Abstract
Transdermal delivery of therapeutic amounts of vitamin D3 is proposed to overcome its variable oral bioavailability, especially for people who suffer from fat malabsorption. The main challenge for this delivery route is to overcome the barrier properties of skin, especially for very lipophilic compounds such as vitamin D3. In this study, the effect of different penetration enhancers, such as oleic acid, dodecylamine, ethanol, oleic acid in propylene glycol, isopropyl myristate, octyldodecanol, and oleyl alcohol in propylene glycol were evaluated in vitro for their effectiveness in delivering vitamin D3 through polyamide filter, polydimethylsiloxane membrane, and porcine skin. A diffusion cell was used to study the transdermal permeability of vitamin D3. Ointment formulations of vitamin D3 were prepared containing the most widely used penetration enhancers, oleic acid, and dodecylamine. The ointment containing oleic acid as chemical penetration enhancer did not improve delivery compared to control. On the other hand, the formulation containing dodecylamine as a penetration enhancer did improve the transdermal delivery of vitamin D3. However, statistical significance and an amount high enough for nutritional supplementation purposes were reached only when the skin was pretreated with 50% ethanol. In these conditions, the ointment delivered an amount of 760-ng vitamin D3 per cm2 of skin. The research shows promise that transdermal delivery could be an effective administration route for vitamin D3 when ethanol and dodecylamine are used as penetration enhancers.
KEY WORDS: dodecylamine, ethanol, penetration enhancer, transdermal delivery, vitamin D3
INTRODUCTION
Vitamin D3 is a naturally occurring form of vitamin D and plays an important role in bone mineralization and skeletal growth. More recently, there is evidence connecting plasma levels of vitamin D3 and its metabolites with cancer, cardiovascular diseases, autoimmune disease, and infection (1). The main source of vitamin D3 prior to oral supplements was the natural synthesis in the skin upon exposure to ultraviolet light. Given the current lifestyle of most people, today, the main source of vitamin D3 has become nutritional supplementation. The recommended daily allowance for normal children and adults is 400 IU/day. The best approach for assessing vitamin D status is measuring the level of 25-hydroxyvitamin D3 (25(OH)D3, calcidiol) in the serum. According to many studies, low 25(OH)D3 levels are associated with increased risk of many chronic diseases such as hypertension, inflammation, diabetes, and cancer (2). Furthermore, high levels of 25(OH)D3 were recently associated with increased risk of pancreatic cancer (3). Therefore, each patient should be recommended an individualized optimal dose of vitamin D supplement.
Vitamin D3 absorption is an important factor to consider when calculating an appropriate dose. When vitamin D3 is administered orally, the absorption takes place in the proximal small intestine with the aid of bile acids. There are many factors that affect vitamin D3 absorption resulting in large differences in the bioavailability of vitamin D3 supplements in some populations. Any diseases resulting in malabsorption of intestinal fat will decrease vitamin D3 absorption (1,4). In patients with celiac disease, biliary obstruction and chronic pancreatitis, the absorption of tritium-labeled (3H)-vitamin D3 fell to 50%, <28%, and <18%, respectively. This is significantly lower than normal subject absorption, which ranges from 62 to 91% (4,5). In another publication, it has been reported that only 50% of the vitamin D3 dose is actually absorbed in cystic fibrosis patients (6). Furthermore, long-term use of bile acid binding medications such as colestipol and cholestyramine will decrease vitamin D3 absorption (5).
It is difficult to estimate the pharmacokinetic properties of vitamin D3 because of the nanomolar concentrations in the body. Less than 25% of vitamin D3 is metabolized to 25(OH)D3. The level of vitamin D3 in the serum is correlated to the vitamin D3 in fat tissue (7). The half-lives of vitamin D3, 25(OH)D3, and 1,25(OH)2D3 are 24 h, 3 weeks, and 4–6 h, respectively (8). The volume of distribution of unmetabolized vitamin D3 is 4 l per kg (9).
Vitamin D3 is available on the market as oral solution, capsule, powder, and tablet. The absorption of vitamin D3 from these formulations can be unreliable especially for patients with fat malabsorption. To address problems related to low bioavailability, other routes of administration can be investigated. Since vitamin D3 is a potent lipophilic drug, having a short plasma elimination half-life, and is needed in a small daily dose, it can be considered as a good candidate for transdermal delivery.
Transdermal delivery has many advantages compared to oral or injection administration, such as avoiding side effects on the gastrointestinal tract, providing continuous drug delivery, and being easy to apply or remove. However, there are currently no quantitative studies regarding the effectiveness of this route of administration for vitamin D3. All studies so far have only investigated topical delivery of vitamin D3.
For example, in a clinical report of two cases with inferior heel pain, the patients were successfully treated with vitamin D3 cream (10). The treatment consisted of applying a cream containing 1200 IU of vitamin D3 per gram twice a day. The calcitriol and Ca2+ levels in the serum were monitored after the treatment and were found to be in the normal range.
Another recent study analyzed the relationship between serum level of 25(OH)D3 and stratum corneum hydration based on conductance measurements and tested the effect of topical application of vitamin D3 on dry skin. The blood level of 25(OH)D3 and skin conductive measurements for 83 subjects were taken. The results showed that subjects with low levels of 25-hydroxvitamin D had low skin conductance, which indicated that the subjects’ skin was drier. Further, 61 subjects from the above group who were in the non-sufficient group were randomly chosen to receive either a vehicle or topical supplementation with vitamin D3 (10 μg/g formulation). The group who received the vitamin D3 supplementation significantly improved dryness compared to the subjects who received treatment without vitamin D3 (11).
Yamagishi et al. delivered calcitriol transdermally in dairy cattle (5 mg/animal) and studied the effects on plasma calcitriol and calcium concentrations. The tail skin of six healthy, non-pregnant Jersey heifers were treated for a duration of 2 days with three different types of patches: an encapsulated 500-μL reservoir solution made of 10 μg/μL calcitriol in vehicle (99% ethanol), 10 μg/μL calcitriol with 10 μg/μL dodecylamine in vehicle, or vehicle alone as control. Cattle treated with calcitriol alone or calcitriol with dodecylamine had significant increases in plasma calcitriol and Ca2+ concentrations on day 2 and days 2 and 3, respectively (12). Further, it has been proven that topical administration of active vitamin D3 (1,25-dihydroxyvitamin D3) and its analogues can be used as first-line treatment for plaque psoriasis (13).
However, transdermal delivery of therapeutic amounts is very difficult to achieve for some compounds because of the barrier properties of the skin. There are specific requirements for a molecule in order to pass through the skin barrier, which are as follows: small molecular weight (<600 Da), an adequate solubility in both lipids and water, and a balanced partition coefficient (logP from −1 to 4) (14). The molecular weight of vitamin D3 is 384.64 g/mol, and the recommended daily dose is very small (0.01–5 mg). Vitamin D3 is soluble in ethanol, acetone, ether, and chloroform and insoluble in water (15). The measured lipid/water partition coefficient is 10.2 (16). Except for its very high lipophilicity, vitamin D3 is an excellent candidate for transdermal delivery.
