Abstract
The objective of this study was to develop novel docetaxel phospholipid nanoparticles (NDPNs) for intravenous administration. Modified solvent diffusion-evaporation method was adopted in the NDPN preparation. Central composite design (CCD) was employed in the optimization of the critical formulation factor (drug content) and process variable (stirring rate) to obtain NDPNs with 215.53 ± 1.9-nm particle size, 0.329 ± 0.02 polydispersity index (PDI), and 75.41 ± 4.81% entrapment efficiency. The morphological examination by transmission electron microscopy revealed spherical structure composed of a drug core stabilized within the phospholipid shell. Enhanced cell uptake of coumarin-6-loaded phospholipid nanoparticles by MCF-7 cell line indicated NDPN-efficient cell uptake. In vitro hemolysis test confirmed the safety of the phospholipid nanoparticles. NDPNs exhibited increased area under the curve (AUC) and mean residence time (MRT) by 3.0- and 3.3-fold, respectively, in comparison with the existing docetaxel parenteral formulation (Taxotere®), indicating a potential for sustained action. Thus, the novel NDPNs exhibit an ability to be an intravenous docetaxel formulation with enhanced uptake, decreased toxicity, and prolonged activity.
KEY WORDS: cancer, delivery vehicle, docetaxel phospholipid nanoparticles, nanoparticles, pharmacokinetics
INTRODUCTION
Cancer is one of the major causes of death in the world. According to World Health Organization (WHO), around 7.6 million people die due to cancer all over the world. Cancer accounts for 13% of the total death in the world, principally due to lung, breast, stomach, liver, and colon cancer. It continues to claim lives with about 13.1 million deaths in 2030. In 2013, breast, lung, bronchus, and colorectal cancers were the most prevalent cancer in women, accounting for 51% of the estimated cancer cases. Breast cancer alone accounts for 29% of cancer in women and is the leading cause of death from cancer [1, 2].
Docetaxel (DTX) is one of the most potent anticancer agents to treat a wide range of tumors, especially breast, ovarian, lung, and head and neck cancers. DTX is a taxane derivative, structurally similar to paclitaxel, but more potent as an inhibitor of microtubule depolymerization. Since DTX exhibits very low water solubility, its marketed formulation (Taxotere®, Sanofi Aventis, Dagenham, UK) for intravenous administration contains a high concentration of Polysorbate 80. The presence of Polysorbate 80 in Taxotere is reported to cause numerous adverse effects, including acute hypersensitivity reactions, fluid retention, and peripheral neuropathy [3–5]. Hence, several approaches have been investigated to eliminate the addition of the Polysorbate 80 and increase DTX solubility and efficacy. They include PLGA nanospheres, PEgylated nanoliposomes, liposomes, nanostructured lipid carriers (NLCs), chitosan nanoparticles, oral solid dispersion, conjugates, and β-cyclodextrin complex [6–14]. Although these systems exhibit specific advantages, limitations exemplified by low drug loading, drug leakage, and polymer toxicity necessitate the development of a safe and effective DTX intravenous formulation.
Phospholipids are endogenous amphiphilic biocompatible macromolecules having a neutral charge. They form complexes with hydrophilic and lipophilic drug molecules, and the resulting colloidal dispersion has been called phytosomes and pharmacosomes [15–17]. Abelcet®, the phospholipid complex of Amphotericin B, forms a nanoassembly when added to aqueous medium. Upon intravenous administration, they have been found to accumulate in tumor tissue, attributed to the enhanced permeability and retention (EPR) effect associated with the poorly developed tumor vasculature that allows the 200–600-nm particles to diffuse freely into the tumor microenvironment [18, 19].
In the present study, we developed novel docetaxel phospholipid nanoparticles (NDPNs) with improved pharmacokinetics which can be safely administered intravenously. NDPNs eliminate the need for surfactants in preparation of parenteral formulation. A combination of phospholipids, distearoyl phosphatidylcholine (DSPC), and distearoyl phosphatidylglycerol (DSPG) (1:1) was selected in NDPNs. We adopted a novel modified solvent diffusion-evaporation method in NDPN preparation. Central composite design was employed for optimization of critical formulation and process parameters, identified by initial experimentation, to obtain optimum particle size, polydispersity index, and entrapment efficiency. The nanoparticle structure was determined by transmission electron microscopy (TEM). A simple hemolysis test was adopted to compare the toxicity of NDPNs with Taxotere. In addition, we compared the pharmacokinetics of NDPN and Taxotere in Wistar rats.
MATERIALS AND METHODS
Materials
Docetaxel trihydrate and paclitaxel were generous gift samples from Mac-Chem Product Pvt. Ltd. (Thane, India). Parenteral grade phospholipids, namely, dimyristoyl phosphatidylcholine (DMPC), dimyristoyl phosphatidylglycerol sodium salt (DMPG-Na), distearoyl phosphatidylglycerol sodium salt (DSPG-Na), distearoyl phosphatidylethanolamine (DSPE), distearoyl phosphatidylcholine (DSPC), Lipoid S 75–3 (saturated phosphatidylcholine 70%), Lipoid E 80-S (phosphatidylcholine 70%), and Lipoid S PC-3 (phosphatidylcholine ≥98%), were obtained as gift samples from Lipoid (GmbH, Germany). Coumarin-6 was purchased from Sigma-Aldrich, Mumbai, India. Dulbecco’s modified Eagle’s medium (DMEM) (high glucose) was purchased from HiMedia, Mumbai, India. Fetal bovine serum (FBS) and penicillin-streptomycin solution were purchased from Invitrogen, NY, USA. All other chemicals used were of analytical grade.
