Abstract
In this study, the use of trimethylchitosan (TMC), by higher solubility in comparison with chitosan, in alginate/chitosan nanoparticles containing cationic β-cyclodextrin polymers (CPβCDs) has been studied, with the aim of increasing insulin uptake by nanoparticles. Firstly, TMCs were synthesized by iodomethane, and CPβCDs were synthesized within a one-step polycondensation reaction using choline chloride (CC) and epichlorohydrine (EP). Insulin–CβCDPs complex was prepared by mixing 1:1 portion of insulin and CPβCDs solutions. Then, nanoparticles prepared in a three-step procedure based on the iono-tropic pregelation method. Nanoparticles screened using experimental design and Placket Burman methodology to obtain minimum size and polydispercity index (pdI) and the highest entrapment efficiency (EE). CPβCDs and TMC solution concentration and pH and alginate and calcium chloride solution concentrations are found as the significant parameters on size, PdI, and EE. The nanoparticles with proper physicochemical properties were obtained; the size, PdI, and EE% of optimized nanoparticles were reported as 150.82 ± 21 nm, 0.362 ± 0.036, and 93.2% ± 4.1, respectively. The cumulative insulin release in intestinal condition achieved was 50.2% during 6 h. By SEM imaging, separate, spherical, and nonaggregated nanoparticles were found. In the cytotoxicity studies on Caco-2 cell culture, no significant cytotoxicity was observed in 5 h of incubation, but after 24 h of incubation, viability was decreased to 50% in 0.5 mμ of TMC concentration. Permeability studies across Caco-2 cells had been carried out, and permeability achieved in 240 min was 8.41 ± 0.39%, which shows noticeable increase in comparison with chitosan nanoparticles. Thus, according to the results, the optimized nanoparticles can be used as a new insulin oral delivery system.
KEY WORDS: alginate, cationic β-cyclodextrin, insulin nanoparticle, oral delivery, trimethyl chitosan
INTRODUCTION
Insulin has a low bioavailability by oral administration, due to its instability against gastric pH, gastrointestinal enzymes, and its low permeability across the intestinal epithelium (1–4).
The oral route is the most convenient route of administration and improves patient compliance, but the absorption of proteins via oral administration depends on several factors such as decreased digestion in the gastrointestinal (GI) tract and increased intercellular and paracellular penetration. Therefore, different types of drug carriers have been employed to protect insulin from the harsh environment of the gastrointestinal tract and deliver it to the target side in the body (5,6).
Nanoparticles containing insulin, which are categorized as the colloidal dispersions composed of biodegradable and biocompatible polymers, have been extensively studied for novel drug delivery system. Chitosan, due to its mucoadhesive properties and its ability to open tight junctions, is among the popular ones (7–11).
Although chitosan nanoparticles have proper physico-chemical properties, these polymeric nanoparticles have several problems such as instability against acidic pH (12–16). The alginate/chitosan polyelectrolyte complex is considered one of the nanoparticulate drug delivery systems in which chitosan can increase paracellular permeability due to its mucoadhesivness and binding to epithelia as well as its ability to opening tight junctions (17–19). Alginate can form a reversible gel by cross-linking with multivalent cations such as Ca2+. This gel matrix aggregates in the acidic pH and preserves the drug molecule in this condition (20). Therefore, the chitosan/alginate complex can improve insulin absorption across the intestinal mucosa and efficiently protect it from the harsh environment of the stomach (2,21–27).
Chitosan is insoluble in neutral and basic pH, while trimethyl chitosan (TMC), the most studied quaternized derivative of chitosan, is soluble up to a pH value of 9.0 due to its permanent cationic charge. The increased solubility of TMC is considered as an advantage compared to nonmodified chitosan. TMC also has a well-known structure and simple method of preparation (28). In recent studies, the transepithelial resistance of Caco-2 cells in the presence of TMC was determined, which shows a reversible decrease indicating a reversible opening of the tight junctions (29,30). Therefore, in this study, TMC was used instead of parent chitosan to increase the nanoparticles’ penetration into entrocytes.
As cyclodextrins (CDs) are able to increase absorption by modifying the mucosal membrane fluidity and can also protect insulin against denaturation, thermal denaturation, and degredation, significant effort has been made to entrap insulin and the cyclodextrin complex into the chitosan and alginate hydrogel matrix (27). CDs are known as cytotoxic elements because of their binding to membrane cholesterol and proceeding by hemolysis, but they are safe in oral administration because of their poor absorption from the GI tract (28,31–33).
β-Cyclodextrins are one of those cyclodextrin derivatives that pose better water solubility than parent CD. In the interaction of protein molecules with CD, hydrophobic side chains penetrate into the CD cavity and make a noncovalent complex that aims to ensure protein stability (34). CDs modified by the quaternary ammonium group cannot bind to cholesterol and consequently are hemocompatible. These ionic CDs have better complexation potential and better solubility. CD polymers also pose lesser damage to the mucosal membrane (34,35). To combine all of the aforementioned advantages, the cationic polymers of βCD were prepared by applying choline chloride (Cc) to provide the quaternary ammonium group and epichlorohydrine (Ep) to form polymeric chains (25,36).
The aim of the present study is to prepare a novel nanoparticulate drug delivery system consisting of TMC, alginate, and cationic β-cyclodextrin (CPβCD) for the oral delivery of insulin.
METHODS AND MATERIALS
Materials
β-Cyclodextrin, epichlorohydrine, and choline chloride purchased from Sigma-Aldrich (Dorset, UK). Low molecular weight Chitoclear® chitosan [viscosity 1% (w/v) solution in acetic acid, 22 mPa S] was provided by Primex (Siglufjordur, Iceland). Sodium alginate was provided by Sigma-Aldrich (Dorset, UK). N-Methylpyrolidone, sodium iodide, iodomethane, phosphoric acid, sodium hydroxide, and calcium chloride was purchased from Merck (Darmstadt, Germany), and human insulin was provided by Exir Pharmaceutical (Lorestan, Iran).
All other chemicals were of pharmaceutical grade and used as received.
Synthesis of Trimethyl Chitosan
For the preparation of trimethyl chitosan, 4 g chitosan was dissolved in acetic acid 1% (v/v) initially. Sodium iodide (2 g) and 50 ml sodium hydroxide (NaOH, 1 M) were added to the mixture. Then, 1.5 ml of methyl iodide (CH3I) was added to the solution under reflux condition at 50°C, and the procedure was repeated three times. The product was then precipitated by adding adequate quantities of acetone.
The precipitates were dissolved in deionized water and dialysized against distilled water using a dialyzing tube with molecular cut-off of 12,000 Da for 3 days. Then, the purified polymer was precipitated using NaOH (5 N). Finally, the precipitation was dried completely using a vacuum oven (37–39).