Upon application on the skin, almost any chemical compound—and especially small molecules—will diffuse to some extent in the skin and through the skin. The purpose of the current study was to find if the amount of vitamin D3 delivered transdermally from ointment formulations is small enough to be negligible or large enough to be useful for supplementation purposes; the influence of penetration enhancers on dermal and transdermal delivery was also investigated.
There are various techniques to enhance drug delivery through the stratum corneum. Both chemical and physical methods could enhance penetration through the skin barrier. One of the most effective methods to enhance transdermal delivery consists of using chemical penetration enhancers such as fatty acids, esters and alcohols, azones, amides, polyols, essential oils, terpenes, and certain polymers (17).
Fatty acids have been used as penetration enhancers for several drugs such as donepezil, rivastigmine, caffeine, and propranolol. Oleic acid is a cis-unsaturated fatty acid and one of the most investigated chemical penetration enhancers. It is commonly used in many transdermal formulations. For example, oleic acid improves the penetration of both salicylic acid and 5-flurouracil through the human skin membrane (2). Oleic acid could enhance penetration across the skin by phase separation (18). There are several advantages to using oleic acid over any penetration other enhancers. Some advantages include that it is a natural compound, is recognized as safe, and is approved by FDA as inactive ingredient in some products such as Vivelle® (19).
There are several studies that investigated the toxicity of oleic acid. According to Loftsson et al., oleic acid irritation depends on the other vehicle additives used because pure oleic acid or propylene glycol did not cause any irritation when they were applied to human skin for 6 h (20). Oleic acid did not cause irritation when it was dissolved in n-propanol or 1-ethyl-1,3-hexanediol either. In contrast, when 5% oleic acid in propylene glycol was applied, minor irritation was detected and the irritation increased with increasing oleic acid concentrations. Moreover, Boelsma and his colleagues demonstrated that oleic acid toxicity depends on the thickness of the stratum corneum (21). They found that when 5% labeled oleic acid/propylene glycol was applied to human stratum corneum for 21 h, no oleic acid was detected in the receptor medium. In the literature, concentrations of oleic acid ranging from 1 to 30% have been proposed for enhancing drug penetration across the skin (22).
Dodecylamine, another common enhancer, is a typical unsaturated fatty amine which is known to increase the skin permeation rate of various drugs (e.g., testosterone (23) and fluorouracil (24)). Dodecylamine has been used as a penetration enhancer for a calcitriol transdermal patch (12).
Several transdermal delivery formulations are available, such as gel, cream, lotion, ointment, microemulsion, liposomes, niosomes, transfersomes, ethosomes, and nanocarriers.
Ointment was chosen as the best transdermal delivery system for the current investigation because the ointment base increases the contact time with skin, which is vital for this highly lipophilic compound (25). Moreover, ointment bases usually increase skin hydration and drug permeability, which are very important advantages over any other type of delivery system (17). Many drugs have been used with ointment bases for transdermal delivery, such as salicylic acid and ketoprofen (26).
For this project, petroleum jelly (Vaseline) was used as an ointment base because it has many benefits over the other bases; for example, petroleum jelly is compatible with many drugs, has an emollient effect, has good stability because of its anhydrous properties, and increases drug permeation because of its occlusive properties (27).
MATERIALS AND METHODS
Materials
Vitamin D3, isotopically labeled vitamin D3, PEG 400, PEG 1500, anhydrous lanolin, white Vaseline, ammonium acetate (mass spectrometry grade), formic acid, HPLC grade acetonitrile, HPLC grade methanol, and phosphate buffer saline (pH = 7.4) were purchased from Sigma-Aldrich (Atlanta, GA). Isopropyl myristate, oleyl alcohol, and octyldodecanol were received as research samples from BASF (Tarrytown, NY). PDMS membranes with a thickness of 0.005″ were purchased from Specialty Silicone Products, Inc. (Ballston Spa, NY). Porcine serum and porcine ear skin (full thickness) were purchased from Bioreclamation (Hicksville, NY) and further processed as previously described (28).
Preparation of Vitamin D3 Ointment
Three formulations with vitamin D3 were prepared: one as control and two with penetration enhancers, as shown in Table I.
Table I.
Ingredients for Vitamin D3 Master Formulation (F1) Without Penetration Enhancer, Formulation 2 (F2) with Oleic Acid, and Formulation 3 (F3) With Dodecylamine
| Ingredient | Amount |
|---|---|
| Vitamin D3 | 0.3 g |
| Penetration enhancer (one option) | 0 (none) for formulation 1 |
| 1 g oleic acid for formulation 2 | |
| 1 g dodecylamine for formulation 3 | |
| PEG 400 | 1.9 g |
| PEG 1500 | 1.0 g |
| Anhydrous lanolin | 1.5 g |
| White Vaseline | q.s. 10.0 g |
Formulation 1 (F1) consists of vitamin D3 ointment without any penetration enhancers and is used as control formulation. Formulation 2 (F2) contains in addition oleic acid as penetration enhancer while formulation 3 (F3) contains dodecylamine as penetration enhancer. To prepare the ointments, vitamin D3, white Vaseline, anhydrous lanoline, the penetration enhancer (except for F1), and PEG 1500 were melted together (70°C) on a steam bath; after they were melted, hot PEG 400 solvent was added slowly and stirred (29).
Physical stability of the ointment preparations was checked by monitoring the color and odor during the experiment, as well as by examining each formulation under the polarized light microscope.
Preparation of Stock, Working, and Calibration Solutions
All stock solutions were prepared in ethanol at concentrations of 1.04 × 107 nM, 6.24 × 105 nM, and 2.58 × 105 nM for vitamin D3, 25-hydroxyvitamin D3, and labeled vitamin D3, respectively. Working solutions of vitamin D3 and 25-hydroxy vitamin D3 were prepared by diluting the stock solutions with 75:25 acetonitrile:water to a final concentration of 5 × 104 nM. The working internal standard solution was also prepared by diluting the stock solution of internal standard with 75:25 acetonitrile:water to a final concentration of 300 nM. A working solution was prepared by mixing equal amounts of vitamin D3 and 25 hydroxyvitamin D3 solution and diluted with the internal standard solution to a final concentration of 2500 nM. All solutions were stored at −20°C when not in use.
The calibration standards were prepared when needed at concentrations of 4, 20, 100, 500, and 2500 nM by serial dilutions of the working solution with 75:25 acetonitrile:water.