HPLC Analysis of Docetaxel
A HPLC method was validated for DTX estimation in all the samples, including in solubility and release experiments. The HPLC system comprised of a Waters 2695 separation module equipped with a quaternary pump, an auto sampler unit, and a Waters 2996 photodiode array detector (PDA). Phenomenex-C18 (5 μm; 250 mm × 4.6 mm) analytical column was used for the estimation. The mobile phase consisted of acetonitrile and water in 65:35 (v/v) proportions. The flow rate was maintained at 1 ml/min and the PDA detector was set at 230 nm. The Empower HPLC software was employed for analysis of the results. The procedure was performed in triplicate, and the average and standard deviations were calculated.
Selection of Phospholipids
Several phospholipids were screened in order to select the optimum phospholipid on the basis of DTX solubility. Briefly, 1:2 molar ratios of DTX and phospholipid in distilled water were dispersed and agitated at 100 rpm for 24 h at 37°C [20]. The amount of dissolved DTX was quantified by HPLC.
Preparation of NDPN
Modified solvent diffusion-evaporation method was employed in the preparation of NDPNs. Weighed quantities of DTX and DSPC/DSPG (1:1) were separately dissolved in 5 and 10 ml methanol, respectively. Both the solutions were mixed at 40°C to obtain a clear solution and added dropwise in to 15 ml aqueous phase with stirring (RH basic 1 IKAMAG® heating plate, IKA, India) at 750 rpm. The solvent was evaporated (Rotavapor® R-210, Buchi, Switzerland) at 35°C under very low pressure (~50 mbar) to obtain NDPN dispersion. Free DTX in dispersion was separated by centrifugation at 8000 rpm for 10 min. The supernatant containing NDPNs was freeze-dried and stored at 2–8°C [21, 22].
The coumarin-6 phospholipid nanoparticles were prepared by the same method, as described above.
Design of Experiments (DoE)
In the screening trials, we evaluated the NDPN formulation and process parameters, including drug content, the ratio of combinations of phospholipids, volumes of the organic and aqueous phases, and stirring and evaporation rates. NDPN particle size, polydispersity index (PDI), and entrapment efficiency formed the responses; these are the critical quality attributes which affect the performance of the nanoparticle dispersion. They were significantly influenced by drug content (critical formulation parameter) and stirring rate (critical process parameter). Therefore, we selected drug content and stirring rate as the critical parameters for optimization by central composite design (CCD) (Design Expert 8.0.7.1) (Table I). The parameters were evaluated at three levels, high, middle, and low (+1, 0, and −1). Other parameters such as the volumes of organic and aqueous phases, evaporation rate, ratios of phospholipids, and amount of phospholipids were kept constant during the optimization study. A total of 13 random experimental trials were designed by the software with five center points, and four axial and four factorial points.
Table I.
Formulation and Process Factors for CCD Study
| Independent factor | Design level | ||
|---|---|---|---|
| Drug content (% w/w) | A | 35.0 | −1 |
| 52.5 | 0 | ||
| 70.0 | +1 | ||
| Stirring rate (rpm) | B | 750.0 | −1 |
| 1050 | 0 | ||
| 1350 | +1 |
Freeze Drying of NDPNs
NDPNs were freeze-dried with 5% w/v PEG-6000 as a cryoprotectant. NDPNs were rapidly frozen in a shell freezer and lyophilized by continuous freeze drying for 48 h. Temperature at −74°C and pressure of 0.045 to 0.055 TORR were maintained in the freeze dryer (Allied frost FD-3, India) during the operation, and the freeze-dried NDPNs were stored at 2–8°C.
Characterization of Nanoparticles
Particle Size, PDI, and Zeta Potential
The size and size distribution of NDPN particles were determined by dynamic light scattering (DLS) (Nano ZS, Malvern Instruments, UK), considering the average of ten measurements. The estimation of zeta potential was based on electrophoretic mobility in an electric field. The values are an average of 20 measurements.
Shape and Morphology
The shape and morphology of NDPNs were studied by transmission electron microscopy (TEM) (Technai F20, FEI, Netherlands). The sample was stained with 1.5% phosphotungstic acid and placed on 400-mesh carbon-coated grid and held at RT for 90 s. The sample was viewed at an accelerating voltage of 100–200 kV.
Entrapment Efficiency
An indirect method was adopted for estimating the percentage of drug encapsulated in NDPNs. Briefly, NDPN dispersion was centrifuged at 8000 rpm for 10 min to sediment the free drug. The supernatant, containing the nanoparticles, was subjected to ultracentrifugation at 40,000 rpm for 30 min. The entrapment efficiency was calculated by the following formula:
Differential Scanning Calorimetry (DSC)
DSC thermograms of the freeze-dried NDPNs, physical mixture, PEG-6000, and DTX were recorded (Mettler Toledo, DSC, 821e, Switzerland). The system was calibrated with indium before the study. The samples (2–8 mg) were weighed, placed in aluminum pans, and heated at a rate of 10°C/min in an atmosphere of nitrogen. The samples were heated over a temperature range of 25–200°C and analyzed with empty pan as a reference.
Powder X-Ray Diffraction (PXRD)
The X-ray diffraction pattern of freeze-dried NDPNs, physical mixture, PEG-6000, and DTX was acquired from X-ray diffractometer (Bruker D8 Advance, Bruker, Germany). Measurements were performed at a voltage of 40 kV and 25 mA. The scanned angle was set from 3° < 2θ > 40° and scanned at rate of 1 min−1.
