Abstract
Dysfunction of the autophagy-lysosomal pathway (ALP) and the ubiquitin-proteasome system (UPS) was thought to be an important pathogenic mechanism in synuclein pathology and Parkinson's disease (PD). In the present study, we investigated the role of sestrin2 in autophagic degradation of α-synuclein and preservation of cell viability in a rotenone-induced cellular model of PD. We speculated that AMP-activated protein kinase (AMPK) was involved in regulation of autophagy and protection of dopaminergic cells against rotenone toxicity by sestrin2. The results showed that both the mRNA and protein levels of sestrin2 were increased in a TP53-dependent manner in Mes 23.5 cells after treatment with rotenone. Genetic knockdown of sestrin2 compromised the autophagy induction in response to rotenone, while overexpression of sestrin2 increased the basal autophagy activity. Sestrin2 presumably enhanced autophagy in an AMPK-dependent fashion, as sestrin2 overexpression activated AMPK, and genetic knockdown of AMPK abrogated autophagy induction by rotenone. Restoration of AMPK activity by metformin after sestrin2 knockdown recovered the autophagy activity. Sestrin2 overexpression ameliorated α-synuclein accumulation, inhibited caspase 3 activation, and reduced the cytotoxicity of rotenone. These results suggest that sestrin2 upregulation attempts to maintain autophagy activity and suppress rotenone cytotoxicity through activation of AMPK, and that sestrin2 exerts a protective effect on dopaminergic cells.
INTRODUCTION
Parkinson's disease (PD) is one of the most common neurodegenerative diseases in the aging population. The main pathological feature of PD is the degeneration of midbrain dopaminergic neurons located in the substantia nigra with the presence of ubiquitinated cytoplasmic inclusions, named Lewy bodies (LB), in the affected brain regions (1, 2). In vivo and in vitro genetic models overexpressing α-synuclein replicate the essential pathological features of PD (3, 4). α-Synuclein is an aggregate-prone protein, which, in pathological status, forms dimeric and higher-order toxic oligomeric species, which then serve as nuclei for the formation of protein aggregates, leading to dysfunction and degeneration of dopaminergic neurons (2, 5, 6).
Autophagy, a conserved degradation pathway for proteins, lipids, and cellular organelles, is dysregulated in PD (7). Autophagy is both induced and impaired in several genetic and chemical models of PD, leading to an accumulation of immature autophagic vesicles and α-synuclein (8, 9). Enhancement of autophagy with overexpression of beclin1 effectively reduced α-synuclein accumulation and ameliorated PD pathology in animal models (10). Meanwhile, deficiency in autophagy-related genes, such as atg7, caused PD-like neurodegeneration (11). Thus, autophagy might be protective in response to an increased burden of misfolded protein and/or chemical intoxication (8, 12–15). In vivo study with CCI-779, a derivative of rapamycin, retarded the progression of α-synuclein-induced neurodegeneration by activation of autophagy, suggesting that the mTOR pathway is a promising target for PD treatment (16). However, pathways regulating autophagy activity under the pathological conditions in PD were not fully elucidated. The mTOR (mammalian target of rapamycin) pathway is one of the most characterized pathways responsible for regulation of autophagy, and it plays an important role during the pathological development of neurodegenerative diseases, including PD (17–21). mTOR senses nutrient and amino acid signals to modulate autophagy activity. AMP-activated protein kinase (AMPK) originally was thought to promote autophagy through its ability to inhibit TOR complex 1 (22–24). Recently, evidence proved that the association of AMPK with ULK1 (unc-51-like kinase 1) and phosphorylation of ULK1 by AMPK was required for autophagy initiation. Meanwhile, TOR complex 1 counteracts the initiation of autophagy through phosphorylation of ULK1 at different sites (25). These results further suggested the functional importance of the coordination of AMPK and TOR signaling in autophagy regulation. It has been reported that pharmacological inhibition of mTOR activated autophagy and produced protective effects in animal models of PD and other neurodegenerative diseases (17, 26).
Sestrin2, a stress-response protein, exhibits oxidoreductase activity in vitro and protects cells from oxidative stress. Recent reports showed that sestrin2 could upregulate autophagic catabolism by inhibiting mTOR (27–29). However, how sestrin2 activates autophagy and its role in PD were not fully understood. Rotenone is an organic pesticide and piscicide (30) that is utilized for the production of the chemical model of PD. It is highly lipid soluble and freely crosses cellular membranes, where it causes mitochondrial dysfunction by inhibiting complex I of the electron transport chain. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and α-synuclein aggregation (31). In this study, we aimed to investigate if sestrin2 promoted autophagy activity through AMPK and protected dopaminergic cells from rotenone-induced cytotoxicity. The results demonstrated that sestrin2 was upregulated, and its induction increased autophagy activity and reduced rotenone cytotoxicity through an AMPK-dependent mechanism.
MATERIALS AND METHODS
Antibodies.
LC3 (1:1,000; 4108; Cell Signaling, Boston, MA), p62 (1:1,000; 12-1107; American Research Products, Waltham, MA), AMPKα (1:1,000; 2603; Cell Signaling), phosphorylated AMPK (Thr172) (1:1,000; 2535; Cell Signaling), caspase 3 (1:1,000; 9661; Cell Signaling), phosphor-S6K (1:1,000; ab2571; Abcam, Cambridge, MA), α-synuclein (1:800; ab1903; Abcam), sestrin2 (1:800; 21346-1-AP; ProteinTech, Chicago, IL), Flag (1:5,000; F1804; Sigma, Saint Louis, MO), TP53 (1:1,000; ab183547; Abcam), Bax (1:1,000; ab5714; Abcam), and actin (1:4,000; A5441; Sigma) were used.