Extraction of Vitamin D3 from Samples
Acetonitrile was used both to extract vitamin D3 and 25-hydroxyvitamin D3 from samples and to precipitate plasma proteins (in the case of porcine serum samples). The extraction method was previously described and validated (30). Briefly, 100 μL of solvent (acetonitrile) with deuterated vitamin D3 standard at a concentration of 300 nM was vortexed with 50 μL of sample for 5 min at 2500 rpm, sonicated for 10 min, and centrifuged at 7000 rpm for 30 min. Subsequently, a portion of the supernatant, around 100 μL, was transferred into small volume glass inserts for autosampler vials and subjected to analysis by LC-MS/MS (10 μL injected).
To evaluate the extraction method, a 5 μL spike from a 5000 nM solution of vitamin D3 and 25-hydroxyvitamin D3 was mixed with 45-μL porcine serum for 10 min to obtain a final concentration of 500 nM vitamin D3 (and metabolite). Then, the extraction procedure and analysis were performed as described above in this section. Each experiment was performed at least in triplicate.
Because porcine serum already contains some vitamin D3, blank corrections were made. Fifty microliters of serum was vortexed with 100 μL of the solvent with deuterated vitamin D3 standard at a concentration of 300 nM to precipitate serum proteins and extract vitamin D3, followed by the general analytical procedure. This experiment was performed at least three times for each batch of serum.
High Performance Liquid Chromatography-Mass Spectrometry
A Waters Acquity ultra performance liquid chromatography system was used with a C18 column (2.1 by 50 mm) packed with 1.7-μm particles. The HPLC method was as follows: the mobile phases used were (A) 2 mM ammonium acetate with 0.1% formic acid and (B) methanol with 0.3% formic acid at a flow rate of 0.3 mL/min and a gradient from 50 to 95% solvent B. The run time for each sample was 7.5 min. The internal standard was isotopically labeled vitamin D3 (6,19,19-D3). The system was equipped with a triple quadrupole mass spectrometer and was used in positive ion electrospray ionization mode. Detection was performed by monitoring the following mass-to-charge transitions: 385.4/159.1 for vitamin D3, 401.5/159.2 for 25(OH)D3, and 388.4/259.2 for isotopically labeled vitamin D3 (internal standard). The retention times were 4.2 min for 25OHD3 and 5.5 min for both vitamin D3 and isotopically labeled vitamin D3. The relative standard deviation (RSD) of the analytical method, determined at three concentration levels in porcine serum and skin homogenate (low 5 nM, medium 50 nM, and high 500 nM) varied between 2.9 and 5.3% for intra-day and inter-day experiments, respectively. Method accuracy varied between 105% for low concentrations to 97% for high concentrations, which indicates that the LC-MS/MS method gave excellent reproducibility and good accuracy in the data set. The linear range evaluated in this study was 4-2500 nM vitamin D3 in serum, skin homogenate, and PBS, with an excellent correlation coefficient (R2 = 1).
Solubility Test
The solubility limit of vitamin D3 in various receptor solutions (20% ethanol in phosphate buffer; porcine serum) was tested to select the best receptor medium for the diffusion cell experiments, in order to achieve sink conditions. First, an excess amount of vitamin D3 powder was allowed to dissolve in 8-mL 20% ethanol in phosphate buffered saline. Separately, an excess amount of vitamin D3 powder was added to a vial containing 4-mL porcine serum with 0.1% sodium azide (to prevent bacterial growth). Each experiment was done in triplicate. The solutions were kept in a 37°C incubator for 24 h. After 24 h, 1.0-mL supernatant was collected and centrifuged at 7000 rpm for 15 min. Aliquots of 50 μL were taken from each vial and further processed using the general sample preparation and analysis procedure for measuring the concentration of vitamin D3. In the case of the samples collected from the serum experiments, analysis was performed after a suitable dilution with mobile phase.
Diffusion Cell Experiments
The vertical Franz diffusion cell (PermeGear, Hellertown, PA) was used to investigate the vitamin D3 release from the three investigated formulations, as well as vitamin D3 delivery through artificial membrane and porcine skin.
The in vitro release of vitamin D3 from the ointment formulation was tested using a polyamide filter (0.22 μm pore size). An “infinite” dose was placed on a filter and mounted on the Franz diffusion cell. The infinite dose means that the amount of drug applied to the membrane is sufficient to achieve and maintain the maximum rate of absorption into the receptor solution. To obtain the steady state conditions where the absorption rate and permeability coefficient can be calculated, doses of >10 mg/cm2 are required. The diffusion surface area was 0.91 cm2 for the cells used in this research. Therefore, 0.334 g of the formulation, which was equivalent to 10 mg of vitamin D3, was applied to the filter membrane. The temperature was maintained around 37°C. The receptor medium contained 8 mL of porcine serum (in which the solubility of vitamin D3 is high enough to maintain sink conditions). Accurate volumes of samples from the receptor phase (100 μl) were withdrawn at time points 0, 0.5, 1, 3, 5, 7, 12, and 24 h. The volume of each withdrawn sample was replaced with fresh receptor medium to keep the volume constant. The amount of vitamin D3 released was determined by LC-MS/MS as described above.
For delivery through an artificial membrane, ointment containing an infinite dose of vitamin D3 was placed on a polydimethylsiloxane (PDMS) membrane and mounted on the Franz diffusion cell.
For investigating transdermal delivery, porcine skin was used instead of a PDMS membrane. The skin’s electrical resistance was measured before and after each diffusion cell experiment to test the skin integrity. A multimeter was used to test the resistance. Skin with a specific electrical resistance of <1.8 KΩ/cm2 was considered damaged and was not used, as previously reported (31).
Skin pretreatment with penetration enhancers was carried out as follows: skin resistance was measured; skin was treated with various penetration enhancers; skin was washed with phosphate buffer; skin resistance was measured before the diffusion cell experiment; the amount of vitamin D in the receptor solution was measured at the times 0, 0.5, 1, 3, 5, 7, 12, and 24 h; and skin resistance was measured again at the end of the experiment.
Assay of Vitamin D3 Concentration in the Skin Using the Tape Stripping Method
To measure the cutaneous delivery of vitamin D3, the skin from the permeation experiments was used after thoroughly washing with distilled water. Adhesive tape was used to remove the stratum corneum from epidermis. Fifteen strips were used to remove the stratum corneum from the epidermal tissues. The pieces of tape were cut so that they covered the diffusion area. The first tape strip was discarded, and the following fifteen tape strips were placed in a vial. The area outside of the flange imprints of the viable epidermis was trimmed; the remaining part, which was exposed to the formulation, was cut into small pieces, placed in a vial, homogenized with a tissue homogenizer for 1 min, and processed further according to previously published procedures (32).