In Vitro Release Kinetics
In vitro release of NDPNs was studied in 0.01 M phosphate-buffered saline (PBS, pH 7.4, 3% Tween 80). The release profile of NDPNs was compared with the marketed formulation (Taxotere). Briefly, 2 ml of the sample (~3 mg of DTX) was filled into a dialysis bag (MWCS 12 kDa). The bag was suspended in beaker containing PBS (20 ml) and agitated in a shaker water bath at 100 rpm at 37°C [23]. At regular intervals, samples were withdrawn (200 μl) and quantified by HPLC. To analyze the release kinetics and mechanism, the data were fitted to zero-order, first-order, Hixson Crowell, Weibull, Higuchi’s, Baker-Lonsdale, and Korsemeyer-Peppas release model:
Cell Culture Experiment
Cell Line
Human breast cancer cell line MCF-7 was purchased from National Center for Cell Sciences, Pune, India. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, NY, USA) containing 10% fetal bovine serum.
Cell Uptake Study
The cell uptake potential of the phospholipid nanoparticles was evaluated in MCF-7 human adenocarcinoma cells. The cells were cultivated in the eight-well cover glass chamber until 70% confluence. Coumarin-6-loaded phospholipid nanoparticles, at 0.250 mg/ml concentration in DMEM, were added to the wells [9] and incubated for 30 min. After which, the cells were rinsed three times with phosphate-buffered saline (PBS) and observed under a fluorescent microscope (Nikon Eclipse, Te2000-S).
Hemolysis Study
NDPNs and Taxotere were evaluated for toxicity at 50 μg/ml concentration. Freshly collected blood samples from Wistar rat were washed three times with PBS and centrifuged at 2800 rpm for 5 min. The erythrocyte suspension (100 μl) was added to the formulations (900 μl) and incubated for 60 min at 37°C with intermittent shaking. The samples were centrifuged at 3000 rpm for 60 min, and the hemoglobin released in the supernatant was quantified by UV–vis spectroscopy at 540 nm. Erythrocytes incubated with saline and distilled water served as negative (0%) and positive controls (100%), respectively, and the data is represented as the percentage of complete hemolysis. The experiments were run in triplicate. The percent hemolysis rate (% HR) was calculated from the following Eq. (5):
where At, Anc, and Apc are the absorbance of test samples, and negative and positive controls, respectively.
In Vivo Pharmacokinetic Study
Animals and Dosing
Female Wistar rats of 100–120 g were obtained from the central animal facility (CAF), NIPER, S.A.S. Nagar, India. The study protocol was approved by the Institutional Animal Ethics Committee (IAEC), National Institute of Pharmaceutical Education & Research (NIPER), S.A.S Nagar, India. The animals were maintained at 25 ± 2°C and 50–60% relative humidity (RH) under natural light/dark conditions for 1 week before the experiment.
Rats were randomly divided into two groups of six animals each. They were administered (dose of 4 mg/kg) Taxotere injection and NDPNs in normal saline intravenously [23]. Blood samples were collected from the retro-orbital plexus under mild anesthesia into microcentrifuge tubes containing heparin (40 IU/ml blood) at 5-, 15-, 30-, 60-, 240-, 480-, and 720-min time points. Plasma was separated by centrifuging the blood samples at 7000 rpm for 5 min and stored at −20°C prior to analysis.
Quantification of DTX in Plasma Samples
DTX was quantified by the HPLC (Waters 2695). Briefly, an aliquot (100 μl) of plasma was mixed with 10 μl of internal standard solution (paclitaxel 20 μg/ml) and 50 μl of drug solution. After vortexing for 45 s, chilled acetonitrile (150 μl) was added as protein precipitating agent and vortexed for 3 min. The mixture was centrifuged at 14,000 rpm for 10 min at 4°C. The supernatant was filtered (0.45 μm PVDF syringe filter, Millipore Millex-HV) and the filtrate (80 μl) was analyzed by HPLC system. Phenomenex-C18 (5 μm; 250 mm × 4.6 mm) analytical column at temperature of 40°C was employed for the estimation. The mobile phase consisted of acetonitrile and water (pH 4.0) in 48:52 (v/v) proportions, maintained at 1.5 ml/min flow rate. DTX was quantified at 230 nm using PDA detector (Waters 2996). Calibration curve was obtained from 50–2000 ng/ml with r2 value of 0.993.
Statistical Analysis
The results of in vivo experiments are reported as mean ± SD (n = 6). The difference between the groups was tested by paired t test using GraphPad Prism 5.0 software. The difference was considered to be statistically significant when P < 0.05.
RESULTS AND DISCUSSION
Selection of Phospholipids
The aim of the solubility study was to select the phospholipid in which DTX exhibited minimum solubility (Fig. 1). Although, in Lipoid S 75–3, DSPG-Na, DSPE, DSPC, and Lipoid S PC-3, DTX exhibited minimum solubility, we selected combination of DSPC and DSPG-Na (1:1) in NDPNs. DTX formed a stable dispersion with DSPC and DSPG-Na. The dispersion retained its size and integrity, which could be due to the negative charge on DSPG-Na, which stabilizes NDPNs and minimizes its aggregation.
Fig. 1.