Synthesis of Cationic β-Cyclodextrin Polymers
Cationic polymers of βCD have been synthesized according to Zhang et al. (27), with minor modifications, in a one-step polycondensation by the portion of 1:15:4 of βCD/Ep/Cc. Briefly, β-CD was completely dissolved in NaOH (0.6 N) in a water bath on the magnetic stirrer at 25°C for 24 h. Subsequently, choline chloride (2.7 g) was added spontaneously, and 6.9 g of epichlorohydrine was added dropwise at a rate of 0.1 ml/min. The temperature of the solution increased up to 60°C and was maintained for 2 h. Finally, the solution was neutralized by a hydrocholoric acid solution (3 N). Following purification by a dialyzing tube with a molecular cut-off of 1000 Da in 24 h, the solution was frozen and lyophilized into a fine cake (27,36).
Synthesis and Preparation of Nanoparticles
In this study, insulin nanoparticles were prepared in a three-step modified ionotropic pregelation method. First, the insulin–βCD complex was prepared in a 1:1 volumetric ratio in which 3 ml of βCD solution in different concentrations was added to equal volumes of insulin solution (0.5 mg/ml, pH = 6.3 ± 0.1) and stirred for 1 h. After that, sodium alginate solution (10 ml) in different concentrations, which was previously stirred for 12–24 h to dissolve, was added to the complex solution and stirred for 10 min. The pH of alginate solution was kept constant at 4.9 in all experiments. Then, the CaCl2 solution with variable concentrations (1.5 ml) was added dropwise for 15 min, while the stirring rate was set at 300 rpm. Finally, the TMC solution (2 ml) in different concentrations and various pH values was added and stirred at 700 rpm for 30 min (27,40). The prepared opalsant nanosuspension was centrifuged at 12,000 rpm for 20 min to settle down the nanoparticles. The transparent aqueous solution in supernatant was kept for insulin determination, and the settled down nanoparticles were resuspended in the double-distilled water that was previously filtered through 0.44-μm filters. Sucrose (5% w/v) was added to the nanocolloid as the lyoprotectant. The nanocolloid was then frozen overnight at −20°C and freeze-dried using a Christ® freeze drier (Martin Christ GmbH, Osterode am Harz, Germany). The nanoparticles were freeze-dried for 48 h, at 0.07 mbar working pressure and −50°C condenser temperature.
Characterization of Nanoparticles
Determination of Size and PdI of Nanoparticles
Photon correlation spectroscopy (PCS) was used for measuring size of the nanoparticles. PCS measures the conversion of intensity of scattering light caused by the Brawnian behavior of particles (41). For the determination of size and PdI of the particles, the measuring sample is diluted in 1:5 ratio by previously filtered deionized water. The experiment was done in triplicate (42).
Determination of Zeta Potential of Nanoparticles
Electrical charge on the surface of particles demonstrated by zeta potential and the related value indicates physical stability of colloidal system. Zeta Sizer 3000Hs (Malvern, UK) was used for determining zeta potential of the particles (43,44).
Determination of Entrapment Efficiency and Loading Efficiency of Nanoparticles
To calculate the entrapment efficiency (EE%) and loading efficiency (LE%) of nanoparticles, the opalsant colloidal nanosuspension has been centrifuged at 12,000 rpm for 20 min, and the nonencapsulated insulin in the transparent supernatant aqueous fluid was measured by high-performance liquid chromatography (HPLC) (n = 3). Then, the EE% and LE% were determined using the equations provided below:
HPLC Analysis
In this study, an Agilent 1260 infinity, equipped with UV–vis detector and C18 column (125 × 4.6 mm × 5 μm) was used for the determination of insulin in the samples. The mobile phase contained a mixture of acetonitrile: phosphate buffer (30:70). The buffer consisted of 500 ml of KHPO4 (0.2 N), comprising 1% triethyl amine, and the pH was adjusted to 2.8 using phosphoric acid 85% v/v. The flow rate and the detector wavelength were adjusted to 1 ml/min and 214 nm, respectively.
The analytical method was proven to be linear in the range of 0.5–70 μg/ml with linear coefficient (R2) of 0.996. The related precision and accuracy (i.e., inter- and intraday variations) were calculated and proven to be in the acceptable range according to ICH guidelines.
Experimental Design Studies
To obtain an optimized nanoparticulate system for drug delivery, the application of statistical experimental design methodology has been found to be very useful in the several last years. Screening and optimization are the most important applications of these methods. Screening is ordinarily feasible by factorial models. This technique is used to recognize the effective factors on a system’s behavior. Optimization, which is usually carried out by response surface methods (RSMs), is used to achieve optimized terms for preparing a drug delivery system with desirable physicochemical features. Although different methods exist to conduct these studies, in this study, the Placket Burman experimental design method was used for screening (26,45,46).
The independent variables (factors) were defined as the concentration and pH of CPβCD, the concentration and pH of TMC, the concentration of alginate, and the concentration of calcium chloride according to preliminary studies. Responses including the size, PdI, and EE% were considered the dependent factors, as illustrated in Table I. Design Expert® software (V.8.8.1, Stat-Ease, Inc. Minneapolis, USA) was used to design the experiments, as well as for screening, modeling, and predictions. According to Design Expert®, 12 runs must be performed for a full screening study. The Placket Burman design is summarized in Table II.
Table I.
Variables Used in Placket Burman Experimental Design
| Independent variables (factors) | Levels | ||
|---|---|---|---|
| −1 | +1 | ||
| Numeric factors | Concentration of β-cyclodextrin (A) | 0.5 mg/ml | 4 mg/ml |
| pH of β-cyclodextrin (B) | 3 | 8 | |
| Concentration of alginate (C) | 0.1 mg/ml | 1 mg/ml | |
| concentration of TMC (D) | 0.5 mg/ml | 2 mg/ml | |
| pH of TMC solution (E) | 3 | 6 | |
| Concentration of CaCl2 (F) | 5 mg/ml | 30 mg/ml | |
| Dependent variables (responses) | Constrains | ||
| Y 1 = size (nm) | Minimize | ||
| Y 2 = PdI | Minimize | ||
| Y 3 = Entrapment efficiency (EE%) | Maximize | ||
Table II.