Vitamin D3 was removed from the tape by a serial liquid extraction method using three volumes of 1 mL acetonitrile (3 mL total), which ensured that all the vitamin D3 was extracted from the tape. Extraction of vitamin D3 from the dermal tissues was also performed by serial liquid extraction using 2 volumes of 2 mL acetonitrile (4 mL total) to extract all the vitamin D3. The content of each vial was vortexed for 5 min, sonicated for 15 min, and filtered. Then, 50 μL supernatant from each vial was mixed with 100 μL acetonitrile containing internal standard and treated as previously described for vitamin D3 extraction. After that, LC-MS was used to measure the amount of vitamin D3 in each of the samples.
The amount of the vitamin D3 in the receptor phase was used to calculate the index of transdermal delivery, and the amount in the SC and epidermal tissues were used to calculate the index of topical delivery.
Vitamin D3 Diffusion and In Vitro Release
There are several different release mechanisms such as zero order, first order, and Higuchi release (33,34). Curve fitting and statistical analysis were used to find the best fit. Microsoft Excel 2010 and SigmaPlot 10 were used to perform the curve fitting.
The Korsmeyer and Peppas model described drug release using Eq. 1: (35)
| 1 |
where Mt/M∞ is the fraction of drug released at time t, k is the kinetic constant of the system, and n is the release exponent. The n value is used to differentiate between different release mechanisms as follows: Fickian diffusion (Higuchi release) for n = 0.5, non-Fickian release for 0.5 < n < 1, zero-order release (case II transport) for n = 1, and super case II transport for n >1.
The cumulative amount of vitamin D3 that diffused through polyamide filter, PDMS membrane, and porcine skin was calculated using Eq. 2:
| 2 |
where Cumn is the cumulative amount of vitamin D3 in the receptor medium, VR is the receptor volume, Cn is the concentration of the nth sample, Vcol. is the collected sample volume, and Ci is the concentration of the ith sample. In this study, the receptor solution volume was 8 mL and the collected volume was 100 μL.
Diffusion parameters were calculated by plotting the cumulative amount permeated in 24 h versus time. The slope of the curve was the flux (J) and the x-intercept of the straight line was lag time. To calculate the apparent permeability coefficient (Kp), the flux was divided by the donor concentration (36).
The enhancement factor (EF) of each penetration enhancer was calculated using Eq. 3 (37):
| 3 |
where Jp is the flux for the ointment containing penetration enhancer and Jc is the flux for the control formulation.
RESULTS
Physical Appearance and Homogeneity
The physical appearance and homogeneity of the prepared ointments were examined visually and under the polarized light microscope. All three formulations were uniform in appearance and had a light yellow color. As Fig. 1 shows, there were no solid or un-dissolved particles in the formulations.
Fig. 1.
Textures of prepared ointments under the polarized light microscope (magnification = ×200) at 25°C
Vitamin D3 Solubility Studies
The solubility limit of vitamin D3 was found to be 6.7 × 102 ± 32 nM (n = 3) in a solution of 20% ethanol in phosphate buffer saline and 2.6 × 104 ± 2.1 × 103 nM (n = 3) in porcine serum.
Investigation of Vitamin D3 Delivery
In Vitro Release and Penetration Through PDMS Artificial Membrane
The amounts of vitamin D3 (ng/cm2) delivered through the filter membrane from F1, F2, and F3 ointments at various time points are shown in Fig. 2. The cumulative amounts released through the filter membrane in 24 h were (mean ± SE) 4.0 × 104 ± 7.3 × 103; 5.2 × 103 ± 1.2 × 103; and 6.6 × 104 ± 1.4 × 103 ng/cm2 for F1, F2, and F3 ointments, respectively.
Fig. 2.
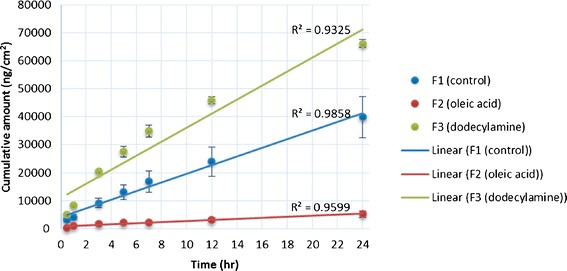
Cumulative permeation of vitamin D3 through a filter from control formulation, oleic acid formulation, and dodecylamine formulation as a function of time. Each point represents the mean ± SE of three trials
The cumulative amounts (ng/cm2) of vitamin D3 that permeated through the PDMS membrane following the application of F1, F2, and F3 ointment are shown in Fig. 3. The amounts of vitamin D3 permeated in 24 h from the F1, F2, and F3 ointments were 1.3 × 103 ± 2.9 × 102 ng/cm2, 3.3 × 103 ± 2.7 × 103 ng/cm2, and 6.4 × 103 ± 7.5 × 102 ng/cm2, respectively.
Fig. 3.

Cumulative permeation of vitamin D3 through PDMS membrane from control formulation, oleic acid formulation, and dodecylamine formulation as a function of time. Each point represents the mean ± SE of three trials
In Vitro Skin Penetration Study
Skin penetration of vitamin D3 from the two formulations with penetration enhancers was studied by comparison to the control formulation. As Fig. 4 shows, both formulations with enhancers delivered more vitamin D3 into both the stratum corneum and epidermis than the control over the first 24 h of the experiment. Compared to F1, formulation F2 delivered four times more compound into the epidermis and two times more into the stratum corneum, while F3 delivered eight times more into the epidermis and ten times more into the stratum corneum.
Fig. 4.
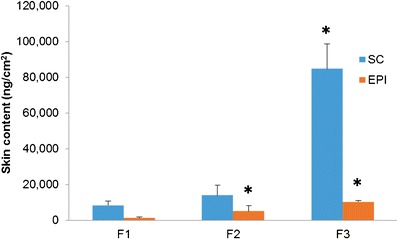
Vitamin D3 delivered to the stratum corneum (SC) and epidermis (EPI)
The cumulative amounts of vitamin D3 penetrated through the skin into the receptor solution (pig serum) over 24 h from F1, F2, and F3 were found to be 170 ± 48, 0, and 360 ± 260 ng/cm2, respectively. For F2, no detectable increase in the concentration of vitamin D3 in the porcine serum was observed. Since these amounts are too low for delivering the daily dose of vitamin, pretreatment of skin with various other penetration enhancers was investigated.
As shown in Fig. 5, skin pretreatment with 5% oleic acid in propylene glycol (5% OA/PG) or with 50% ethanol resulted in higher transdermal delivery of vitamin D3 in contrast to experiments that did not include pretreatment.
Fig. 5.