Solubility of DTX in presence of different phospholipids
Mechanistic Understanding of Particle Formation by Solvent Diffusion-Evaporation Method
In the marketed DTX formulation (Taxotere), Polysorbate 80 and ethanol (50:50 v/v) added as co-solvents cause hypersensitivity reaction and fluid retention [24]. Hence, in order to avoid these adverse reactions, NDPNs were formulated by modified solvent diffusion-evaporation method. The nanodispersion is formed by a difference in the surface tension of the organic and aqueous phases. The force exerted by the liquid exhibiting high surface tension (water) on the surrounding liquid is stronger than the force exerted by methanol, which exhibits lesser surface tension. This difference results in turbulence and thermal inequalities in the system, leading to the continuous formation of eddies of methanol at the liquid interface, which generates interfacial convective flows. Consequently, violent spreading is observed due to miscibility of methanol and water, which again break down into smaller droplets and so on until it forms submicron droplets. The solvent present in the submicron droplets flow away because of its low surface tension and precipitate submicron phospholipid nanoparticles [25].
Optimization of Nanoparticles
In order to optimize the NDPN formulation and its process, the relation between the components of the formulation and process variables were studied and optimized by adopting the QbD principles. The DoE was adopted to optimize the critical formulation and process factors based on their effect on critical quality attributes (CQAs), those characteristics that affect the performance of NDPNs [26]. The CQAs of NDPNs include particle size, polydispersity index (PDI), and entrapment efficiency.
Effect of Independent Factors on Particle Size (y1), PDI (y2), and Entrapment Efficiency (y3)
Thirteen experiments were conducted to optimize the drug content and stirring rate on NDPN particle size (y1), PDI (y2), and entrapment efficiency (y3). From the study, particle size ranged from 170 to 508 nm, PDI from 0.270 to 0.534, and entrapment efficiency from 7.06 to 62.68%. Table II presents the data of CCD experimental runs.
Table II.
Results of CCD Experiments
| Run | Drug content (% w/w) |
Stirring rate (rpm) |
Particle size (y1) (nm) |
PDI (y2) | Entrapment efficiency (%) (y3) |
|---|---|---|---|---|---|
| 1 | 52.5 | 1050 | 243.3 | 0.270 | 16.51 |
| 2 | 27.7 | 1050 | 419.5 | 0.448 | 7.060 |
| 3 | 52.5 | 1050 | 338.0 | 0.405 | 56.41 |
| 4 | 52.5 | 625 | 209.1 | 0.279 | 54.68 |
| 5 | 35.0 | 750 | 239.8 | 0.420 | 41.75 |
| 6 | 52.5 | 1050 | 253.2 | 0.348 | 44.38 |
| 7 | 35.0 | 1350 | 170.4 | 0.435 | 21.28 |
| 8 | 52.5 | 1050 | 246.2 | 0.468 | 47.38 |
| 9 | 77.2 | 1050 | 291.1 | 0.534 | 62.38 |
| 10 | 52.5 | 1050 | 342.8 | 0.370 | 49.30 |
| 11 | 70.0 | 750 | 270.0 | 0.328 | 56.87 |
| 12 | 70.0 | 1350 | 515.0 | 0.528 | 13.30 |
| 13 | 52.5 | 1474 | 333.2 | 0.447 | 45.73 |
ANOVA was applied to determine the significance and the magnitude of the effects of the main variables and interaction between variables. The results (Table III) confirm the adequacy of the models (P value <0.05). It identified the significant factors that affect the responses y1, y2, and y3 of NDPNs. In particle size and PDI, the drug content (A) and stirring rate (B) were not significant; however, their interaction was significant (AB, AB2, and A2). In entrapment efficiency, stirring rate was the significant factor.
Table III.
ANOVA for Particle Size (y1), PDI (y2), and Entrapment Efficiency (y2)
| Source | Sum of squares | Degree of freedom | F value | Prob > F P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| y1 | y2 | y3 | y1 | y2 | y3 | y1 | y2 | y3 | y1 | y2 | y3 | |
| Model | 83705.0 | 0.051 | 2173.85 | 5 | 3 | 2 | 5.67 | 4.29 | 5.00 | 0.028 | 0.04 | 0.034 |
| A-Drug content (% w/w) | 08239.4 | 0.001 | 911.090 | 1 | 1 | 1 | 2.79 | 0.47 | 4.19 | 0.145 | 0.51 | 0.070 |
| B-Stirring rate | 10799.8 | 0.018 | 1262.75 | 1 | 1 | 1 | 3.66 | 4.68 | 5.81 | 0.104 | 0.06 | 0.039 |
| A2 | – | 0.023 | – | – | 1 | – | – | 5.86 | – | – | 0.04 | – |
| AB | 24716.5 | – | – | 1 | – | – | 8.37 | – | – | 0.027 | – | – |
| B2 | 0538.54 | – | – | 1 | – | – | 0.18 | – | – | 0.684 | – | – |
| AB2 | 38677.1 | – | – | 1 | – | – | 13.1 | – | – | 0.011 | – | – |
| Residual | 0.031 | 2.029 | 1953.44 | 6 | 8 | 9 | – | – | – | – | – | – |
| Lack of fit | 0.010 | 0.033 | 1011.32 | 2 | 4 | 5 | 1.40 | 0.49 | 0.85 | 0.345 | 0.74 | 0.570 |
| Pure error | 0.021 | 0.025 | 942.110 | 4 | 4 | 4 | – | – | – | – | – | – |
| Cor total | 0.083 | 0.001 | 4127.29 | 11 | 11 | 11 | – | – | – | – | – | – |
Underlined entries are significant model terms with P value <0.05
The final mathematical models obtained from the design are as follows:
| 2 |
| 3 |
The positive sign indicates a synergistic effect, while the negative sign indicates an antagonistic effect.
