, 16% ET, and 20% water appears to be the most appropriate for IA injection for the treatment of RA.
ACKNOWLEDGMENTS
The authors are supported by National Natural Science Foundation of China (No. 81274099), Anhui Provincial Natural Science Foundation (No. 1408085QH183), Anhui Provincial Natural Science Foundation (No. 11040606 M219), Natural Science Foundation of the Anhui Higher Education Institutions of China (No. KJ2012A184), and Anhui Provincial Training Plan for a Thousand of Talents. The content is solely the responsibility of the authors and does not necessarily represent the official views of Anhui University of Chinese Medicine or Hospira Inc.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
REFERENCES
- 1.Gordon DA, Hastings DE. Clinical features of rheumatoid arthritis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. 3. New York: Mosby; 2003. pp. 765–80. [Google Scholar]
- 2.Tarner IH, Müller-Ladner U. Drug delivery systems for the treatment of rheumatoid arthritis. Expert Opin Drug Deliv. 2008;5(9):1027–37. doi: 10.1517/17425247.5.9.1027. [DOI] [PubMed] [Google Scholar]
- 3.Pugner KM, Scott DI, Holmes JW, Hieke K. The costs of rheumatoid arthritis: an international long-term view. Semin Arthritis Rheum. 2000;29:305–20. doi: 10.1016/S0049-0172(00)80017-7. [DOI] [PubMed] [Google Scholar]
- 4.Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182–8. doi: 10.1016/j.semarthrit.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Buchner E, Beitze D, Schmidt-Weber CB, Kaever V, Emmrich F, et al. Amelioration of rat experimental arthritides by treatment with the alkaloid sinomenine. Int J Immunopharmacol. 1996;18(10):529–43. doi: 10.1016/S0192-0561(96)00025-2. [DOI] [PubMed] [Google Scholar]
- 6.Xu M, Liu L, Qi C, Deng B, Cai X. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Planta Med. 2008;74(12):1423–9. doi: 10.1055/s-2008-1081346. [DOI] [PubMed] [Google Scholar]
- 7.Qian SS, Chen YL, Gui SY, Wang J, Zhou YJ, Chen L. Enhanced penetration of sinomenine fomulations following skin pretreatment with a polymer microneedle patch. Lat Am J Pharm. 2014;33(3):464–9. [Google Scholar]
- 8.Gerwin N, Hops C, Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Deliv Rev. 2006;58:226–42. doi: 10.1016/j.addr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Larsen C, Ostergaard J, Larsen SW, Jensen H, Jacobsen S, Lindegaard C, et al. Intra-articular depot formulation principles: role in the management of postoperative pain and arthritic disorders. J Pharm Sci. 2008;97(11):4622–54. doi: 10.1002/jps.21346. [DOI] [PubMed] [Google Scholar]
- 10.Lee KW, Nguyen TH, Hanley T, Boyd BJ. Nanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugs. Int J Pharm. 2009;365(1–2):190–9. doi: 10.1016/j.ijpharm.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Shah JC, Sadhale Y, Chilukuri DM. Cubic phase gels as drug delivery systems. Adv Drug Deliv Rev. 2001;47(2–3):229–50. doi: 10.1016/S0169-409X(01)00108-9. [DOI] [PubMed] [Google Scholar]
- 12.Chen YL, Ma P, Gui SY. Cubic and hexagonal liquid crystals as drug delivery systems. Bio Med Res Int. 2014 doi: 10.1155/2014/815981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clogston J, Caffrey M. Controlling release from the lipidic cubic phase. Amino acids, peptides, proteins and nucleic acids. J Control Release. 2005;107(1):97–111. doi: 10.1016/j.jconrel.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Mezzenga R, Schurtenberger P, Burbidge A, Michel M. Understanding foods as soft materials. Nat Mater. 2005;4(10):729–40. doi: 10.1038/nmat1496. [DOI] [PubMed] [Google Scholar]