Cell culture and treatment.
The hybrid Mes 23.5 cell line, derived from somatic cell fusion of rat embryonic mesencephalon cells with murine N18TG2 neuroblastoma cells, were provided by W. D. Le from Shanghai Jiaotong University (Shanghai, China). Mes 23.5 cells display many properties of developing neurons of the substantia nigra (SN) zona compacta and offer several advantages for such initial studies, including greater homogeneity than primary cultures. Mes 23.5 cells were seeded on polylysine-precoated 24-well plates (Corning, NY) at a density of 104 cells/cm2 and maintained in Dulbecco's modified Eagle's medium (DMEM; GIBCO, NY) containing Sato's components (Sigma) and 5% fetal bovine serum (FBS; Sigma) at 37°C in a 95% air–5% CO2 humidified atmosphere incubator.
Differentiated PC12 cells were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China), and were grown in tissue culture flasks at 37°C under an atmosphere of 5% CO2 in DMEM containing 10% fetal bovine serum (complete media). The culture medium was replenished at 2- to 3-day intervals based on the doubling time. Cells were treated with the indicated concentrations of rotenone (Sigma-Aldrich) as previously reported to generate the in vitro PD model. For inhibition or activation of autophagy and AMPK, 3-methyladenine, bafilomycin A1 (bafA1), compound C, and metformin were purchased from Sigma-Aldrich.
Assessment of cell viability.
Cytotoxicity was measured using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Cells were cultured in 96-well plates (1,000 cells per well) for 24 h, and then cells were treated with rotenone at the indicated concentrations or vehicle for 12 to 48 h and incubated with MTT for an additional 1 to 2 h. The percentage of growth inhibition was calculated as OD of the vehicle minus the OD of treated samples divided by the OD of the vehicle times 100, where optical density (OD) was measured at 570 nm with a microplate reader (BieTek, Burleigh, Queensland, Australia). In each experiment, quintuplicate wells were used for each drug concentration, and assays were repeated in three independent experiments.
Quantitative real-time PCR.
Total RNA was extracted with the RNAiso reagent kit (TaKaRa, Dalian, China). cDNA was generated by reverse transcription of 2 μg of total RNA using random primers and PriMescript RT reagent kit (TaKaRa) in a total reaction volume of 20 μl according to the manufacturer's instructions. The sequences of forward and reverse oligonucleotide primers, specific to sestrin2, p62, and a housekeeping gene (the β-actin gene), were designed using Primer5 software. The primers used are the following: for sestrin2, forward, 5′-CTGGTGACCCCCTCAGCGGATA-3′; reverse, 5′-ATGGGCACGGAAGGTTGGGG-3′; for p62, forward, 5′-TGTGGAACATGGAGGGAAGAG-3′; reverse, 5′-TGTGCCTGTGCTGGAACTTTC-3′; for β-actin, forward, 5′-CTGGCCGAACTCATCCAAG-3′; reverse, 5′-CTGCCTCATGCGTTCCATC-3′. Real-time quantitative PCR was performed in an iCycler 5 (Bio-Rad, Hercules, CA). A 20-fold dilution of each cDNA was amplified in a 20-μl volume using the Fast Start DNA master plus SYBR green 1 master mix (Roche Applied Science, Indianapolis, IN), with 200 nM final concentrations of each primer. PCR cycle conditions were 95°C for 10 s and then 35 cycles of 95°C for 15 s and 60°C for 31 s. The amplification specificity was evaluated with melting curve analysis. Threshold cycle (CT), which correlates inversely with the target mRNA levels, was calculated using the second derivative maximum algorithm in the software provided with the iCycler. For each cDNA, the mRNA levels were normalized to β-actin mRNA.
RNA interference of TP53, sestrin2, and AMPK.
Small interfering RNAs (siRNA) targeted the following mRNA: TP53, GAAGAAAATTTCCGCAAAA; sestrin2, GACCATGGCTACTCGCTGA; and AMPK α1/α2, AAGAGAAGCAGAAGCACGACGTT. Negative siRNA (TAAGGCTATGAAGAGATAC) was synthesized by GenePharma (Shanghai, China). The siRNA oligonucleotides used to target the AMPK α1/α2 gene were validated previously (32). For transfection, cells were plated in 9-cm dishes at 30% confluence, and siRNA duplexes (200 nM) were introduced into the cells using Lipofectamine 2000 (11668-019; Invitrogen, Carlsbad, CA) according to the manufacturer's recommendations.
Expression of GFP-LC3 and sestrin2-Flag.
The activation of autophagy was detected following transfection of cells with green fluorescent protein (GFP)-LC3 and sestrin2-3×Flag expression plasmids. The presence of several intense fluorescent dots in cells is indicative of the accumulation of autophagosomes. The transfection of cells with expression plasmids was performed using Lipofectamine 2000. For each condition, the number of GFP-LC3 dots per cell was determined with a fluorescence microscope from at least 100 GFP-LC3-positive cells.
The sestrin2-3×Flag expression plasmid was generated by PCR from the clone for sestrin2 (GC091F05; purchased from Genechem, China) with the primers CGGAATTCCATGATCGTGGCGGACTCCGAG (forward) and CGGGATCCGGTCATGTAGCGGGTGATGGC (reverse) and subsequently digested with EcoRI and BamHI and cloned into the EcoRI and BamHI sites of the 3×Flag coding sequence (Invitrogen). Transfection of cells with expression plasmids was performed using Lipofectamine 2000.
Western blot analysis.