Equation 1 illustrates that particle size decreases with an increase in drug content. However, the size increases with an increase in stirring rate. The interaction of drug content and stirring rate increases the particle size. A similar response was obtained with interaction of drug content with B2.
Equation 2 illustrates that both drug content and stirring rate increased PDI. Similar response was observed with A2.
Equation 3 illustrates that drug content increases the entrapment efficiency while stirring rate caused a reduction.
The 3D surface plots further explain the relationship between the variables and responses. Figure 2a presents the effect of stirring rate and drug content on particle size. The optimum particle size was obtained at decreased stirring rate and drug content. Figure 2b presents the effect of drug content and stirring rate on PDI. Minimum PDI was obtained at decreased stirring rate and drug content of 51.63% w/w. Similarly, optimum entrapment efficiency at decreased stirring rate and at maximum drug content was observed (Fig. 2c).
Fig. 2.
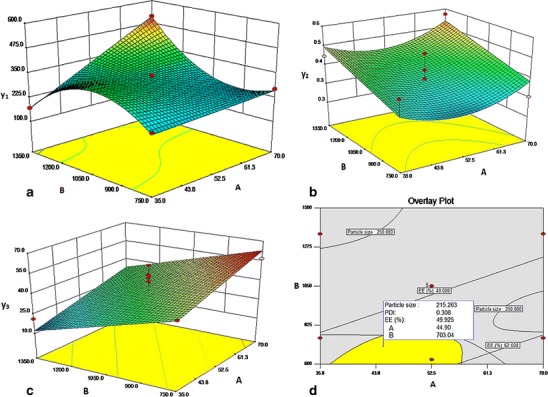
Effect of drug content (a) and stirring rate (b) on particle size (a), PDI (b), and entrapment efficiency (c). Overlay plot for optimized parameters of NDPN (d)
The stirring rate at 750 rpm resulted in the optimum size, PDI, and entrapment efficiency in NDPNs. At lower stirring rates, the collision between the solvent droplets is less, providing enough time to disperse into submicron-sized particles. At higher stirring rates, although the particle size decreases, there is increase in PDI, probably due to breakage of the nanoparticles being formed due to attrition leading to smaller particles but with increased PDI (Fig. 2b). Drug content at 51.63% w/w resulted in optimum size, PDI, and entrapment efficiency in NDPNs. When drug content is 51.63% w/w, there is sufficient phospholipid to envelope the drug molecule. Further increase in drug content increases the percentage of drug entrapment which reaches an optimum (Fig. 2c).
The optimum conditions in NDPN formulation predicted by the model are drug content of 51.63% and stirring rate of 750 rpm to obtain particle size from 170 to 275 nm, PDI from 0.27 to 0.35, and entrapment efficiency within 40 to 62%. NDPNs exhibited optimized zeta potential of −37.05 ± 0.77 mV. Experimental batches prepared with the optimum values yield results within the predicted limits, thus confirming the reliability of the optimization process. Figure 2d is an overlay plot with the design space (yellow area) for obtaining the nanoparticles.
Freeze Drying of NDPNs
NDPNs were freeze-dried with PEG-6000 (5% w/v) as a cryoprotectant to form to an intact fluffy cake which easily re-dispersed in water upon shaking with no significant (P > 0.05) changes in particle size ((217.60 ± 3.66 nm before and 295.96 ± 57.53 nm after freeze drying).
Characterization of Nanoparticles
Shape and Morphology
TEM image of NDPNs presents a spherical structure with a transparent layer of phospholipids around drug particles (Fig. 3). The polar groups of the phospholipid molecules are oriented toward the surface, while the non-polar tails face the core in which the drug appears to be present. A good correlation was obtained in the values of particle size obtained by Zetasizer and TEM.
Fig. 3.
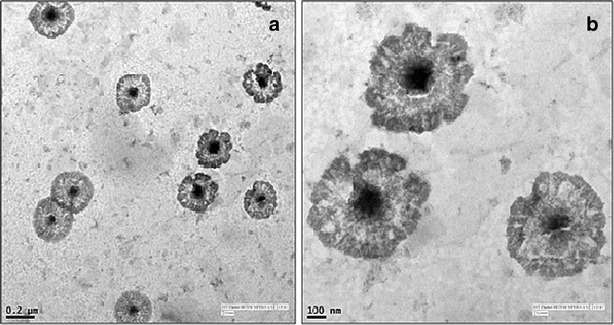
TEM image of NDPNs at a ×5000 and b ×19,000
DSC Analysis
DSC study was undertaken to determine the physical state of drug in the nanoparticles. The physical mixture exhibits endothermic peaks at 61.84°C (movement of phospholipid head group), 82.14°C (loss of water), 112.89°C (melting of DSPC), 124.82°C (melting of DSPG), and 169.24°C (melting of DTX). PEG-6000 exhibits a peak at 63.47°C (Fig. 4). In NDPNs, DTX endothermic peak at 169.24°C was absent, indicating possible entrapment of DTX within the phospholipid nanoparticle.
Fig. 4.