Plackett Burman Experimental Design Runs (n = 3)
| No | Independent variables | Dependent variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| βCD conc. (A) (mg/ml) | βCD pH (B) | Alginate conc. (C) (mg/ml) | TMC conc. (D) (mg/ml) | TMC pH (E) | CaCl2 conc. (F) (mg/ml) | Size (Y 1) (nm) | PdI (Y 2) | EE (Y 3) (%) | |
| 1 | 4 | 3 | 1 | 0.5 | 3 | 5 | 1505.6 ± 68.16 | 0.84 ± 0.14 | 96.63 ± 1.06 |
| 2 | 4 | 8 | 0.1 | 2 | 6 | 5 | 286 ± 65.5 | 0.29 ± 0.1 | 99.2 ± 0.26 |
| 3 | 4 | 8 | 1 | 0.5 | 6 | 30 | 2023 ± 419 | 0.97 ± 0.03 | 98.4 ± 0.17 |
| 4 | 4 | 3 | 0.1 | 0.5 | 6 | 30 | 552.3 ± 82.9 | 0.57 ± 0.06 | 96.9 ± 0.25 |
| 5 | 0.5 | 3 | 0.1 | 0.5 | 3 | 5 | 350.6 ± 164.5 | 0.54 ± 0.1 | 99.7 ± 0.06 |
| 6 | 0.5 | 3 | 1 | 2 | 6 | 5 | 1886.6 ± 940 | 0.73 ± 0.25 | 100.5 ± 0.0 |
| 7 | 0.5 | 8 | 0.1 | 0.5 | 3 | 30 | 974.3 ± 486 | 0.62 ± 0.09 | 99.6 ± 0.15 |
| 8 | 0.5 | 8 | 1 | 0.5 | 6 | 5 | 2393 ± 1219 | 0.85 ± 0.15 | 99.6 ± 0.43 |
| 9 | 0.5 | 3 | 0.1 | 2 | 6 | 30 | 1011.6 ± 635.2 | 0.76 ± 0.31 | 99.8 ± 0.11 |
| 10 | 0.5 | 8 | 1 | 2 | 3 | 30 | – | – | – |
| 11 | 4 | 3 | 1 | 2 | 3 | 30 | – | – | – |
| 12 | 4 | 8 | 0.1 | 2 | 3 | 5 | 187 ± 20.07 | 0.34 ± 0.01 | 99.8 ± 0.11 |
EE% entrapment efficiency
Morphological Studies
Morphology of prepared particles was examined by scanning electron microscopy (SEM). A few amount of freeze-dried nanoparticles were diluted by deionized water. Samples were then dispersed on a lamel and permitted to dry; then, the gold-coated samples were observed by an electron microscope.
In Vitro Release Studies
In this study, in order to investigate the release profile of nanoparticles in acidic and neutral media, both simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) were used for in vitro release studies.
For the preparation of simulated gastric fluid (SGF), hydrochloric acid solution (0.2 N, 39 ml) was added to sodium chloride solution (0.2 N, 250 ml), and the volume was adjusted to 1000 ml with deionized water, while the pH was maintained at 2.2. Simulated intestinal fluid (SIF) was prepared by dissolving KH2PO4 (6.8 g) in 250 ml of deionized water. The solution was mixed with NaOH (0.2 N, 77 ml), and finally, the volume was adjusted to 1000 ml using deionized water, while the pH was maintained at 6.8.
The proper amount of lyophilized powder equivalent to 70 mg of insulin, considering the loading efficiency (LE%) and the amount of lyoprotectant used for freeze drying, was weighted and dispersed in the SGF. Throughout the entire study, the temperature was kept constant at 37 ± 1.0°C, and the release medium was gently agitated at 50 rpm using the USP basket apparatus I. The experimental condition was adjusted in a manner to ensure that the sink condition was established.
In predetermined time intervals of 15, 30, 45, 60, 75, 90, and 120 min, 1 ml of the solution was sampled and replaced by a freshly prepared, preheated medium. The samples were centrifuged at 12,000 rpm for 20 min, and the supernatant was collected for insulin determination using the previously mentioned HPLC method. After 120 min postincubation, the release medium was changed to SIF, and samples were taken at 150, 195, 240, 300, and 360 min.
Caco-2 Cell Culture
Caco2 cells were provided from Pasteur institute (Tehran, Iran) at passage numbers of 30–40. Cells were cultured on 25-cm2Nunc plastic flasks (Roskilde, Denmark). The medium contained modified Eagle’s medium (MEM) supplemented with 1% v/v nonessential amino acids (90% v/v), fetal bovine serum (FBS, 9% v/v), and penicillin-streptomycin (100 U/ml, 1% v/v). Cells were incubated in a humidified atmosphere containing 5% CO2 and 95% air at 37°C. The culture medium was changed every second day. Cells were passaged after 7 days until the desired confluency is reached.
Permeability Studies
For performing the permeability studies on Caco-2 cell monolayer, insulin was used in free solution form and in the form of nanocolloids prepared from nonmodified chitosan–alginate- CPβCD and TMC–alginate–CPβCD.
Caco-2 cells with passage numbers of 30–45 were obtained as mentioned before. The cells were seeded on polyethylene terephthalate (PET) membrane filters with a pore size of 0.4 μm (Grenier Bio one, Manore, NC, USA) in 12-well plate at the cell density of 2 × 104 cell/cm2. The medium included modified Eagle’s medium mentioned previously in this publication. The culture medium was added to both apical (1 ml) and basolateral (2 ml) compartments and was changed every second day for 18 days. The cells were incubated at 37°C in an atmosphere of 5% CO2 at 90% humidity. To ensure the formation of cell monolayer, the transepithelial electrical resistance (TEER) was determined every second day using an EVOM2, epithelial voltammeter for TEER (World Precision Instruments, Sarasota, FL, USA) equipped with chopstick electrode set. The experiment was performed when the TEER values were more than the values of 600–700 Ω cm2 to ensure the formation of a monolayer. On the other hand, the transwell membrane was studied visually by optical microscopy to ensure the formation of a monolayer.
One hour before the experiment, the medium in both apical and basolateral sides was changed to a transport medium, i.e., white MEM buffered with n-(2-hydroxyethyl) piperazine-n-(2-ethanosulfonic acid) (HEPES) at pH 7.4, and the cells were allowed to equilibrate for 1 h.
For permeability studies, 1 ml of 0.40 mg/ml final concentration of insulin in the form of nanocolloids prepared was added to the apical side of the cell culture dish. Samples of 50 μl were collected from the basolateral part at predetermined times of 0, 30, 60, 90, 120, 180, and 240 min and replaced with equal volumes of fresh white MEM-HEPES medium. The samples were analyzed for the insulin content using the previously mentioned HPLC method. The study was performed in triplicate.
Statistical Analysis
All experiments had been done in triplicate, and results were reported as ,mean ± SD. Statistical significant differences were determined by one-way analysis of variance (ANOVA), which proceeded with appropriate tests designed by SPSS (V. 19.0.0, IBM statistics, New York, USA), and the differences were considered significant when p < 0.05.
For performing a Placket Burman experimental design, Design Expert® software (V.8.8.1, Stat-Ease, Inc., Minneapolis, USA) was used for modeling, prediction, and screening.