Protein was extracted from cells using cell lysis solution supplemented with protease inhibitors (04693159001; Roche) and phosphorylase inhibitors (04906845001; Roche). Protein concentration was determined with a bicinchoninic acid (BCA) protein assay kit (23227; Pierce, Rockford, IL). Equal amounts of proteins were fractionated on Tris-glycine SDS-polyacrylamide gels, subjected to electrophoresis, and transferred to nitrocellulose membranes. Membranes were blocked with Tris-buffered saline (TBS) containing 5% (wt/vol) dry milk with 0.1% Tween 20, washed with TBS containing 0.1% Tween 20 (TBST), and then incubated overnight at 4°C with specific primary antibodies in nonfat milk containing 0.1% NaN3. After washing in TBST, membranes were incubated with fluorescence-conjugated secondary antibodies (1:10,000; anti-rabbit [711-035-152] and anti-mouse [715-035-150] antibodies; Jackson ImmunoResearch, PA) at room temperature for 1 h. Immunoreactivity was detected using an Odyssey infrared imager (Li-COR Biosciences, Lincoln, NE). The signal intensity of primary antibody binding was quantitatively analyzed with ImageJ software (W. S. Rasband, NIH, Bethesda, MD) and was normalized to a loading control, β-actin.
Immunocytochemistry and fluorescence imaging.
For immunofluorescence microscopic examination, Mes 23.5 cells were plated on 12-mm poly-l-lysine-coated coverslips and cultured for 24 h, and then cells were treated with siRNA and drugs. Cells were washed in phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde in PBS at 4°C for 10 min, and then washed again with PBS. The cells were permeabilized with 0.25% Triton X-100 and then were blocked with 10% normal goat serum (NGS) for 15 min. Primary antibodies included a mouse monoclonal antibody against Flag (F1804; Sigma) diluted in PBS that was added to the cells and left overnight at 4°C. The coverslips were washed three times before incubation with secondary antibodies using the same procedure as that for the primary antibodies. The coverslips were mounted on slides with mounting medium (F4680; Sigma-Aldrich) and were examined with a laser scanning confocal microscope (Zeiss, Jena, German).
Statistical analysis.
All data are presented as means ± standard errors of the means (SEM). Data were subjected to one-way analysis of variance (ANOVA) using the Prism software statistical package (GraphPad Software, San Diego, CA). When a significant group effect was found, post hoc comparisons were performed using the Newman-Keuls t test to examine special group differences. Independent group t tests were used for comparing two groups. All calculations were performed using the SPSS 14.0 software package (SPSS Inc., Chicago, IL).
RESULTS
Rotenone increased both sestrin2 gene transcription and protein expression.
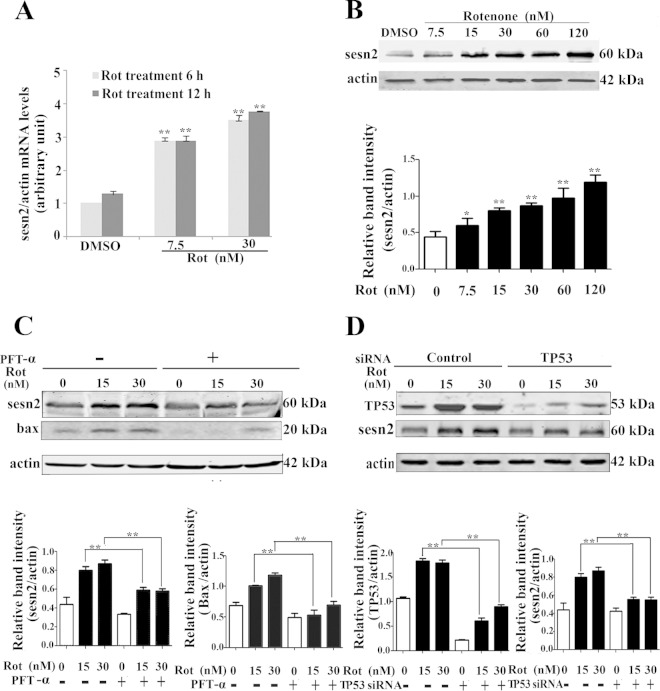
To determine the effects of rotenone on sestrin2 expression, we treated Mes 23.5 cells with various concentrations of rotenone. After 6 h of treatment, rotenone induced significant transcriptional upregulation of sestrin2 (Fig. 1A), and the enhancement of sestrin2 gene transcription was in a dose-dependent manner (7.5 nM and 30 nM) (Fig. 1A). Further studies with immunoblotting showed that rotenone dose dependently enhanced sestrin2 protein expression (Fig. 1B). Sestrin2 belongs to the sestrin family, which is transcriptionally regulated by the Forkhead transcription factor (FoxO) and TP53 (33). Thus, we investigated if sestrin2 upregulation was TP53 dependent. Pifithrin-α (PFT-α), an inhibitor affecting TP53 nucleic translocation, significantly inhibited the upregulation of sestrin2 protein levels by rotenone (Fig. 1C). Furthermore, using siRNA specifically targeted to TP53, sestrin2 upregulation was significantly attenuated by knockdown of TP53 (Fig. 1D). These results suggest that sestrin2 is induced through a TP53-dependent mechanism.
FIG 1.