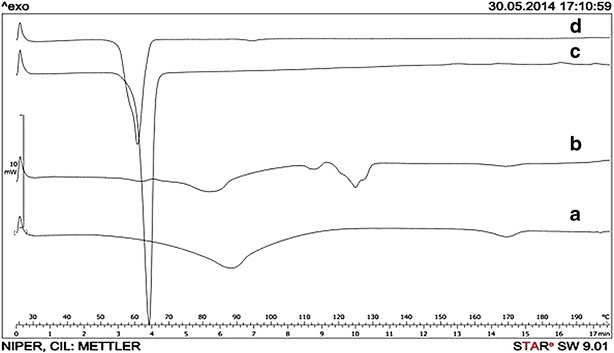
DSC thermograms of DTX (a), physical mixture (b), PEG-6000 (c), and freeze-dried NDPNs (d)
PXRD Analysis
X-ray diffraction (XRD) study was undertaken to determine the effect of the nanoparticle on the crystallinity of DTX. Figure 5 presents the XRD data of freeze-dried NDPNs, physical mixture, PEG-6000, and DTX. DTX exhibits characteristic XRD patterns, which were absent in NDPNs. This pattern could be due to DTX entrapment in the core of the phospholipid nanoparticles. Our finding was supported by the DSC analysis where the drug’s endotherm peak has disappeared.
Fig. 5.

X-ray diffraction pattern of DTX (a), physical mixture (b), PEG-6000 (c), and freeze-dried NDPNs (d)
Release Kinetics
Figure 6 presents the drug release profiles from NDPNs and Taxotere. NDPNs exhibited a biphasic release profile with 26.43% of drug released in 24 h followed by prolonged release of up to 3 days (78.35%), while Taxotere released almost 80% of drug within 20 h. The release data was fitted into different release models. Weibull model was best suited (r2 = 0.9972) for the release of DTX from NDPNs. This is an empirical model which has been widely employed to study the release data of both rapid and extended release drug delivery systems. It is applicable for any drug release mechanism involving dissolution, diffusion, and mixed dissolution-diffusion rate limited process [27]. The slope of model equation (shape factor) is an indicator of the mechanism of transport of drug through the delivery system. The estimated value of shape factor ≤0.75 implies Fickian diffusion while value in the range of 0.75–1 indicates a combined mechanism of Fickian diffusion and swelling control release [28]. From NDPNs, DTX exhibited a shape factor value of 0.8 indicating a combined mechanism of release. Sustained release of DTX from NDPNs would lead to enhanced plasma concentration and reduced biodistribution in comparison to Taxotere, which in turn may reduce the harmful effects of DTX to normal tissues.
Fig. 6.

Release profile of NDPNs
Cell Uptake Study
The cell uptake potential of NDPNs was studied in human adenocarcinoma (MCF-7) cells by fluorescence microscopy. Since DTX does not exhibit fluorescence, NDPNs of coumarin-6, a fluorescent hydrophobic dye with poor aqueous solubility (0.25 μg/ml), were employed. The dye has been commonly employed as a model for studying cell uptake and internalization potential of nanoparticles [29, 30]. The MCF-7 cells were selected for the uptake study considering that the ultimate goal of NDPN formulation was in optimization of breast cancer treatment. Figure 7 presents the internalization of the coumarin-6 phospholipid nanoparticles in the MCF-7 cells. There was an enhanced uptake of the nanoparticles, indicated visually by the intense fluorescence observed within the cells, confirming the potential of NDPN formulation for increased uptake by cancer cells.
Fig. 7.

Cell uptake study in MCF-7 cells. a Transmitted light channel and b FITC channel
Hemolysis Study
This study was undertaken to evaluate the hemolytic activities of Taxotere and NDPNs. Surfactants have been reported to interact with the lipid bilayer of red blood cells and cause hemolysis [31]. NDPNs exhibited significantly less (P < 0.05) hemolytic activity (50 μg/ml DTX) in comparison to Taxotere (Fig. 8). Polysorbate 80 present in the commercial formulation as solubilizing agent causes severe hemolysis. The biocompatibility of NDPNs can be attributed to phospholipid, which is a component of biological membranes. Hence, NDPNs are a novel formulation for safe intravenous administration of docetaxel.
Fig. 8.
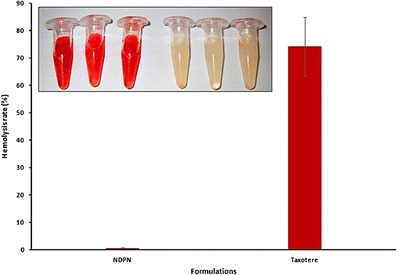
In vitro hemolysis of NDPNs and Taxotere. Insert images (from left): the first three tubes are Taxotere and last three tubes are NDPNs (n = 3)
Pharmacokinetic Study
The comparative effect of NDPNs and Taxotere on the DTX pharmacokinetic parameters was evaluated. Figure 9 presents the plasma concentration-time profiles after intravenous administration of the two formulations. NDPNs exhibited enhanced DTX plasma concentrations with enhancement in the mean residence time (MRT) (11.85 ± 3.89 vs. 3.58 ± 0.20 h), area under the curve (AUC0-12h), and prolonged T1/2 (10.42 ± 4.35 vs. 5.57 ± 0.86 h) in comparison to Taxotere (Table IV). NDPNs exhibited considerably reduced clearance (2.21 ± 0.36 vs. 6.73 ± 0.81 l/h/kg) in comparison to Taxotere. NDPNs were detectable even after 12 h while Taxotere concentration was undetectable after 4 h in the blood plasma. This enhancement in plasma DTX concentration for an extended period of time can be attributed to the reduction in clearance owing to the presence of phospholipid, which probably retards the elimination rate of the drug. Thus, NDPNs aid in safe intravenous delivery of DTX with prolonged circulating time, and enhanced cell uptake has the potential for increased efficacy.
Fig. 9.