RESULTS
Synthesis of Trimethyl Chitosan
TMC as a quaternized derivative was synthesized, and the chemical structure has been studied by 1H-NMR spectroscopy, as illustrated in Fig. 1. Considering the chemical structure of chitosan and TMC, which is shown in Fig. 2, the signals developed at 2.3 and 2.7 ppm are assumed to be related to the –N(CH3)2 and N+(CH3)3, respectively; the signal at 3.02 ppm is assigned to the O-methylated sites (−OCH3). The signals at 3.2–3.5 ppm correspond to hydrogens in the sugar skeleton of chitosan. The peaks at 4.6 ppm are related to the anomeric protons of the sugar. The degree of quaternization was calculated by integration and shown to be 36.4%.
Fig. 1.
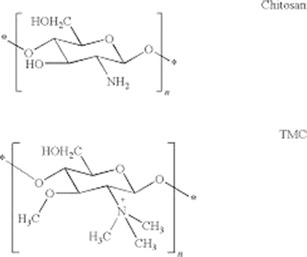
Chemical structure of chitosan and trimethyl chitosan (TMC)
Fig. 2.
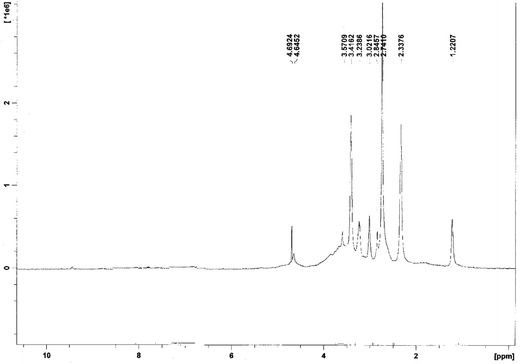
H-NMR spectrum of TMC (trimethyl chitosan)
Experimental Design
Although iono-tropic gelation is a known method for preparing hydrogels using polyelectrolytes, because several factors are effective in the preparation of nanoparticles, the optimization of effective factors is necessary.
Design Expert® software was used to study the effects of independent factors, including the concentration and pH of the βCD solution, the concentration and pH of TMC solution, and finally, the concentration of sodium alginate and CaCl2 solution on the size, pdI, and EE% of nanoparticles, and the Placket Burman experimental design study was considered. The experimental results are summarized in Table II. During the preparation of formulations 10 and 11, large aggregates were developed, and consequently, the related large aggregates were not examined for physico-chemical properties including size, PdI, and EE%.
Particle Size
According to the results shown in Table II, the size of nanoparticles varies in the range of 187 ± 20.07 nm to 2393 ± 1219 nm.
The 3-D graph of alterations in size due to changes in values of independent factors is illustrated in Fig. 3a–c. The particle size is increased by increasing the alginate concentration; particle size was observed to be greatest when the alginate concentration was highest (Fig. 3a).
Fig. 3.
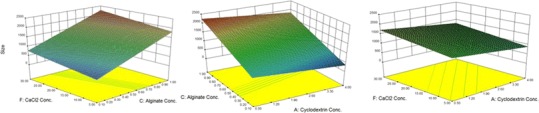
3-D plots for size
Although the particle size also increased by increasing the concentration of CaCl2 (Fig. 3b), but higher β-CD concentrations resulted in smaller particle sizes (Fig. 3c).
Statistical analysis was done by Design Expert® to choose the best significant statistical model to predict particle size variations (p < 0.05).The regression analysis of variance showed that the linear coefficients of A, C, and F factors, which indicate the βCD, sodium alginate, and CaCl2 concentrations, respectively, are significant in the suggested model (p < 0.05). The characteristics of the identified significant models are summarized in Table III.
Table III.
Characteristics of the Models Fitted to Responses
| Dependent variables (responses) | Best-fitted model | R 2 | Adj R2 | Pred R 2 | Adeq Precision |
|---|---|---|---|---|---|
| Size (Y 1) | Linear | 0.9665 | 0.9498 | 0.9130 | 17.131 |
| PdI (Y 2) | Linear | 0.8943 | 0.8414 | 0.7269 | 10.998 |
| EE% (Y 3) | Linear | 0.9097 | 0.8645 | 0.7611 | 10.812 |
Polydispersity Index
PdI is defined as an index demonstrating the hemogenicity of nanocolloids, and numerically is between 0 and 1. In all of the results, the PdI varies between 0.29 and 0.97, and βCD, alginate, and CaCl2 concentrations were found to affect its quantity (Table II).
A3-D graph of changes in PdI in accordance with changes in the values of independent factors is illustrated in Fig. 4a–c. As shown in Fig. 4a, PdI is increased by increasing the CaCl2 concentration. Higher alginate concentrations provide particles with higher PdI values (Fig. 4b).
Fig. 4.
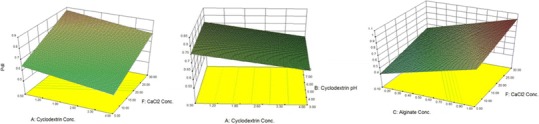
3-D plots for PdI
Statistical analysis was done using Design Expert® software to choose the best fitted, significant, statistical model to predict changes in PdI (p < 0.05). The regression analysis of variance showed that the linear coefficients for factors A, C, and F, which demonstrate βCD, alginate, and CaCl2 concentrations, respectively, are significant in the suggested model (p < 0.05). The proposed model, defined as the equation provided below, and its characteristics are demonstrated in Table III.
Entrapment Efficiency
According to the results shown in Table II, the EE% of particles is observed to vary between 99.84% and 100.03%.
A3-D graph of the changes in EE% in accordance with the changes in values of independent factors is illustrated in Fig. 5. As shown in this figure, a higher EE% was associated with higher concentrations of TMC and βCD and lower pH values of β-CD.
Fig. 5.
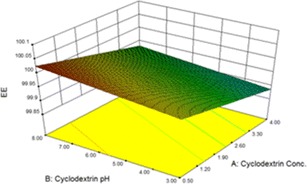
3-D plots for EE%
Due to the statistical analysis done using Design Expert® software, the regression analysis of variance showed that the linear coefficients of A, B, and D factors are significant in the suggested model (p < 0.05), while A, B, and D are defined as the βCD concentration, the p value of βCD, and the TMC concentration, respectively. The fitted model defined by the following equation and its related characteristics is summarized in Table III.
Preparation of Optimized Nanoparticles and Model Validation
The predicted quantities of independent variables required to prepare optimized nanoparticles are shown in Table IV.
Table IV.
Optimized Independent Variables and the Related Predicted Values
| No. | Optimized independent variables | Predicted dependent variables (responses) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cyclodextrin conc. (A) | Cyclodextrin pH (B) | Alginate conc. (C) | TMC conc. (D) | TMC pH (E) | CaCl2 conc. (F) | Y 1 = size (nm) | Y 2 = PdI | Y 3 = EE% | |
| 1 | 4.0 | 8.0 | 0.1 | 2.0 | 4.5 | 5.0 | 370 | 0.346 | 97% |
EE% entrapment efficiency
Nanoparticles were prepared by suggested formulation in the laboratory condition (Table IV), and their observed physico-chemical properties were determined as illustrated in Table V. As shown in this table, the calculated error percent is <5% in all cases, indicating the proper accuracy and significance of the model.