Sestrin2 was upregulated by rotenone (Rot). (A) Mes 23.5 cells were treated with DMSO or rotenone at the indicated concentrations for 6 h and 12 h. After rotenone treatment, RNA was extracted, followed by real-time PCR detection of sestrin2 mRNA levels. Actin was used as an internal control. (B) Mes 23.5 cells were treated with DMSO or rotenone at the indicated concentrations for 12 h. After rotenone treatment, cells were harvested and immunoblotted for sestrin2 protein levels. (C) Cells were pretreated with PFT-α (10 μM) for 1 h and treated with rotenone for an additional 12 h. Cells were harvested and immunoblotted for detection of sestrin2 and Bax protein levels. Actin was used as a loading control. (D) TP53 was knocked down by transient transfection with TP53 siRNA for 48 h. Control cells were transfected with control siRNA. TP53 knockdown cells then were treated with DMSO or the indicated concentrations of rotenone for 12 h. Cells were harvested and immunoblotted for detection of TP53 and sestrin2. The band density of sestrin2, Bax, and TP53 relative to that of actin was analyzed by densitometry. Values are the means ± SEM from three independent experiments. *, P < 0.05; **, P < 0.01.
Sestrin2 upregulation contributed to autophagy induction.
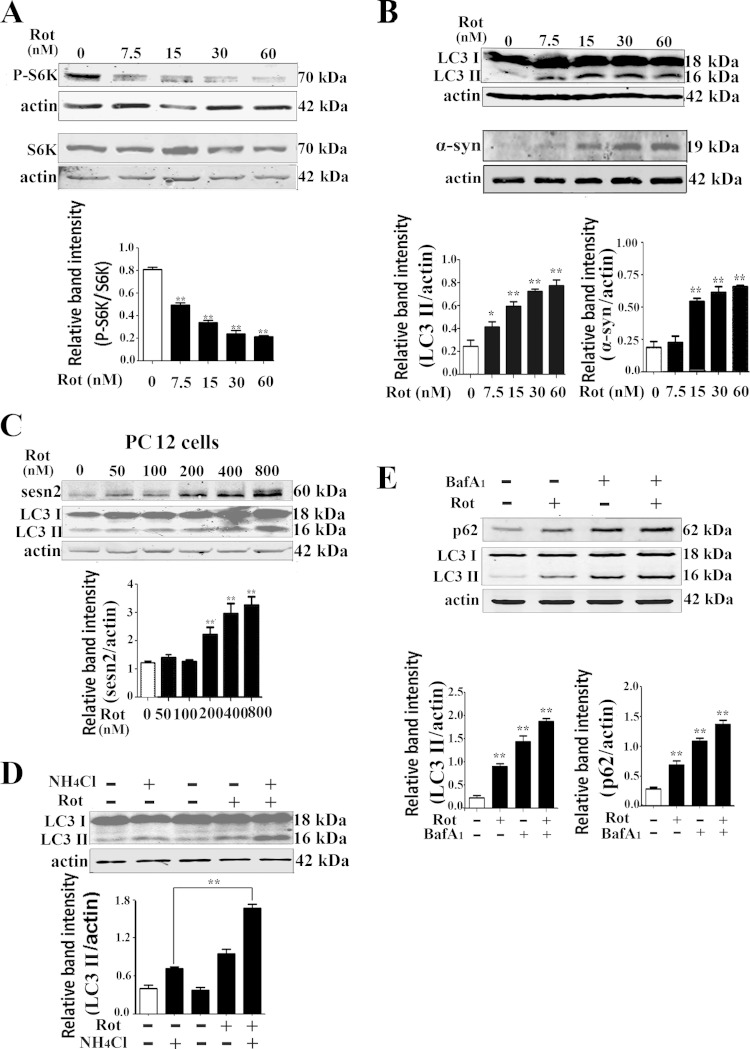
A previous report showed that 6-OHDA (6-hydroxydopamine) triggered a progressive reduction of mTOR activity (19), and we postulated that rotenone, an environmental toxin, would trigger the same response in a dopaminergic neuronal cell line. Our results showed that the phosphorylated p70S6K, a substrate of mTOR, was significantly diminished by rotenone treatment (Fig. 2A). Sestrin2 is a regulator of mTOR in response to environmental stress. Downregulation of sestrin2 could suppress autophagy induced by various stimuli (34). Thus, we asked if sestrin2 upregulation contributed to maintaining autophagy activity in the rotenone PD model. Rotenone caused significant increases in α-synuclein protein levels (Fig. 2B) and LC3-II protein levels in both Mes 23.5 and PC12 cells (Fig. 2B and C). To elucidate if the increase in LC3-II was due to dysfunction of late-stage autophagy activity, NH4Cl, a lysosomotropic compound elevating/neutralizing the lysosomal/vacuolar pH and blocking the lysosomal degradation, was used to monitor the turnover of LC3. The results showed that the rotenone-induced elevation in LC3-II level was further increased after treatment with NH4Cl (Fig. 2D). Bafilomycin A1, a vacuolar type H+-ATPase (V-ATPase) inhibitor that blocks lysosomal function, also was used in the present study. Similarly, rotenone-induced elevation in LC3-II level was increased further after the treatment with bafilomycin A1. Meanwhile, the levels of p62 protein, a ubiquitin-binding scaffold protein that binds directly to LC3 and is itself degraded by autophagy, also was monitored. Rotenone caused an increase in p62 protein level, and the effect also was strengthened by bafilomycin A1 (Fig. 2E); however, the mRNA levels of p62 were not significantly increased in rotenone (data not shown).
FIG 2.