- 15.Ubbink J, Burbidge A, Mezzenga R. Food structure and functionality: a soft matter perspective. Soft Matter. 2008;4(8):1569–81. doi: 10.1039/b802183j. [DOI] [PubMed] [Google Scholar]
- 16.Mohammady SZ, Pouzot M, Mezzenga R. Oleoylethanolamide-based lyotropic liquid crystals as vehicles for delivery of amino acids in aqueous environment. Biophys J. 2009;96(4):1537–46. doi: 10.1016/j.bpj.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barauskas J, Landh T. Phase behavior of the phytantriol/water system. Langmuir. 2003;19(23):9562–5. doi: 10.1021/la0350812. [DOI] [Google Scholar]
- 18.Wagner E. Panthenol and phytantriol in cosmetics. Parfümerie Kosmetic. 1994;75(4):260–7. [Google Scholar]
- 19.Dong Y, Larson I, Hanley T, Boyd BJ. Bulk and dispersed aqueous phase behavior of phytantriol: effect of vitamin E acetate and F127 polymer on liquid crystal structure. Langmuir. 2006;22(23):9512–8. doi: 10.1021/la061706v. [DOI] [PubMed] [Google Scholar]
- 20.Barauskas J, Johnsson M, Tiberg F. Self-assembled lipid superstructures: beyond vesicles and liposomes. Nano Lett. 2005;5(8):1615–9. doi: 10.1021/nl050678i. [DOI] [PubMed] [Google Scholar]
- 21.Chang CM, Bodmeier R. Low viscosity monoglyceride-based drug delivery systems transforming into a highly viscous cubic phase. Int J Pharm. 1998;173(1–2):51–60. doi: 10.1016/S0378-5173(98)00180-X. [DOI] [Google Scholar]
- 22.Borgheti-Cardoso LN, Depieri LV, Diniz H, Calzzani RA, Fantini MC, Lyomasa MM, et al. Self-assembling gelling formulation based on a crystalline-phase liquid as a non-viral vector for siRNA delivery. Eur J Pharm Sci. 2014;58(16):72–82. doi: 10.1016/j.ejps.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Carvalho FC, Campos ML, Peccinini RG, Gremião MP. Nasal administration of liquid crystal precursor mucoadhesive vehicle as an alternative antiretroviral therapy. Eur J Pharm Biopharm. 2013;84(1):219–27. doi: 10.1016/j.ejpb.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Phelps J, Bentley MV, Lopes LB. In situ gelling hexagonal phases for sustained release of an anti-addiction drug. Colloids Surf B: Biointerfaces. 2011;87(2):391–8. doi: 10.1016/j.colsurfb.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 25.Han K, Pan X, Chen M, Wang R, Xu Y, Feng M, et al. Phytantriol-based inverted type bicontinuous cubic phase for vascular embolization and drug sustained release. Eur J Pharm Sci. 2010;41(5):692–9. doi: 10.1016/j.ejps.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed AR, Dashevsky A, Bodmeier R. Drug release from and sterilization of in situ cubic phase forming monoglyceride drug delivery systems. Eur J Pharm Biopharm. 2010;75(3):375–80. doi: 10.1016/j.ejpb.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Réeff J, Gaignaux A, Goole J, De Vriese C, Amighi K. New sustained-release intraarticular gel formulations based on monolein for local treatment of arthritic diseases. Drug Dev Ind Pharm. 2013;39(11):1731–41. doi: 10.3109/03639045.2012.730529. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh AK, Jasti BR. Parenteral routes of delivery. In: Theory and practice of contemporary pharmaceutics. 1st ed. USA: CRC Press; 2004. p. 387–423.