Table V.
Observed Responses and Prediction Errors (n = 3)
| No. | Dependent variables (responses) | ||||||
|---|---|---|---|---|---|---|---|
| Size (nm) | PdI | EE% | LE% | ||||
| Observed response (mean ± SD) | Prediction error (%) | Observed response (mean ± SD) | Prediction error (%) | Observed response (mean ± SD) | Prediction error (%) | Observed response (mean ± SD) | |
| 1 | 150.82 ± 21 | −59.2% | 0.362 ± 0.036 | 4.62% | 93.2% ± 4.1 | 3.91% | 5.43% ± 0.21 |
EE% entrapment efficiency, LE% loading efficiency
Morphological Studies
Morphology of the freeze-dried nanoparticles prepared from the optimized formulation depicted in Table IV has been studied using SEM. As shown in Fig. 6, spherical to subspherical nanoparticles were prepared.
Fig. 6.

SEM images of morphological study of nanoparticles
In Vitro Release Study
According to obtained data, a few amount of insulin released in 120 min of postincubation, which is regarded as the release in SGF medium (i.e., pH = 2.2) and considered to be 4.9 ± 0.24%, while just after changing the release medium to SIF (i.e., pH = 6.8), the sharp increase in release rate of insulin from nanoparticles could be observed (Fig. 7) in a manner that, after 350 min postincubation, 98.7 ± 0.13% of insulin is released from nanoparticles.
Fig. 7.
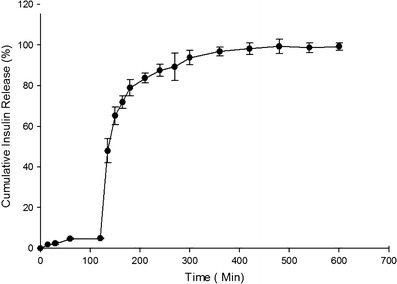
In vitro release profile of insulin from nanoparticles (n = 3)
Permeation Studies Across Caco-2 Cells
Insulin permeability across Caco-2 cell monolayer has been studied using insulin-free solution, chitosan–alginate–CPβCD nanoparticles, and TMC–alginate–CPβCD nanoparticles. Cell monolayer formation was confirmed by TEER value and assessment of membrane filter by optical microscopy. Insulin permeation in TMC–alginate–CPβCD nanoparticles had a noticeable increase in comparison with chitosan–alginate–CPβCD nanoparticles (p < 0.05). All the data demonstrated in Fig. 8 show permeability of mentioned nanoparticles achieve to 8.75 ± 0.39 in 240 min.
Fig. 8.
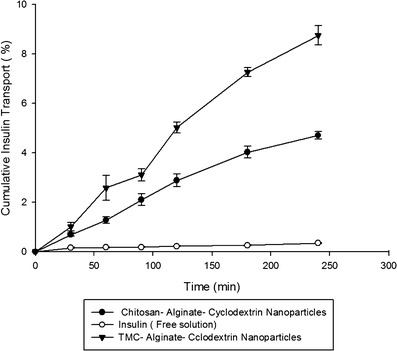
Cumulative insulin transport across Caco-2 cell monolayer
DISCUSSION
Polymeric nanoparticles are known as nanocolloidal drug carriers that can be produced by natural, synthetic, and semisynthetic polymers. Particle size is the most important feature of the nanomeric systems, which is considered a significant feature in drug loading, drug release, and particulate system stability (26). Many studies have proven that nanoparticles exhibit better characteristics than microparticles in drug delivery because of their smaller size and better mobility, which leads to better cellular uptake and better target delivery (47). Many studies have assessed the use of PLGA (48–50), chitosan (51–53), and albumin (54) in peptide delivery.
The mechanism by which chitosan can open tight junctions and act as a permeation enhancer interrupts the integration of tight junctions by electrostatic interaction with the intestinal mucosa (55,56).
In recent years, ionic gelation and polyelectrolyte complexation (PEC) have been extensively used as a suitable technique to prepare the nanoparticles of sensitive molecules such as peptides and proteins. In these methods, procedures such as sonication and the presence of organic solvents can be avoided, which reduces the possible damage to peptides and proteins (57). The PEC method can prepare nanoparticles with a high EE% and zeta potential, and ionic gelation can provide drug retention and eventually higher bioavailability.
Chitosan and alginate are considered apolycation and a polyanion, respectively, which can form a polyelectrolyte complex and take part in peptide and protein delivery.
In this study, the preparation of insulin nanoparticles begins first by preparing an ionic pre-gel of alginate and CaCl2 according to iono-tropic pregelation and proceeds by complexation between alginate and TMC. Then, a TMC polyelectrolyte solution is added to form a polyelectrolyte complex that alginate pre-gel locates as its core (58).
Insulin in low concentrations can develop much more stable complexes, while in higher concentrations, insulin can be aggregated, consequently reducing the electrostatic attraction between the peptide and cationic βCD (27).
Cyclodextrins are considered helicoidal molecules with hydrophobic cavities and hydrophilic external surfaces. A portion of insulin hydrophobic side chains is located in the cavities by a noncovalent interaction, while the electrostatic attraction between negatively charged insulin and quaternary ammonium groups of cationic βCDs results in a stronger connection than nonmodified βCDs. Cyclodextrins are also used for delivery of small interfering RNA (siRNA). CALAA01, a cyclodextrin-based nanoparticulate system containing siRNA, has been proven to be effective in phase 1 clinical trial (59). Moreover, cationic βCDs have better solubility and biocompatibility (33).
The inclusion of insulin bulky molecules in βCD’s cavities is called inclusion complexation. Therefore, by increasing the concentration of βCD, the particle size and pdI are expected to decrease. Nevertheless, this increase in the βCD concentration can result in a decrease in the EE%. These cationic polymers increase insulin solubility, and some of the insulin extrudes alginate hydrogel (37).
By increasing the pH of the βCD solution, the EE% would decrease. This could be due to a reduction of the positive charge as a consequence of increasing the pH. Therefore, the electrostatic interaction and eventually the solubility would decrease (37).
In the pre-gel preparing section, the order of adding CaCl2 and polymer to alginate solution is important and influences the particle size, which may be due to the different nature of alginate’s interaction with CaCl2 and the polymer. In fact, negatively charged alginate can first cross-link with Ca2+, as a cationic ion, and the hydrogel can be developed. Second, the previously developed hydrogel resulting from a negative charge on its surfaces can complex with positively charged TMC. Cross-linking cannot be established when the complexation forms at first (41).
Pre-gel formation time out and the stirring speed are also effective parameters for determining the size and EE%. Therefore, they were fixed as a constant in all tests.