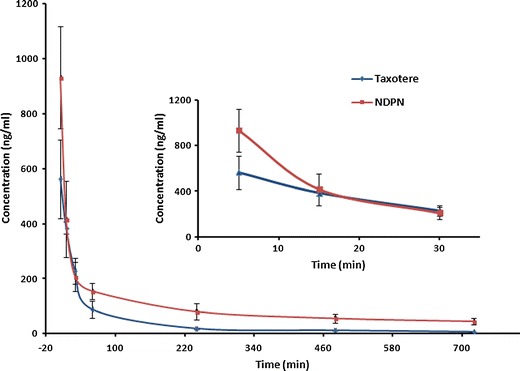
Plasma concentrations of DTX after intravenous administration of Taxotere and NDPNs to rats (4 mg/kg) (n = 6). Insert figure is the detail view of mean DTX plasma concentration-time profiles from above two formulations over period from 5 to 30 min
Table IV.
Pharmacokinetic Parameters of DTX After Intravenous Administration of Taxotere and NDPNs to Rats (4 mg/kg)
| Parameters | Taxotere | NDPN |
|---|---|---|
| Cmax (ng/ml) | 562.86 ± 143.19 | 932.20 ± 186.05* |
| Cl (l/h/kg) | 6.73 ± 0.81 | 2.21 ± 0.36* |
| T1/2(h) | 5.57 ± 0.86 | 10.42 ± 4.35* |
| MRT(h) | 3.58 ± 0.20 | 11.8 ± 3.89* |
| AUC0-12h (ng/ml·h) | 598.48 ± 72.34 | 1806.29 ± 282.94* |
Data are represented as mean value ± SD, n = 6
C max maximum plasma concentration, Cl clearance, T 1/2 biological half life, MRT mean residence time, AUC 0-12h area under the curve
*P < 0.05, significant difference from Taxotere
CONCLUSIONS
In the present study, we developed novel docetaxel phospholipid nanoparticles. Docetaxel is lipophilic molecule necessitating the need of solubilizing agents in the development of parenteral formulation. The phospholipid nanoparticles proved to be suitable for intravenous administration with added advantages of safety, cell uptake with a potential for prolonged duration of action. The oral bioavailability enhancement of drug phospholipid complex has been widely reported, including studies from our laboratory; however, the viability of phospholipid nanoparticles for intravenous administration with reduced toxicity and enhanced bioavailability indicates its potential for effective parenteral delivery, as well.
Acknowledgments
The authors wish to express their gratitude to the Mr. Ashok K. Datusalia, Piyush Dave, Prashant Gupta (Department of Pharmacology and Toxicology), and Charan Singh (Department of Pharmaceutical Technology) for their valuable support in conducting the animal studies. We thank Lipoid (GmbH, Germany) for providing gift samples of phospholipids.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Mettlin C. Global breast cancer mortality statistics. CA Cancer J Clin. 1999;49(3):138–144. doi: 10.3322/canjclin.49.3.138. [DOI] [PubMed] [Google Scholar]
- 3.Norris LB, Qureshi ZP, Bookstaver PB, Raisch DW, Sartor O, Chen H, et al. Polysorbate 80 hypersensitivity reactions: a renewed call to action. J Community Oncol. 2010;7(9):425–428. doi: 10.1016/S1548-5315(11)70418-1. [DOI] [Google Scholar]
- 4.Lee SW, Yun MH, Jeong SW, In CH, Kim JY, Seo MH, et al. Development of docetaxel-loaded intravenous formulation, nanoxel-pm using polymer-based delivery system. J Control Release. 2011;155(2):262–271. doi: 10.1016/j.jconrel.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Upadhyay KK, Bhatt AN, Castro E, Mishra AK, Chuttani K, Dwarakanath BS, et al. In vitro and in vivo evaluation of docetaxel loaded biodegradable polymersomes. Macromol Biosci. 2010;10(5):503–512. doi: 10.1002/mabi.201090008. [DOI] [PubMed] [Google Scholar]
- 6.Chen ZK, Cai MX, Yang J, Lin LW, Xue ES, Huang J, et al. Chemotherapy with PLGA microspheres containing docetaxel decreases angiogenesis in human hepatoma xenograft. Med Oncol. 2012;29(1):62–69. doi: 10.1007/s12032-010-9762-2. [DOI] [PubMed] [Google Scholar]
- 7.Esmaeili F, Dinarvand R, Ghahremani MH, Amini M, Rouhani H, Sepehri N, et al. Docetaxel-albumin conjugates: preparation, in vitro evaluation and biodistribution studies. J Pharm Sci. 2009;98(8):2718–2730. doi: 10.1002/jps.21599. [DOI] [PubMed] [Google Scholar]
- 8.Hwang HY, Kim IS, Kwon IC, Kim YH. Tumor targetability and antitumor effect of docetaxel-loaded hydrophobically modified glycol chitosan nanoparticles. J Control Release. 2008;128(1):23–31. doi: 10.1016/j.jconrel.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Li K, Pan J, Liu B, Feng SS. Folic acid conjugated nanoparticles of mixed lipid monolayer shell and biodegradable polymer core for targeted delivery of docetaxel. Biomaterials. 2010;31(2):330–338. doi: 10.1016/j.biomaterials.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 10.Loxley A. Solid lipid nanoparticles for the delivery of pharmaceutical actives. Drug Deliv Technol 2009;9(8).