Autophagy was enhanced after rotenone treatment. (A) Mes 23.5 cells were treated with rotenone at the indicated concentrations, and immunoblotting detected phosphorylated and total levels of p70S6K (Thr389). (B) After rotenone treatment, cell extracts were used for immunoblotting LC3 and α-synuclein proteins. (C) PC12 cells were treated with rotenone for 12 h and immunoblotted for detection of sestrin2 and LC3 protein levels. (D) Mes 23.5 cells were treated with DMSO and 15 nM rotenone, respectively, and harvested for detection of LC3 levels. Two hours before harvesting, NH4Cl (ammonium chloride, 10 mM) was added to block autophagic degradation. (E) Mes 23.5 cells were treated with DMSO or 15 nM rotenone for 12 h and harvested for detection of LC3 and p62 levels. Two hours before harvesting, 100 nM bafilomycin A1 was added to block autophagic degradation. Actin was used as a loading control. Band density of phosphorylated levels relative to total levels of p70S6K and band density of sestrin2, p62, or LC3-II relative to actin were analyzed with ImageJ and Prism software. Values are the means ± SEM from three independent experiments. *, P < 0.05; **, P < 0.01.
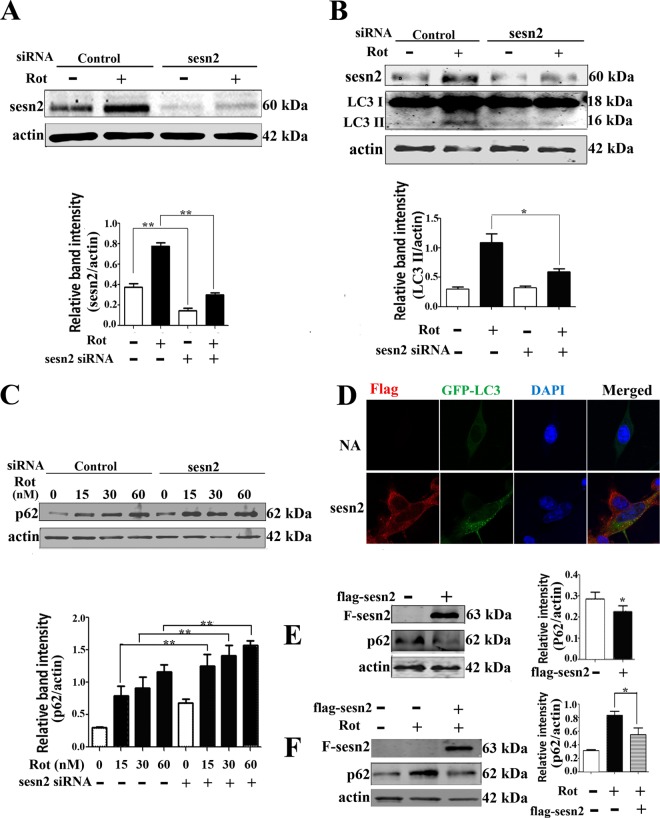
Using siRNA specific to sestrin2, we successfully lowered the sestrin2 protein level and abrogated sestrin2 upregulation induced by rotenone (Fig. 3A). In accordance with our hypothesis, sestrin2 knockdown significantly blocked the upregulation of LC3-II levels (Fig. 3B). Sestrin2 knockdown also significantly aggravated the rotenone-induced accumulation of p62 (Fig. 3C). Sestrin2 expression vectors next were constructed and cotransfected with GFP-LC3. Fluorescence imaging showed an increase in GFP-LC3 patches in sestrin2-overexpressing cells compared to a diffuse distribution in the control cells (Fig. 3D). Immunoblotting results showed decreased p62 levels (Fig. 3E) and alleviated rotenone-induced p62 accumulation (Fig. 3F) in sestrin2-overexpressing cells compared to the level of the control. These results demonstrated that sestrin2 upregulation contributed to autophagy enhancement in our in vitro PD model.
FIG 3.
Sestrin2 upregulation contributed to autophagy enhancement. (A) Sestrin2 was knocked down by transient transfection with sestrin2 siRNA for 48 h. Control cells were transfected with control siRNA. After sestrin2 knockdown, cells were treated with 20 nM rotenone and immunoblotted for sestrin2 protein. (B and C) Sestrin2 knockdown cells and control cells were treated with 20 nM rotenone and immunoblotted for LC3 (B) and p62 (C). (D) Florescent detection of LC3 (green) after cotransfecting Flag-sestrin2 plasmids (250 ng/well) with pcDNA4 GFP-LC3 plasmids for 48 h. Cells treated with equal amounts of empty Flag plasmids but no Flag-sestrin2 plasmids were used as controls. Spotted distribution of GFP indicated enhanced autophagy activity. DAPI, 4′,6-diamidino-2-phenylindole. NA, negative control. (E) Cells were transiently transfected with control or Flag-sestrin2 plasmids for 48 h, and Flag and p62 protein levels were detected. (F) Cells were transfected with control or Flag-sestrin2 plasmids for 48 h and then treated with rotenone for an additional 12 h for detection of a change in p62 protein levels. Actin was used as a loading control. Protein band density of p62 relative to that of actin was analyzed by densitometry. Values are the means ± SEM from three independent experiments. *, P < 0.05; **, P < 0.01.
Sestrin2 upregulation enhanced autophagy through phosphorylation of AMPK.