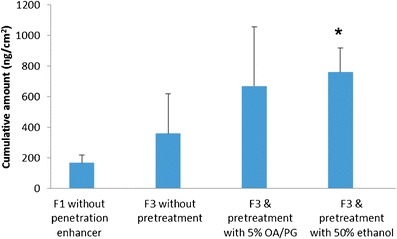
Cumulative amount for the control and F3 formulations with or without skin pretreatment; values represent mean ± S.E. for three trials; OA/PG represents oleic acid in propylene glycol; pretreatment with 5% OA/PG was done at 4°C and pretreatment with 50% ethanol was done at 37°C
DISCUSSION
Vitamin D3 Solubility Studies
To choose the optimal receptor medium for the diffusion cell experiments, the solubility of vitamin D3 in both phosphate buffer (PBS) containing 20% ethanol and porcine serum was measured. The receptor medium used for the in vitro vitamin D3 transdermal delivery experiments was serum because it provided sink conditions (much higher solubility for the vitamin).
The solubility limit of vitamin D3 in water is very low, of only 170 nM (38). In order to provide sink conditions, the receptor medium should have an adequate capacity to solubilize vitamin D3 (39). Adding 20% ethanol to PBS is the classical approach to increase the solubility of lipophilic drugs in the receptor medium (40). However, the disadvantage of using a high content of ethanol is that it can negatively impact skin integrity. Furthermore, the solubility limit of vitamin D3 is still too low even in 20% ethanol solutions. Another way to increase the solubility is to use porcine serum because serum naturally contains vitamin D binding protein and albumin which help to increase the solubility of vitamin D3 in the receptor medium (41). As expected, the solubility limit of vitamin D3 in porcine serum (2.6 × 104 nM) was found to be much higher than the solubility in water (1.7 × 102 nM) or in 20% ethanol in PBS (6.7 × 102 nM). This increased solubility is the main reason for using porcine serum as receptor medium instead of 20% ethanol solution. As an added advantage, the porcine serum helped maintain the integrity of the porcine skin and provided more physiological conditions for the transdermal delivery experiment (42–44).
The average vitamin D3 level in blank serum was found to be 22.4 ± 1.4 nM (n = 4). This level was used as the baseline concentration for all transdermal delivery experiments. The actual vitamin D3 concentrations were determined by subtracting the normal concentration of the serum from the concentration of the sample. In addition, the concentration of 25(OH)D3 was monitored in all samples and was found to be absent in non-biological solutions and constant in biological samples (porcine serum).
Investigation of Vitamin D3 Delivery
In Vitro Release and Penetration Through PDMS Artificial Membrane
Release of vitamin D3 across a polyamide filter and penetration through a polydimethylsiloxane (PDMS) membrane were evaluated for all three ointment formulations in order to characterize them before tests on skin (45–47). The maximum release rates that can be achieved by the investigated formulations were observed when the only barrier between the ointments and the receptor solution was a porous filter. The presence of dodecylamine as a penetration enhancer in the F3 ointment helped promote the release of vitamin D3 compared to the control formulation. On other hand, oleic acid—the penetration enhancer in the F2 ointment—decreased the release of vitamin D3 compared to the control. This is most likely due to the lipophilic complex formed between oleic acid and vitamin D3 (48,49). This was not unexpected, since a similar decrease in the release rate has also been reported for alfuzosin hydrochloride gel when oleic acid was used as penetration enhancer (36).
Because of the barrier properties of PDMS, the amount of vitamin D3 permeated through the membrane was lower than in the case of the filter for all the formulations. As previously shown, PDMS membranes have barrier properties that are very similar to the stratum corneum of the skin (46,50). The highest vitamin D3 delivery rate was achieved by the F3 formulation, as in the case of the experiments with the polyamide filter. In addition, the F2 formulation delivered more vitamin D3 than the control, emphasizing the role of oleic acid in permeabilizing the membrane.
As Table II shows, the flux through the filter for the formulation containing dodecylamine was 1.5 times higher than the value obtained without an enhancer. While F2 also contains a penetration enhancer, oleic acid, the flux through the filter was lower than for the control. Both the flux and permeability for F2 and F3 through PDMS membrane are 2–5 times higher than the values achieved without an enhancer (F1). The formulation containing dodecylamine as a penetration enhancer improved the flux of vitamin D3 for both the filter and PDMS membrane compared to the control. Kinetic modeling showed that the release exponent for all formulations was around 1, indicating that the drug release mechanism was zero order.
Table II.
Diffusion Data for Vitamin D3 Ointment Through Filter Membrane and PDMS Membrane (mean ± SE of three trials)
| Type of barrier | Parameter | F1 | F2 | F3 |
|---|---|---|---|---|
| Filter | Flux [ng/cm2/hr] | 1.5 × 103 ± 8.3 × 101 | 1.9 × 102 ± 1.7 × 101 | 2.5 × 103 ± 3.0 × 102 |
| Permeability coefficient [cm/hr] | 5.1 × 10−5 ± 2.8 × 10−6 | 6.3 × 10−6 ± 5.7 × 10−7 | 8.4 × 10−5 ± 1.0 × 10−5 | |
| PDMS membrane | Flux [ng/cm2/hr] | 4.7 × 101 ± 3.2 × 100 | 1.1 × 102 ± 1.4 × 101 | 2.5 × 102 ± 1.0 × 101 |
| Permeability coefficient [cm/hr] | 1.6 × 10−6 ± 1.1 × 10−7 | 3.6 × 10−6 ± 4.6 × 10−7 | 8.2 × 10−6 ± 3.4 × 10−7 |
In Vitro Skin Penetration Study
Porcine skin was used to investigate the transdermal permeation of vitamin D3 from all the formulations. Skin integrity was verified by measuring its electrical resistance before and after the transdermal delivery experiment. The electrical resistance values indicated that the skin sections maintained integrity for the experiments with all three ointment formulations, as well as when the skin was pretreated with either 50% ethanol at 37°C or 5% oleic acid in propylene glycol at 4°C.
As Fig. 4 shows, the amount of vitamin D3 retained in the skin at the end of the 24-h transdermal delivery experiments for the formulations containing oleic acid or dodecylamine was higher than for the control formulation. A higher vitamin D3 concentration in the skin tissue could increase the flux across the skin, from the formulation into the blood stream—provided a long enough time is given (51). The formulation with dodecylamine showed the highest amount of vitamin D3 delivered topically.
The average cumulative amount delivered transdermally by the formulation containing dodecylamine as a penetration enhancer was higher than that delivered by the control formulation, although statistical significance was not reached. For the formulation containing oleic acid, no detectable amount of vitamin D3 permeated through the porcine skin during the first 24 h of the experiment. Longer times were not investigated since the barrier function of excised skin can decrease significantly after the first 24 h (28,40). Apparently, more than 24 h are needed in the case of F2 to start permeation into the receptor solution. On average, F3 provided an enhancement ratio of 2.2 over the control formulation.