- 29.Yuan Y, Cui Y, Zhang L, Zhu H, Guo Y, Zhong B, et al. Thermosensitive and mucoadhesive in situ gel based on poloxamer as new carrier for rectal administration of nimesulide. Int J Pharm. 2012;430(1–2):114–9. doi: 10.1016/j.ijpharm.2012.03.054. [DOI] [PubMed] [Google Scholar]
- 30.Shah VP, Tsong Y, Sathe P, Liu JP. In vitro dissolution profile comparison-statistics and analysis of the similarity factor f2. Pharm Res. 1998;15:889–96. doi: 10.1023/A:1011976615750. [DOI] [PubMed] [Google Scholar]
- 31.Polli JE, Rekhi GS, Augsburger LL, Shah VP. Methods to compare dissolution profiles and a rationale for wide dissolution specifications for metoprolol tartrate tablets. J Pharm Sci. 1997;86(6):690–700. doi: 10.1021/js960473x. [DOI] [PubMed] [Google Scholar]
- 32.Wadsten-Hindrichsen P, Bender J, Unga J, Engstrom S. Aqueous self-assembly of phytantriol in ternary systems: effect of monoolein, distearoylphosphatidylglycerol and three water-miscible solvents. J Colloid Interface Sci. 2007;315(2):701–13. doi: 10.1016/j.jcis.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Israelachvili J. The science and applications of emulsions-an overview. Colloid Surf A Physicochem Eng Asp. 1994;91(3):1–8. doi: 10.1016/0927-7757(94)02743-9. [DOI] [Google Scholar]
- 34.Engström S, Engström L. Phase behaviour of the lidocaine-monoolein-water system. Int J Pharm. 1992;79(1–3):113–22. doi: 10.1016/0378-5173(92)90102-8. [DOI] [Google Scholar]
- 35.Neustadt DH. Intra-articular injections for osteoarthritis of the knee. Cleve Clin J Med. 2006;73(10):897–911. doi: 10.3949/ccjm.73.10.897. [DOI] [PubMed] [Google Scholar]
- 36.Réeff J, Gaignaux A, Goole J, Siepmann J, Siepmann F, Jerome C, et al. Characterization and optimization of GMO-based gels with long term release for intraarticular administration. Int J Pharm. 2013;451(1–2):95–103. doi: 10.1016/j.ijpharm.2013.04.079. [DOI] [PubMed] [Google Scholar]
- 37.Fong W, Hanley T, Boyd BJ. Stimuli responsive liquid crystals provide ‘on-demand’ drug delivery in vitro and in vivo. J Control Release. 2009;135(3):218–26. doi: 10.1016/j.jconrel.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Chang CM, Bodmeier R. Swelling of and drug release from monoglyceride-based drug delivery systems. Int J Pharm. 1997;147(2):135–42. doi: 10.1016/S0378-5173(96)04805-3. [DOI] [PubMed] [Google Scholar]
- 39.Lara MG, Bentley MV, Collett JH. In vitro drug release mechanism and drug loading studies of cubic phase gels. Int J Pharm. 2005;293(1–2):241–50. doi: 10.1016/j.ijpharm.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Geraghty PB, Attwood D, Collett JH, Dandiker Y. The in vitro release of some antimuscarinic drugs from monoolein/water lyotropic liquid crystalline gels. Pharm Res. 1996;13(8):1265–71. doi: 10.1023/A:1016036908947. [DOI] [PubMed] [Google Scholar]
- 41.Phan S, Fong WK, Kirby N, Hanley T, Boyd BJ. Evaluating the link between self-assembled mesophase structure and drug release. Int J Pharm. 2011;421(1):176–82. doi: 10.1016/j.ijpharm.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 42.Rizwan SB, Hanley T, Boyd BJ, Rades T, Hook S. Liquid crystalline systems of phytantriol and glyceryl monooleate containing a hydrophilic protein: characterisation, swelling and release kinetics. J Pharm Sci. 2009;98(11):4191–204. doi: 10.1002/jps.21724. [DOI] [PubMed] [Google Scholar]