- 11.Musumeci T, Ventura CA, Giannone I, Ruozi B, Montenegro L, Pignatello R, et al. PLA/PLGA nanoparticles for sustained release of docetaxel. Int J Pharm. 2006;325(1–2):172–179. doi: 10.1016/j.ijpharm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 12.Qiu Y, Gao Y, Hu K, Li F. Enhancement of skin permeation of docetaxel: a novel approach combining microneedle and elastic liposomes. J Control Release. 2008;129(2):144–150. doi: 10.1016/j.jconrel.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 13.Yousefi A, Esmaeili F, Rahimian S, Atyabi F, Dinarvand R. Preparation and in vitro evaluation of a pegylated nano-liposomal formulation containing docetaxel. Sci Pharm. 2009;77:453–464. doi: 10.3797/scipharm.0806-08. [DOI] [Google Scholar]
- 14.Zhai G, Wu J, Xiang G, Mao W, Yu B, Li H, et al. Preparation, characterization and pharmacokinetics of folate receptor-targeted liposomes for docetaxel delivery. J Nanosci Nanotechnol. 2009;9(3):2155–2161. doi: 10.1166/jnn.2009.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavan KB, Subhashis D, Niranjan BM. Pharmacosomes as a novel vesicular drug delivery system. Int J Noval Trends Pharml Sci. 2013;3(3):46–52. [Google Scholar]
- 16.Semalty A, Semalty M, Rawat BS, Singh D, Rawat MS. Pharmacosomes: the lipid-based new drug delivery system. Expert Opin Drug Deliv. 2009;6(6):599–612. doi: 10.1517/17425240902967607. [DOI] [PubMed] [Google Scholar]
- 17.Singh C, Bhatt TD, Gill MS, Suresh S. Novel rifampicin-phospholipid complex for tubercular therapy: synthesis, physicochemical characterization and in-vivo evaluation. Int J Pharm. 2014;460(1–2):220–227. doi: 10.1016/j.ijpharm.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 18.Fang J, Nakamura H, Maeda H. The epr effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11(17–18):812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Singh G, Pai RS. Optimized self-nanoemulsifying drug delivery system of atazanavir with enhanced oral bioavailability: in vitro/in vivo characterization. Expert Opin Drug Deliv. 2014;11(7):1023–1032. doi: 10.1517/17425247.2014.913566. [DOI] [PubMed] [Google Scholar]
- 21.Larabi M, Gulik A, Dedieu JP, Legrand P, Barratt G, Cheron M. New lipid formulation of amphotericin b: spectral and microscopic analysis. Biochim Biophys Acta. 2004;1664(2):172–181. doi: 10.1016/j.bbamem.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Larabi M, Yardley V, Loiseau PM, Appel M, Legrand P, Gulik A, et al. Toxicity and antileishmanial activity of a new stable lipid suspension of amphotericin b. Antimicrob Agents Chemother. 2003;47(12):3774–3779. doi: 10.1128/AAC.47.12.3774-3779.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Wang D, Zhang J, Pan W. Preparation and pharmacokinetics of docetaxel based on nanostructured lipid carriers. J Pharm Pharmacol. 2009;61(11):1485–1492. doi: 10.1211/jpp.61.11.0007. [DOI] [PubMed] [Google Scholar]
- 24.Huang XX, Zhou CL, Wang H, Chen C, Yu SQ, Xu Q, et al. Pharmacokinetics, efficacy, and safety evaluation of docetaxel/hydroxypropyl-sulfobutyl-beta-cyclodextrin inclusion complex. AAPS Pharmscitech. 2011;12(2):665–672. doi: 10.1208/s12249-011-9631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mora-Huertas CE, Fessi H, Elaissari A. Influence of process and formulation parameters on the formation of submicron particles by solvent displacement and emulsification-diffusion methods critical comparison. Adv Colloid Interf Sci. 2011;163(2):90–122. doi: 10.1016/j.cis.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Singare DS, Marella S, Gowthamrajan K, Kulkarni GT, Vooturi R, Rao PS. Optimization of formulation and process variable of nanosuspension: an industrial perspective. Int J Pharm. 2010;402(1–2):213–220. doi: 10.1016/j.ijpharm.2010.09.041. [DOI] [PubMed] [Google Scholar]
- 27.Papadopoulou V, Kosmidis K, Vlachou M, Macheras P. On the use of the weibull function for the discernment of drug release mechanisms. Int J Pharm. 2006;309(1–2):44–50. doi: 10.1016/j.ijpharm.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 28.Adibkia K, Siahi Shadbad MR, Nokhodchi A, Javadzedeh A, Barzegar-Jalali M, Barar J, et al. Piroxicam nanoparticles for ocular delivery: physicochemical characterization and implementation in endotoxin-induced uveitis. J Drug Target. 2007;15(6):407–416. doi: 10.1080/10611860701453125. [DOI] [PubMed] [Google Scholar]
- 29.Rivolta I, Panariti A, Lettiero B, Sesana S, Gasco P, Gasco MR, et al. Cellular uptake of coumarin-6 as a model drug loaded In solid lipid nanoparticles. J Physiol Pharmacol. 2011;62(1):45–53. [PubMed] [Google Scholar]
- 30.Gao H, Yang Z, Zhang S, Cao S, Shen S, Pang Z, et al. Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Sci Rep. 2013;3:1–8. doi: 10.1038/srep02534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang G, Tang B, Liu Z, Gou J, Zhang Y, Xu H, et al. Novel hydrophobin-coated docetaxel nanoparticles for intravenous delivery: in vitro characteristics and in vivo performance. Eur J Pharm Sci. 2014;60:1–9. doi: 10.1016/j.ejps.2014.04.016. [DOI] [PubMed] [Google Scholar]