The lag time for formulations F1 and F2 was much longer (from 12 to more than 24 h) than the lag time for F3 (∼7 h). Overall, the lag time for all formulations was very long as the highly lipophilic vitamin D3 needs time to diffuse from the ointment to the skin and also to penetrate through skin and interact with skin lipids.
Furthermore, the cumulative amounts of vitamin delivered by the ointment formulations with penetration enhancers were too low for delivering transdermally the recommended daily dose of vitamin D3 (on a reasonably small area of skin). In order to further decrease lag time and improve vitamin D3 transdermal delivery, skin pretreatment with other penetration enhancers was investigated, as previously proposed (17,52–54).
The following penetration enhancers were used to treat the skin before applying the ointment formulation: 5% OA/PG, isopropyl myristate, octyldodecanol, 5% oleyl alcohol in propylene, and 50% ethanol. It was found that none of these pretreatment agents helped increase the cumulative amount of vitamin delivered, except for 5% oleic acid in propylene glycol and 50% ethanol in phosphate buffer.
Several studies indicated that the combination of oleic acid with propylene glycol improves the transdermal penetration of drugs (22,55,56). In this research, oleic acid was applied to skin samples as a solution at 5% in propylene glycol, for durations ranging from 4 to 12 h and at a temperature of either 4 or 37°C. Pretreatment at body temperature for 12 h was found to decrease the barrier function of the skin too much, as determined by skin resistance measurements, and was not used further. In order to maintain the skin integrity, a shorter pretreatment time of 4 h at 37°C was investigated. However, this pretreatment failed to improve transdermal delivery of vitamin D3. As an alternative, pretreatment for a longer time at a lower temperature was investigated. When skin samples were pretreated with 5% OA/PG for 12 h at 4°C, the skin kept its integrity and the cumulative amounts (ng/cm2) of vitamin delivered by F1, F2 and F3 were approximately double (by comparison to experiments with untreated skin). The amount delivered by the ointment with dodecylamine (F3) continued to be the highest. However, despite improvements in transdermal delivery, pretreatment for 12 h at 4°C was considered to be unlikely to have practical applicability.
On the other hand, pretreatment of skin with 50% ethanol in phosphate buffer for 4 h at 37°C was sufficient to significantly increase transdermal delivery. The cumulative amount of vitamin D3 delivered from F3 in this case was 760 ng/cm2, statistically larger than the amount delivered by the control formulation (Fig. 5). Further advantages of pretreatment with ethanol were that it improved delivery after a shorter exposure time, and it was applicable at physiological temperature. It has been previously reported that ethanol undergoes a synergistic effect when it is combined with chemical permeation enhancers (39,53,57). In addition to the direct effect on skin permeability, ethanol solubilizes the enhancer in the stratum corneum which amplifies the lipid modulating effect. Also, ethanol is used as a penetration enhancer in many lipophilic drug formulations such as those for estradiol and fentanyl (58,59). Our finding is similar to previous research showing that solutions of 25–50% ethanol increase the skin permeability of fluorescein (57). Vitamin D is a micronutrient that is usually administered daily in cases of deficiency; people who suffer from fat malabsorption and vitamin D deficiency would take vitamin D transdermally daily for long periods of time and their skin will already contain the penetration enhancers from previous doses—which can improve the transdermal flux for multiple doses. Further in vivo studies will be required to fully explore transdermal delivery of vitamin D.
Overall, these findings suggest that vitamin D3 can penetrate the skin in therapeutic quantities even with its high lipophilicity, provided that proper penetration enhancers are used. However, this may not be true for all lipophilic compounds. Vitamin D3 is a particular case since the blood of mammalian species contains a high concentration of proteins that can strongly bind it, such as albumin and vitamin D binding protein (41,60). These proteins help solubilize vitamin D3 in the blood and increase the rate of transdermal delivery.
CONCLUSIONS
This study sought to investigate transdermal delivery of vitamin D3 by using different chemical penetration enhancers. To our best knowledge, this is the first report investigating quantitatively the transdermal delivery of vitamin D3, which opens the prospect for further research regarding this delivery route for vitamin D3. Combining dodecylamine and ethanol as penetration enhancers significantly improved the transdermal delivery of vitamin D3 when compared to the control formulation. Based on the recommended daily dose of vitamin D3 (400 IU or 10 μg) and the results of this study, delivery of the recommended daily dose could potentially be achieved by covering a surface of skin of 3.6 ×3.6 cm with vitamin D3 ointment containing both ethanol and dodecylamine. Although these results have only been demonstrated for porcine skin, it is possible that similar results will be obtained in vivo in humans since porcine skin is known to be the best alternative to human skin for in vitro testing of transdermal delivery. This research suggests that transdermal delivery could be an effective way for humans to receive the recommended daily dose of vitamin D3. Transdermal delivery of vitamin D3 could be especially helpful for people who suffer from fat malabsorption.
Acknowledgments
The authors would like to acknowledge funding from the Albany College of Pharmacy and Health Sciences (Scholarship of Discovery for FMM). The authors’ work was independent of the funders.
Conflict of Interest
The authors have no conflict of interest to declare.