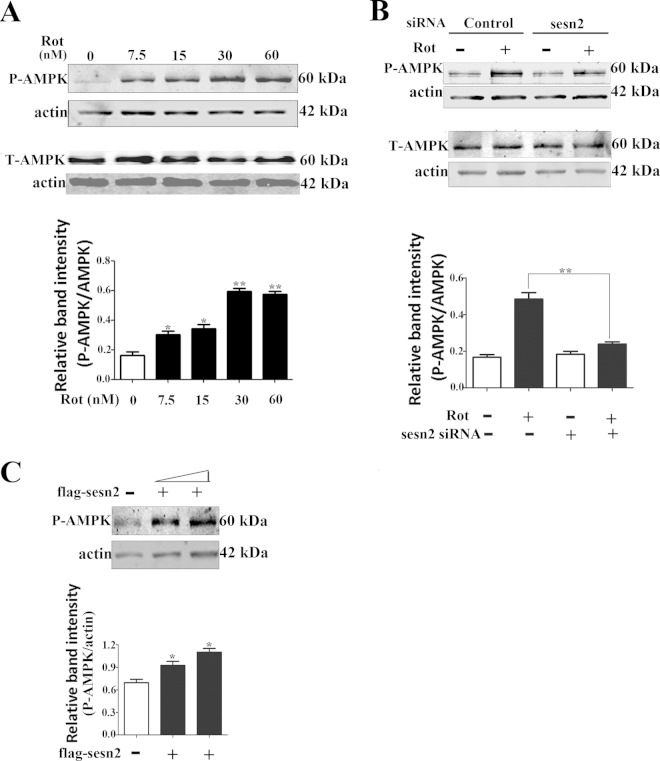
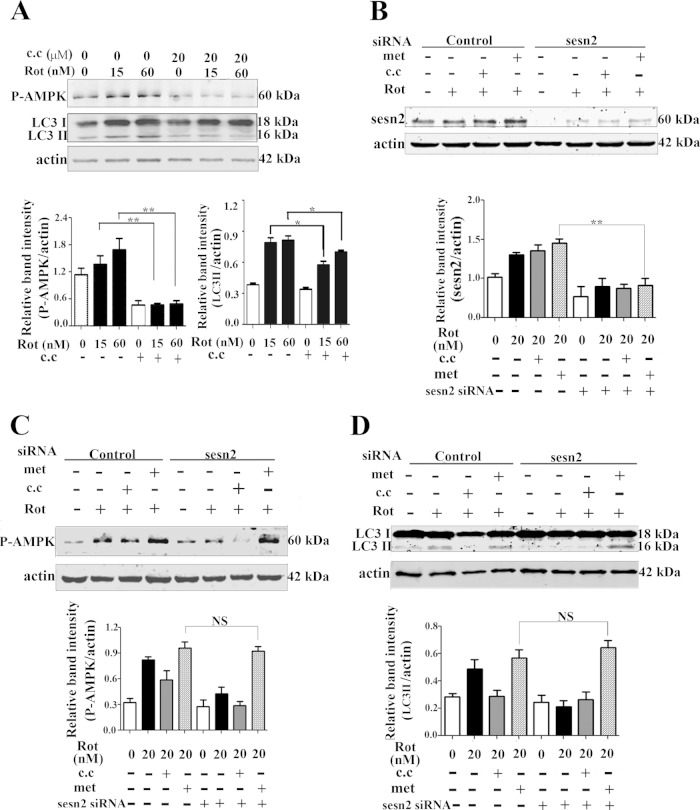
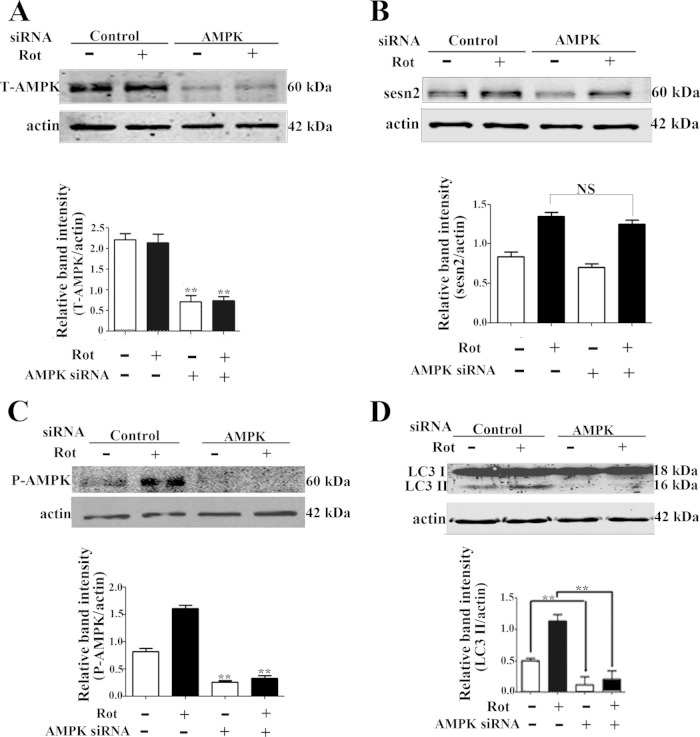
Sestrin2 forms a complex with AMPK, and AMPK activation is required for the negative regulation of mTOR by sestrin2 (27). In accordance with a previous report (35), the present results showed that AMPK was activated by rotenone, evidenced by significant upregulation of phosphorylated AMPK levels (Thr172) but not total AMPK levels (Fig. 4A). We tested if AMPK activation was influenced by sestrin2. The results showed that the basal AMPK phosphorylation was not significantly affected by knockdown of sestrin2, which might be explained as a proposed complicated network for maintaining AMPK homeostasis (36). Sestrin2 knockdown significantly compromised AMPK activation induced by rotenone, with no significant influence on total AMPK levels (Fig. 4B). In contrast, sestrin2 overexpression caused significant AMPK activation in Mes 23.5 cells (Fig. 4C). The role of AMPK activation on autophagy was determined with two drugs, the widely used AMPK inhibitor compound C and the activator metformin. Compound C treatment significantly blocked both AMPK activation, evidenced by downregulation of phosphorylated levels of AMPK (Thr172), and autophagy enhancement by rotenone, evidenced by decreased levels of LC3-II, suggesting a role for AMPK in rotenone-induced autophagy activation (Fig. 5A). Metformin, an AMPK activator, did not significantly influence the levels of sestrin2 (Fig. 5B); however, it reactivated AMPK after sestrin2 knockdown (Fig. 5C). Furthermore, metformin reactivated autophagy to a basal level after sestrin2 knockdown (Fig. 5D). Finally, we constructed siRNA specifically targeted to AMPK α1/α2 catalytic subunits. Both the total AMPK levels and AMPK activation were diminished by AMPK siRNA (Fig. 6A and C). The upregulation of sestrin2 by rotenone showed no significant difference with or without AMPK siRNA (Fig. 6B); however, the autophagy upregulation was robustly blocked by AMPK knockdown (Fig. 6D).