The most effective parameter for size, EE%, and LE% is the massive ratio of alginate/chitosan, which determines the amount of polycations needed to capture the maximum amount of insulin in the particles. Therefore, since TMC is the last layer of nanoparticles and its influence on insulin loading is a major factor, by increasing the concentration of TMC while the concentration of alginate is kept constant, a decrease in the alginate/chitosan ratio and an increase in the EE% can be expected.
Mundargi et al. have reported pH-sensitive microspheres for oral insulin delivery using Eudagit L 100, Eudragit RS and their blends. They showed that, in gastric fluid with pH value of 1.2, the insulin was not released, while the maximum insulin release was found after 4–6 h postincubation in intestinal media with pH value of 6.8 (60). In the present study, results obtained from in vitro release indicated the preparation of pH-sensitive nanoparticles. Alginate, which is present in the structure of nanoparticles, is assumed to develop a gel in acidic pHs, and therefore, the release of drug from nanoparticles can be retarded in SGF. Sormento et al. have also reported alginate nanoparticles for oral delivery of insulin. They reported the burst release of insulin in acidic media due to weak interaction forces between peptide and the polymer (61,62). In this study, as shown in Fig. 7, low burst effect indicate well-established electrostatic interactions between positively charged trimethyl chitosan, calcium chloride as cross-linking agent, negatively charged insulin–CPβCD complex and alginate. Mundargi et al. have reported development of poly(N-vinylcaprolactam-co-methacrilic acid) hydrogel for pH-sensitive insulin delivery. They reported no significant release of insulin from hydrogel microparticles in gastric medium but complete release, approximately 100%, of insulin in pH value of 7.4 (63).
A significant increase in permeability of insulin entrapped in the studied nanoparticulate system has been proven in permeability studies across the Caco-2 cell monolayer. This indicates that these nanoparticles can cross-epithelial enterocytes efficiently and possess better oral absorption. This may be due to the positive charge of TMC, which can enhance mucoadhesion.
CONCLUSIONS
In this study, a cationic derivative of βCD was synthesized, and insulin nanoparticles were prepared using a modified iono-tropic pregelation method. Design Expert® software and Placket Burman methodology were used to define significant parameters on the size, pdI, and EE% of particles. The morphology of prepared nanoparticles was studied using SEM, and the results revealed spherical to subspherical nanoparticles. The release study showed the slow release of entrapped insulin. The cytotoxicity studies revealed no significant cytotoxicity 5 h postincubation, but concentration-dependent cytotoxicity 24 h postincubation. The permeability studies showed significantly higher permeability of prepared nanoparticles across the Caco-2 cell monolayer.
Based on these facts, the prepared insulin nanoparticles using the modified iono-tropic pregelation method can be considered a good candidate for oral insulin delivery.
ACKNOWLEDGMENTS
This study was made possible by financial supports from Deputy of Research, Tehran University of Medical Sciences.
Conflict of Interest
The authors declare no conflict of interests.
REFERENCES
- 1.Reis CP, Ribeiro AJ, Houng S, Veiga F, Neufeld RJ. Nanoparticulate delivery system for insulin: design, characterization and in vitro/in vivo bioactivity. Eur J Pharm Sci. 2007;30(5):392–7. doi: 10.1016/j.ejps.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Sarmento B, Martins S, Ribeiro A, Veiga F, Neufeld R, Ferreira D. Development and comparison of different nanoparticulate polyelectrolyte complexes as insulin carriers. Int J Pept Protein Res. 2006;12(2):131–8. doi: 10.1007/s10989-005-9010-3. [DOI] [Google Scholar]
- 3.Ubaidulla U, Khar RK, Ahmed FJ, Panda AK. Development and in-vivo evaluation of insulin-loaded chitosan phthalate microspheres for oral delivery. J Pharm Pharmacol. 2007;59(10):1345–51. doi: 10.1211/jpp.59.10.0003. [DOI] [PubMed] [Google Scholar]
- 4.Lin Y-H, Liang H-F, Chung C-K, Chen M-C, Sung H-W. Physically crosslinked alginate/N, O-carboxymethyl chitosan hydrogels with calcium for oral delivery of protein drugs. Biomaterials. 2005;26(14):2105–13. doi: 10.1016/j.biomaterials.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Pillai O, Panchagnula R. Insulin therapies—past, present and future. Drug Discov Today. 2001;6(20):1056–61. doi: 10.1016/S1359-6446(01)01962-6. [DOI] [PubMed] [Google Scholar]
- 6.Elsayed A, Remawi MA, Qinna N, Farouk A, Badwan A. Formulation and characterization of an oily-based system for oral delivery of insulin. Eur J Pharm Biopharm. 2009;73(2):269–79. doi: 10.1016/j.ejpb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Tiwari AK, Gajbhiye V, Sharma R, Jain NK. Carrier mediated protein and peptide stabilization. Drug Deliv. 2010;17(8):605–16. doi: 10.3109/10717544.2010.509359. [DOI] [PubMed] [Google Scholar]
- 8.Trapani A, Lopedota A, Franco M, Cioffi N, Ieva E, Garcia-Fuentes M, et al. A comparative study of chitosan and chitosan/cyclodextrin nanoparticles as potential carriers for the oral delivery of small peptides. Eur J Pharm Biopharm. 2010;75(1):26–32. doi: 10.1016/j.ejpb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi H, Yamamoto H, Niwa T, Hino T, Kawashima Y. Enteral absorption of insulin in rats from mucoadhesive chitosan-coated liposomes. Pharm Res. 1996;13(6):896–901. doi: 10.1023/A:1016009313548. [DOI] [PubMed] [Google Scholar]
- 10.Radwan MA, Aboul-Enein HY. The effect of oral absorption enhancers on the in vivo performance of insulin-loaded poly(ethylcyanoacrylate) nanospheres in diabetic rats. J Microencapsul. 2002;19(2):225–35. doi: 10.1080/02652040110081406. [DOI] [PubMed] [Google Scholar]