REFERENCES
- 1.Grossmann RE, Tangpricha V. Evaluation of vehicle substances on vitamin D bioavailability: a systematic review. Mol Nutr Food Res. 2010;54(8):1055–61. doi: 10.1002/mnfr.200900578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol Am Soc Nephrol. 2008;3(5):1535–41. doi: 10.2215/CJN.01160308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstein SJ, Stolzenberg-Solomon RZ, Kopp W, Rager H, Virtamo J, Albanes D. Impact of circulating vitamin D binding protein levels on the association between 25-hydroxyvitamin D and pancreatic cancer risk: a nested case–control study. Cancer Res. 2012;72(5):1190–8. doi: 10.1158/0008-5472.CAN-11-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol Med J Limited. 2011;91(2):115–24. doi: 10.2340/00015555-0980. [DOI] [PubMed] [Google Scholar]
- 5.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khazai NB, Judd SE, Jeng L, Wolfenden LL, Stecenko A, Ziegler TR, et al. Treatment and prevention of vitamin D insufficiency in cystic fibrosis patients: comparative efficacy of ergocalciferol, cholecalciferol, and UV light. J Clin Endocrinol Metab. 2009;94(6):2037–43. doi: 10.1210/jc.2008-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vieth R. Vitamin D, toxicity, policy, and science. J Bone Miner Res. 2007;22(S2):V64–8. doi: 10.1359/jbmr.07s221. [DOI] [PubMed] [Google Scholar]
- 8.Wootton AM. Improving the measurement of 25-hydroxyvitamin D. Clin Biochem Rev. 2005;26(1):33. [PMC free article] [PubMed] [Google Scholar]
- 9.Vieth R. The pharmacology of vitamin D, including fortification strategies. Vitamin D. Amsterdam: Elsevier; 2005. [Google Scholar]
- 10.Lyftogt J. Treating inferior heel pain with vitamin D3 dermal cream: a clinical report on two case histories. Australas Musculoskelet Med. 2008;13(2):75. [Google Scholar]
- 11.Russell M. Assessing the relationship between vitamin D3 and stratum corneum hydration for the treatment of xerotic skin. Nutrients. 2012;4(9):1213–8. doi: 10.3390/nu4091213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamagishi N, Namioka T, Okura N, Sato S, DaNil K, Furuhama K, et al. Application of a reservoir-type calcitriol transdermal patch in dairy cattle. J Vet Med Sci. 2009;71(6):845–8. doi: 10.1292/jvms.71.845. [DOI] [PubMed] [Google Scholar]
- 13.Segaert S, Ropke M. The biological rationale for use of vitamin d analogs in combination with corticosteroids for the topical treatment of plaque psoriasis. J Drugs Dermatol. 2013;12(8):e129–37. [PubMed] [Google Scholar]
- 14.Chandrashekar N, Rani RS. Physicochemical and pharmacokinetic parameters in drug selection and loading for transdermal drug delivery. Indian J Pharm Sci. 2008;70(1):94. doi: 10.4103/0250-474X.40340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kangarlou S, Haririan I, Gholipour Y. Physico-mechanical analysis of free ethyl cellulose films comprised with novel plasticizers of vitamin resources. Int J Pharm. 2008;356(1):153–66. doi: 10.1016/j.ijpharm.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 16.Raska I, Jr, Toropov A. Comparison of QSPR models of octanol/water partition coefficient for vitamins and non vitamins. Eur J Med Chem. 2006;41(11):1271–8. doi: 10.1016/j.ejmech.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Pathan IB, Setty CM. Chemical penetration enhancers for transdermal drug delivery systems. Trop J Pharm Res. 2009;8(2):173–9. doi: 10.4314/tjpr.v8i2.44527. [DOI] [Google Scholar]
- 18.Shah SNH, Shahzad Y, Badshah A, Meidan VM, et al. Developing an efficacious diclofenac diethylamine transdermal formulation. Food Drug Anal (藥物食品分析) 2012;20(2):464–70. [Google Scholar]
- 19.Ibrahim SA, Li SK. Efficiency of fatty acids as chemical penetration enhancers: mechanisms and structure enhancement relationship. Pharm Res. 2010;27(1):115–25. doi: 10.1007/s11095-009-9985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loftsson T, Gildersleeve N, Bodor N. The effect of vehicle additives on the transdermal delivery of nitroglycerin. Pharm Res. 1987;4(5):436–7. doi: 10.1023/A:1016402900093. [DOI] [PubMed] [Google Scholar]
- 21.Boelsma E, Tanojo H, Bodde H, Ponec M. Assessment of the potential irritancy of oleic acid on human skin: evaluation in vitro and in vivo. Toxicol In Vitro. 1996;10(6):729–42. doi: 10.1016/S0887-2333(96)00053-7. [DOI] [PubMed] [Google Scholar]
- 22.Larrucea E, Arellano A, Santoyo S, Ygartua P. Combined effect of oleic acid and propylene glycol on the percutaneous penetration of tenoxicam and its retention in the skin. Eur J Pharm Biopharm. 2001;52(2):113–9. doi: 10.1016/S0939-6411(01)00158-8. [DOI] [PubMed] [Google Scholar]
- 23.An N-M, Kim D-D, Shin Y-H, Lee C-H. Development of a novel soft hydrogel for the transdermal delivery of testosterone. Drug Dev Ind Pharm. 2003;29(1):99–105. doi: 10.1081/DDC-120016688. [DOI] [PubMed] [Google Scholar]
- 24.Aungst BJ, Blake JA, Hussain MA. Contributions of drug solubilization, partitioning, barrier disruption, and solvent permeation to the enhancement of skin permeation of various compounds with fatty acids and amines. Pharm Res. 1990;7(7):712–8. doi: 10.1023/A:1015859320604. [DOI] [PubMed] [Google Scholar]
- 25.Ueda CT, Shah VP, Derdzinski K, Ewing G, Flynn G, Maibach H, et al. Topical and transdermal drug products. Pharmacopeial Forum. 2009;35:750–64. [Google Scholar]
- 26.Herkenne C, Alberti I, Naik A, Kalia YN, Mathy F-X, Préat V, et al. In vivo methods for the assessment of topical drug bioavailability. Pharm Res. 2008;25(1):87–103. doi: 10.1007/s11095-007-9429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ammar H, Ghorab M, El-Nahhas S, Kamel R. Evaluation of chemical penetration enhancers for transdermal delivery of aspirin. Asian J Pharm Sci. 2007;2:96–105. [Google Scholar]
- 28.Hosmer JM, Shin SH, Nornoo A, Zheng H, Lopes LB. Influence of internal structure and composition of liquid crystalline phases on topical delivery of paclitaxel. J Pharm Sci. 2011;100(4):1444–55. doi: 10.1002/jps.22370. [DOI] [PubMed] [Google Scholar]
- 29.Kezutyte T, Drevinskas T, Maruska A, Rimdeika R, Briedis V. Study of tolnaftate release from fatty acids containing ointment and penetration into human skin ex vivo. Acta Pol Pharm. 2011;68(6):965. [PubMed] [Google Scholar]
- 30.Adamec J, Jannasch A, Huang J, Hohman E, Fleet JC, Peacock M, et al. Development and optimization of an LC-MS/MS-based method for simultaneous quantification of vitamin D2, vitamin D3, 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3. J Sep Sci. 2011;34(1):11–20. doi: 10.1002/jssc.201000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davies D, Ward R, Heylings J. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol In Vitro. 2004;18(3):351–8. doi: 10.1016/j.tiv.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Pellett M, Roberts M, Hadgraft J. Supersaturated solutions evaluated with an in vitro stratum corneum tape stripping technique. Int J Pharm. 1997;151(1):91–8. doi: 10.1016/S0378-5173(97)04897-7. [DOI] [Google Scholar]
- 33.Shoaib MH, Tazeen J, Merchant HA, Yousuf RI. Evaluation of drug release kinetics from ibuprofen matrix tablets using HPMC. Pak J Pharm Sci. 2006;19(2):119–24. [PubMed] [Google Scholar]
- 34.Liu Y, Zhu Y, Wei G, Lu W. Effect of carrageenan on poloxamer-based in situ gel for vaginal use: improved in vitro and in vivo sustained-release properties. Eur J Pharm Sci. 2009;37(3):306–12. doi: 10.1016/j.ejps.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Bonacucina G, Cespi M, Misici-Falzi M, Palmieri GF. Rheological, adhesive and release characterisation of semisolid Carbopol/tetraglycol systems. Int J Pharm. 2006;307(2):129–40. doi: 10.1016/j.ijpharm.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 36.Prasanthi D, Lakshmi P. Effect of chemical enhancers in transdermal permeation of alfuzosin hydrochloride. ISRN Pharm. 2012;2012(Article ID 965280):8. doi: 10.5402/2012/965280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho C-W, Shin S-C. Enhanced controlled transdermal delivery of mexazolam using ethylene-vinyl acetate. Iran J Pharm Res. 2011;11(1):3–12. [PMC free article] [PubMed] [Google Scholar]
- 38.SciFinder Scholar [Internet]. [cited 2014 Nov]. Available from: https://scifinder.cas.org/.