FIG 4.
Sestrin2 upregulation contributed to AMPK activation. (A) Cells were treated with rotenone and immunoblotted for phosphorylated AMPK (Thr172) and total AMPK. (B) After sestrin2 knockdown, cells were treated with 20 nM rotenone and immunoblotted for phosphorylated AMPK (Thr172) and total AMPK (T-AMPK). (C) After sestrin2 overexpression, cells were immunoblotted for phosphorylated AMPK (Thr172). Band density of phosphorylated levels of AMPK (P-AMPK) relative to that of actin was analyzed by densitometry. Values are the means ± SEM from three independent experiments. *, P < 0.05; **, P < 0.01.
FIG 5.
Sestrin2 regulated autophagy through AMPK. (A) The effects of compound C (c.c) on phosphorylated AMPK (P-AMPK) and LC3. Cells were treated with rotenone (15 and 60 nM) alone or rotenone combined with compound C (20 μM) for 12 h and then immunoblotted for LC3 and phosphorylated AMPK (Thr172). (B to D) Cells were transiently transfected with control siRNA or sestrin2 siRNA for 48 h. Cells were incubated with rotenone (20 nM) alone or rotenone (20 nM) combined with 20 μM compound C or 2 mM metformin (met), respectively, for an additional 12 h. Cells were harvested and immunoblotted for sestrin2 protein (B), phosphorylated AMPK (Thr172) (C), and LC3 (D). Protein band density of sestrin2, P-AMPK, and LC3-II protein levels relative to that of actin was analyzed by densitometry. Values are the means ± SEM from three independent experiments. NS, nonsignificant; *, P < 0.05; **, P < 0.01.
FIG 6.
AMPK knockdown attenuated autophagy enhancement. AMPK was knocked down by transient transfection with AMPK siRNA for 48 h. Control cells were transfected with control siRNA. After AMPK knockdown, cells were treated with DMSO or 20 nM rotenone for an additional 12 h and immunoblotted for total AMPK (T-AMPK) (A), sestrin2 (B), phosphorylated AMPK (Thr172) (C), and LC3 (D). Values are the means ± SEM from three independent experiments. NS, nonsignificant; *, P < 0.05; **, P < 0.01.
Sestrin2 upregulation increased α-synuclein degradation and inhibited caspase 3 activation.
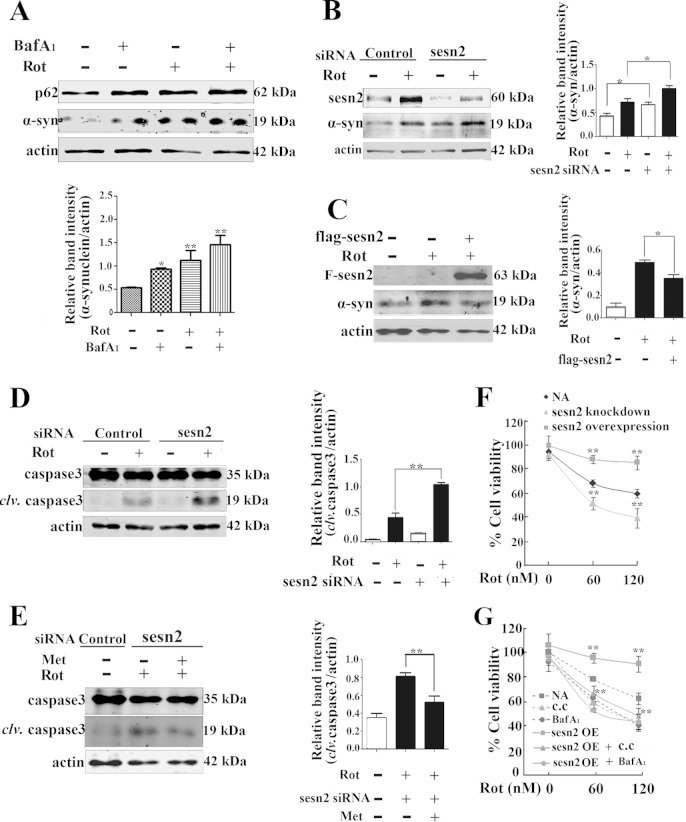
In accordance with previous reports (9, 37), the autophagy inhibitor bafilomycin A1 (bafA1), a V-ATPase inhibitor that causes an increase in lysosomal/vacuolar pH, led to a significant accumulation of α-synuclein (Fig. 7A). We further assessed if sestrin2 was involved in the degradation of α-synuclein. As expected, results showed that genetic knockdown of sestrin2 alone caused an accumulation of α-synuclein and severely aggravated the accumulation of α-synuclein induced by rotenone (Fig. 7B), while sestrin2 overexpression alleviated α-synuclein accumulation (Fig. 7C).
FIG 7.