- 11.Bayat A, Dorkoosh FA, Dehpour AR, Moezi L, Larijani B, Junginger HE, et al. Nanoparticles of quaternized chitosan derivatives as a carrier for colon delivery of insulin: ex vivo and in vivo studies. Int J Pharm. 2008;356(1–2):259–66. doi: 10.1016/j.ijpharm.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 12.Krauland AH, Alonso MJ. Chitosan/cyclodextrin nanoparticles as macromolecular drug delivery system. Int J Pharm. 2007;340(1–2):134–42. doi: 10.1016/j.ijpharm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Vila A, Sánchez A, Tobío M, Calvo P, Alonso MJ. Design of biodegradable particles for protein delivery. J Control Release. 2002;78(1–3):15–24. doi: 10.1016/S0168-3659(01)00486-2. [DOI] [PubMed] [Google Scholar]
- 14.Tiyaboonchai W, Woiszwillo J, Sims RC, Middaugh CR. Insulin containing polyethylenimine–dextran sulfate nanoparticles. Int J Pharm. 2003;255(1–2):139–51. doi: 10.1016/S0378-5173(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 15.Fasano A. Innovative strategies for the oral delivery of drugs and peptides. Trends Biotechnol. 1998;16(4):152–7. doi: 10.1016/S0167-7799(97)01170-0. [DOI] [PubMed] [Google Scholar]
- 16.Kidron M, Bar-On H, Berry EM, Ziv E. The absorption of insulin from various regions of the rat intestine. Life Sci. 1982;31(25):2837–41. doi: 10.1016/0024-3205(82)90673-7. [DOI] [PubMed] [Google Scholar]
- 17.Martin Werle HT, Bernkop-Schnurch A. Modified chitosans for oral drug delivery. J Pharm Sci. 2008;98(5):1643–56. doi: 10.1002/jps.21550. [DOI] [PubMed] [Google Scholar]
- 18.Artursson P, Lindmark T, Davis S, Illum L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2) Pharm Res. 1994;11(9):1358–61. doi: 10.1023/A:1018967116988. [DOI] [PubMed] [Google Scholar]
- 19.Van der Lubben IM, Verhoef JC, Borchard G, Junginger HE. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur J Pharm Sci. 2001;14(3):201–7. doi: 10.1016/S0928-0987(01)00172-5. [DOI] [PubMed] [Google Scholar]
- 20.El-Sherbiny IM. Enhanced pH-responsive carrier system based on alginate and chemically modified carboxymethyl chitosan for oral delivery of protein drugs: preparation and in-vitro assessment. Carbohydr Polym. 2010;80(4):1125–36. doi: 10.1016/j.carbpol.2010.01.034. [DOI] [Google Scholar]
- 21.Shilpa A, Agrawal S, Ray AR. Controlled delivery of drugs from alginate matrix. J Macromol Sci Pure Part C: Polym Rev. 2003;43(2):187–221. doi: 10.1081/MC-120020160. [DOI] [Google Scholar]
- 22.Chen Y, Siddalingappa B, Chan PHH, Benson HAE. Development of a chitosan-based nanoparticle formulation for delivery of a hydrophilic hexapeptide, dalargin. Pept Sci. 2008;90(5):663–70. doi: 10.1002/bip.21055. [DOI] [PubMed] [Google Scholar]
- 23.Sinha VR, Singla AK, Wadhawan S, Kaushik R, Kumria R, Bansal K, et al. Chitosan microspheres as a potential carrier for drugs. Int J Pharm. 2004;274(1–2):1–33. doi: 10.1016/j.ijpharm.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 24.George M, Abraham TE. pH sensitive alginate–guar gum hydrogel for the controlled delivery of protein drugs. Int J Pharm. 2007;335(1–2):123–9. doi: 10.1016/j.ijpharm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Huang L, Xin J, Guo Y, Li J. A novel insulin oral delivery system assisted by cationic β-cyclodextrin polymers. J Appl Polym Sci. 2010;115(3):1371–9. doi: 10.1002/app.30775. [DOI] [Google Scholar]
- 26.Mahjub R, Dorkoosh F, Amini M, Khoshayand M, Rafiee-Tehrani M. Preparation, statistical optimization, and in vitro characterization of insulin nanoparticles composed of quaternized aromatic derivatives of chitosan. AAPS Pharm Sci Technol. 2011;12(4):1407–19. doi: 10.1208/s12249-011-9716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang N, Li J, Jiang W, Ren C, Li J, Xin J, et al. Effective protection and controlled release of insulin by cationic β-cyclodextrin polymers from alginate/chitosan nanoparticles. Int J Pharm. 2010;393(1–2):213–9. doi: 10.1016/j.ijpharm.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Sieval AB, Thanou M, Kotzkb AF, Verhoefa JC, Brussee JC, Junginger HE. Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohydr Polym. 1998;36:157–65. doi: 10.1016/S0144-8617(98)00009-5. [DOI] [Google Scholar]
- 29.Thanou M, Verhoef JC, Junginger HE. Oral drug absorption enhancement by chitosan and its derivatives. Adv Drug Deliv Rev. 2001;52(2):117–26. doi: 10.1016/S0169-409X(01)00231-9. [DOI] [PubMed] [Google Scholar]
- 30.Shao Z, Li Y, Chermak T, Mitra A. Cyclodextrins as mucosal absorption promoters of insulin. II. Effects of β-cyclodextrin derivatives on α-chymotryptic degradation and enteral absorption of insulin in rats. Pharm Res. 1994;11(8):1174–9. doi: 10.1023/A:1018997101542. [DOI] [PubMed] [Google Scholar]
- 31.Sajeesh S, Sharma CP. Cyclodextrin–insulin complex encapsulated polymethacrylic acid based nanoparticles for oral insulin delivery. Int J Pharm. 2006;325(1–2):147–54. doi: 10.1016/j.ijpharm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Irie T, Uekama K. Cyclodextrins in peptide and protein delivery. Adv Drug Deliv Rev. 1999;36(1):101–23. doi: 10.1016/S0169-409X(98)00057-X. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Xiao H, Li J, Zhong Y. Drug carrier systems based on water-soluble cationic β-cyclodextrin polymers. Int J Pharm. 2004;278(2):329–42. doi: 10.1016/j.ijpharm.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Rowsen L, Moses KJD, Sharma CP. Beta cyclodextrin±insulin-encapsulated chitosan/alginate matrix: oral delivery system. J Appl Polym Sci. 1999;75:1089–96. [Google Scholar]
- 35.Li J, Guo Z, Xin J, Zhao G, Xiao H. 21-Arm star polymers with different cationic groups based on cyclodextrin core for DNA delivery. Carbohydr Polym. 2010;79(2):277–83. doi: 10.1016/j.carbpol.2009.08.006. [DOI] [Google Scholar]
- 36.Li J, Loh XJ. Cyclodextrin-based supramolecular architectures: syntheses, structures, and applications for drug and gene delivery. Adv Drug Deliv Rev. 2008;60(9):1000–17. doi: 10.1016/j.addr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Xin J, Guo Z, Chen X, Jiang W, Li J, Li M. Study of branched cationic β-cyclodextrin polymer/indomethacin complex and its release profile from alginate hydrogel. Int J Pharm. 2010;386(1–2):221–8. doi: 10.1016/j.ijpharm.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 38.Kowapradit J, Apirakaramwong A, Ngawhirunpat T, Rojanarata T, Sajomsang W, Opanasopit P. Methylated N-(4-N, N-dimethylaminobenzyl) chitosan coated liposomes for oral protein drug delivery. Eur J Pharm Sci. 2012;47(2):359–66. doi: 10.1016/j.ejps.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Jia Z, Shen D, Xu W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr Res. 2001;333(1):1–6. doi: 10.1016/S0008-6215(01)00112-4. [DOI] [PubMed] [Google Scholar]