- 39.Watkinson R, Herkenne C, Guy RH, Hadgraft J, Oliveira G, Lane M. Influence of ethanol on the solubility, ionization and permeation characteristics of ibuprofen in silicone and human skin. Skin Pharmacol Physiol. 2008;22(1):15–21. doi: 10.1159/000183922. [DOI] [PubMed] [Google Scholar]
- 40.Hosmer J, Reed R, Bentley MVL, Nornoo A, Lopes LB. Microemulsions containing medium-chain glycerides as transdermal delivery systems for hydrophilic and hydrophobic drugs. AAPS PharmSciTech. 2009;10(2):589–96. doi: 10.1208/s12249-009-9251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musteata ML, Musteata FM. Overview of extraction methods for analysis of vitamin D and its metabolites in biological samples. Bioanalysis. 2011;3(17):1987–2002. doi: 10.4155/bio.11.195. [DOI] [PubMed] [Google Scholar]
- 42.Amrish C, Kumar SP. Transdermal delivery of ketorolac. Yakugaku Zasshi J-STAGE. 2009;129(3):373–9. doi: 10.1248/yakushi.129.373. [DOI] [PubMed] [Google Scholar]
- 43.Varshosaz J, Hajhashemi V, Soltanzadeh S. Lipid nanocapsule-based gels for enhancement of transdermal delivery of ketorolac tromethamine. J Drug Deliv. 2011;2011. [DOI] [PMC free article] [PubMed]
- 44.Prasanthi D, Lakshmi P. Terpenes: effect of lipophilicity in enhancing transdermal delivery of alfuzosin hydrochloride. J Adv Pharm Technol Res. 2012;3(4):216. doi: 10.4103/2231-4040.104712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Musteata FM. Pharmacokinetic applications of microdevices and microsampling techniques. Bioanalysis. 2009;1(1):171–85. doi: 10.4155/bio.09.18. [DOI] [PubMed] [Google Scholar]
- 46.Shah SNH, Shahzad Y, Akash MSH, Ali M, Bukhari SNI, Hassan SS. Rabbit skin and polydimethylsiloxane as model membranes to evaluate permeation kinetics from topical formulation. Pak J Zool. 2013;45(1):159–66. [Google Scholar]
- 47.Chen Y, Wang M, Fang L. Biomaterials as novel penetration enhancers for transdermal and dermal drug delivery systems. Drug Deliv. 2013;20(5):199–209. doi: 10.3109/10717544.2013.801533. [DOI] [PubMed] [Google Scholar]
- 48.Funke AP, Schiller R, Motzkus HW, Günther C, Müller RH, Lipp R. Transdermal delivery of highly lipophilic drugs: in vitro fluxes of antiestrogens, permeation enhancers, and solvents from liquid formulations. Pharm Res. 2002;19(5):661–8. doi: 10.1023/A:1015314314796. [DOI] [PubMed] [Google Scholar]
- 49.Gao S, Singh J. In vitro percutaneous absorption enhancement of a lipophilic drug tamoxifen by terpenes. J Control Release. Elsevier; 1998;51(2):193–9. [DOI] [PubMed]
- 50.Moss GP, Sun Y, Wilkinson SC, Davey N, Adams R, Martin GP, et al. The application and limitations of mathematical modelling in the prediction of permeability across mammalian skin and polydimethylsiloxane membranes. J Pharm Pharmacol. Wiley Online Library; 2011;63(11):1411–27. [DOI] [PubMed]
- 51.Obata Y, Otake Y, Takayama K. Feasibility of transdermal delivery of prochlorperazine. Biol Pharm Bull. 2010;33(8):1454–7. doi: 10.1248/bpb.33.1454. [DOI] [PubMed] [Google Scholar]
- 52.Saqib Z, Asgar A, Mohammed A, Abdul A. Transdermal drug delivery of labetalol hydrochloride: feasibility and effect of penetration enhancers. Asian J Pharm. 2010;2(4). [DOI] [PMC free article] [PubMed]
- 53.Rajan R, Vasudevan DT. Effect of permeation enhancers on the penetration mechanism of transfersomal gel of ketoconazole. J Adv Pharm Technol Res. 2012;3(2):112. doi: 10.4103/2231-4040.97286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahlstrom L, Cross S, Mills P. The effects of freezing skin on transdermal drug penetration kinetics. J Vet Pharmacol Ther. 2007;30(5):456–63. doi: 10.1111/j.1365-2885.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 55.Moreira TS, de Sousa VP, Pierre MBR. A novel transdermal delivery system for the anti-inflammatory lumiracoxib: influence of oleic acid on in vitro percutaneous absorption and in vivo potential cutaneous irritation. AAPS PharmSciTech. Springer; 2010;11(2):621–9. [DOI] [PMC free article] [PubMed]
- 56.Moreira TS, de Sousa VP, Pierre MBR. Influence of oleic acid on the rheology and in vitro release of lumiracoxib from poloxamer gels. J Pharm Pharm Sci. 2010;13(2):286–302. doi: 10.18433/j34880. [DOI] [PubMed] [Google Scholar]
- 57.Kim Y-C, Park J-H, Ludovice PJ, Prausnitz MR. Synergistic enhancement of skin permeability by N-lauroylsarcosine and ethanol. Int J Pharm. 2008;352(1):129–38. doi: 10.1016/j.ijpharm.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 58.Santos P, Watkinson A, Hadgraft J, Lane M. Formulation issues associated with transdermal fentanyl delivery. Int J Pharm. 2011;416(1):155–9. doi: 10.1016/j.ijpharm.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 59.Ta V, Chin WK, White AA. Allergic contact dermatitis to testosterone and estrogen in transdermal therapeutic systems. Dermatitis. 2014;25(5):279. doi: 10.1097/DER.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 60.Laing CJ, Cooke NE. Vitamin D-binding protein. In: David Feldman, J. Wesley Pike and Francis H. Glorieux, editor. Elsevier; 2005. p. 117–52.