- 40.Kotzé AF, Lueßen HL, de Leeuw BJ, de Boer BG, Coos Verhoef J, Junginger HE. Comparison of the effect of different chitosan salts and N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2) J Control Release. 1998;51(1):35–46. doi: 10.1016/S0168-3659(97)00154-5. [DOI] [PubMed] [Google Scholar]
- 41.Rajaonarivony M, Vauthier C, Couarraze G, Puisieux F, Couvreur P. Development of a new drug carrier made from alginate. J Pharm Sci. 1993;82(9):912–7. doi: 10.1002/jps.2600820909. [DOI] [PubMed] [Google Scholar]
- 42.Helgason T, Awad T, Kristbergsson K, McClements DJ, Weiss J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN) J Colloid Interface Sci. 2009;334(1):75–81. doi: 10.1016/j.jcis.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Motwani SK, Chopra S, Talegaonkar S, Kohli K, Ahmad FJ, Khar RK. Chitosan–sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: formulation, optimisation and in vitro characterisation. Eur J Pharm Biopharm. 2008;68(3):513–25. doi: 10.1016/j.ejpb.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Bunjes H. Characterization of solid lipid nano- and microparticles. Lipospheres in drug targets and delivery. New York: CRC Press; 2005. pp. 41–66. [Google Scholar]
- 45.Wu L, Zhang J, Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63(6):456–69. doi: 10.1016/j.addr.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Julienne MC, Alonso MJ, Gómez Amoza JL, Benoit JP. Preparation of poly(D, L-lactide/glycolide) nanoparticles of controlled particle size distribution: application of experimental designs. Drug Dev Ind Pharm. 1992;18(10):1063–77. doi: 10.3109/03639049209069315. [DOI] [Google Scholar]
- 47.Hu W, Enying L, Yao LG. Optimization of drawbead design in sheet metal forming based on intelligent sampling by using response surface methodology. J Mater Process Technol. 2008;206(1–3):45–55. doi: 10.1016/j.jmatprotec.2007.12.002. [DOI] [Google Scholar]
- 48.Chen V, Ma Y. Nanoparticles—a review. Trop J Pharm Res. 2006;5(1):561–73. [Google Scholar]
- 49.Fresta M, Puglisi G, Giammona G, Cavallaro G, Micali N, Furneri PM. Pefloxacine mesilate‐ and ofloxacin‐loaded polyethylcyanoacrylate nanoparticles. Characterization of the colloidal drug carrier formulation. J Pharm Sci. 1995;84(7):895–902. doi: 10.1002/jps.2600840721. [DOI] [PubMed] [Google Scholar]
- 50.Mundargi RC, Babu VR, Ransawamy V, Patel P, Aminbhavi TM. Nano/ micro technologies for delivering maromolecular therapeutics using poly ( D, L-lactide-co-glycolide) and its derivatives. J Control Release. 2008;125:193–209. doi: 10.1016/j.jconrel.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Wada R, Hyon SH, Ikada Y. Lactic acid oligomer microspheres containing hydrophilic drugs. J Pharm Sci. 1990;79(10):919–24. doi: 10.1002/jps.2600791016. [DOI] [PubMed] [Google Scholar]
- 52.Uchida T, Yagi A, Oda Y, Nakada Y, Goto S. Instability of bovine insulin in poly (lactide-co-glycolide)(PLGA) microspheres. Chem Pharm Bull. 1996;44(1):235–6. doi: 10.1248/cpb.44.235. [DOI] [PubMed] [Google Scholar]
- 53.Ganguly K, Chaturvedi K, More UA, Nadagouda MN, Aminbhavi TM. Polysacharide-based micro/nano hydrogels for delivering macromolecular therapeutics. J Control Release. 2014;193:162–73. doi: 10.1016/j.jconrel.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 54.Werle M, Takeuchi H, Bernkop-Schnürch A. Modified chitosans for oral drug delivery. J Pharm Sci. 2009;98(5):1643–56. doi: 10.1002/jps.21550. [DOI] [PubMed] [Google Scholar]
- 55.Di Colo G, Zambito Y, Zaino C. Polymeric enhancers of mucosal epithelia permeability: synthesis, transepithelial penetration‐enhancing properties, mechanism of action, safety issues. J Pharm Sci. 2008;97(5):1652–80. doi: 10.1002/jps.21043. [DOI] [PubMed] [Google Scholar]
- 56.Agnihotri SA, Nadagouda MN, Aminbhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release. 2004;100:5–28. doi: 10.1016/j.jconrel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 57.Reis CP, Veiga FJ, Ribeiro AJ, Neufeld RJ, Damgé C. Nanoparticulate biopolymers deliver insulin orally eliciting pharmacological response. J Pharm Sci. 2008;97(12):5290–305. doi: 10.1002/jps.21347. [DOI] [PubMed] [Google Scholar]
- 58.Sadeghi A, Dorkoosh F, Avadi M, Saadat P, Rafiee-Tehrani M, Junginger H. Preparation, characterization and antibacterial activities of chitosan N-trimethyl chitosan (TMC) and N-diethylmethyl chitosan (DEMC) nanoparticles loaded with insulin using both the ionotropic gelation and polyelectrolyte complexation methods. Int J Pharm. 2008;355(1):299–306. doi: 10.1016/j.ijpharm.2007.11.052. [DOI] [PubMed] [Google Scholar]
- 59.Chaturvedi K, Ganguly K, Kulkarni AR, Kulkarni VH, Nadaqouda MN, Rudzinski WE, et al. Cyclodextrin-based siRNA delivery nanocarriers: a state of the art review. Expert Opin Drug Deliv. 2011;8(11):1455–68. doi: 10.1517/17425247.2011.610790. [DOI] [PubMed] [Google Scholar]
- 60.Mundargi RC, Rangaswamy V, Aminabhavi TM. pH sensetive oral insulin delivery systems using Eudragit microspheres. Drug Dev Ind Pharm. 2011;37(8):977–85. doi: 10.3109/03639045.2011.552908. [DOI] [PubMed] [Google Scholar]
- 61.Sarmento B, Ribeiro A, Veiga F, Sampaio P, Neufeld R, Ferreira D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm Res. 2007;24(12):2108–206. doi: 10.1007/s11095-007-9367-4. [DOI] [PubMed] [Google Scholar]
- 62.Sarmento B, Ferreira D, Veiga F, Ribeiro A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr Polym. 2006;66(1):1–7. doi: 10.1016/j.carbpol.2006.02.008. [DOI] [Google Scholar]
- 63.Mundargi RC, Rangaswamy V, Aminabhavi TM. Poly (N-vinylcaprolactam-co-methacrilic acid) hydrogel microparticles for oral insulin delivery. J Microencapsul. 2011;28(5):384–94. doi: 10.3109/02652048.2011.576782. [DOI] [PubMed] [Google Scholar]