Sestrin2 upregulation enhanced α-synuclein degradation and inhibited caspase 3 activation. (A) Mes 23.5 cells were treated with 20 nM rotenone for 10 h and then with 100 nM bafilomycin A1 for an additional 2 h. Cells were harvested and immunoblotted for p62 and α-synuclein. (B) After sestrin2 knockdown, cells were incubated with rotenone for an additional 12 h and then were harvested and immunoblotted for sestrin2 and α-synuclein. (C) After sestrin2 overexpression, cells were incubated with rotenone for an additional 12 h. Cells were harvested and immunoblotted for Flag and α-synuclein. (D) After sestrin2 knockdown, cells were treated with 20 nM rotenone for an additional 24 h and immunoblotted for caspase 3. clv, cleaved. (E) After sestrin2 knockdown, cells were treated with rotenone for 20 h and then with metformin (2 mM) for an additional 4 h. Cells were harvested and immunoblotted for caspase 3. (F) After sestrin2 knockdown or sestrin2 overexpression, cells were treated with rotenone for an additional 12 h. Cell viability was assessed with an MTT assay. (G) Cells were treated with rotenone alone or rotenone combined with compound C (20 μM) for 12 h or rotenone for 10 h and bafilomycin A1 (100 nM) for an additional 2 h. Cell viability was assessed with MTT assay. After sestrin2 overexpression (OE), cells were subjected to the same treatment, and cell viability was assessed with MTT assay. Values are the means ± SEM from three independent experiments. *, P < 0.05; **, P < 0.01.
A previous report showed that rotenone influenced cell viability through a mechanism not fully elucidated and might involve apoptosis (38). The influence of sestrin2 knockdown and overexpression on activation of caspase 3 was investigated in the present study. Results showed that after 24 h of incubation with or without rotenone, sestrin2 knockdown alone caused the activation of caspase 3 and aggravated the rotenone-induced activation of caspase 3 (Fig. 7D). Metformin, an AMPK activator, which abrogated the blockage of autophagy by sestrin2 knockdown, also could partially block activation of caspase 3 after sestrin2 knockdown (Fig. 7E). Finally, using MTT assay, we verified the influence of sestrin2 on cell viability. Twelve hours after rotenone treatment, sestrin2 knockdown reduced the cell viability to 40% of the control at a high rotenone dose (120 nM) (Fig. 7F). In contrast, sestrin2 overexpression preserved cell viability. However, the protective effects of sestrin2 overexpression on cell viability were attenuated by treatment with compound C or bafilomycin A1 (Fig. 7G). These results verified that sestrin2 protects cells against rotenone toxicity through AMPK-dependent autophagy activation.
DISCUSSION
Aging is one of the most prominent susceptible factors of PD (39, 40). Sestrins are members of a stress-responsive protein family, transcriptionally regulated by TP53 and FoxO (40, 41). Acting as an antiaging protein, sestrin2 is connected to reactive oxygen species (ROS) generation and is involved in many age-related disorders (42–44). Sestrin2 exerts its physiological function mainly through its negative regulation of mTOR (27, 33). Inhibition of mTOR by sestrin2 activated autophagy, which is also considered a mechanism that attenuates the aging process (45). The present study found that rotenone induced a robust increase in mRNA and protein levels of sestrin2. The increase in sestrin2 was TP53 dependent.
Sestrin2 upregulation maintained autophagy activity in rotenone PD model.
It is reasonable that in the early stage when the lysosome was not severely disrupted, autophagy enhancement might be a complementary response to sustain a normal degradation function with aging (8). The increases in autophagy biomarkers and a number of autophagosomes have been found in PD animal models or postmortem brain tissues (46, 47). It is now generally agreed that although the autophagy may be induced, the completion of the autophagy process is compromised in various PD models (9, 48, 49). Many studies showed that maintaining the level of autophagy might be helpful in PD (10). Moreover, elucidating pathways regulating autophagy activity might help to understand more pathogenic mechanisms of PD. Here, with a toxic rotenone model, we found that autophagy was induced with a concomitant increase in autophagy substrate p62 after rotenone exposure. Inhibition of lysosomal acidification further increased levels of LC3-II and p62. α-Synuclein is reported as an autophagy substrate. The present study found that protein levels of α-synuclein were significantly increased after rotenone treatment. In another independent study, we found that rotenone had no significant effect on α-synuclein mRNA (J.-J. Guan, L.-M. Fang, Y.-S. Hou, H.-D. Xu, F. Wu, J.-C. Wu, and Z.-H. Qin, unpublished observations). These data indicate that autophagy was induced initially in response to rotenone intoxication, but the autophagic degradation process was impaired. Genetic knockdown of sestrin2 abrogated autophagy induction and further increased the accumulation of autophagy substrates. Thus, upregulation of sestrin2 may be a mechanism for the cell to attempt the restoration of normal autophagy flux (50).
Our study suggested that sestrin2 upregulation was TP53 dependent. Rotenone is a toxicant that also was proposed to induce endoplasmic reticulum (ER) stress (51). As reports showed that in some circumstances TP53 was positively regulated by ER stress, which implies that sestrin2 upregulation is part of the mechanism that responds to ER stress (52) in the present study. This was in accordance with a previous report that ER stress induced sestrin2 upregulation and regulated autophagy (53).
AMPK activation was required for enhancement of autophagy by sestrin2.
Reports show that sestrin2 activates AMPK through upstream kinase LKB1 (liver kinase B1) (54, 55). AMPK is proposed to be a mediator of benefits in longevity and calorie restriction (56, 57). Activation of AMPK maintains survival of neurons through regulating mitochondrial biogenesis and preserving the energy balance. Recent reports proved that natural products, such as resveratrol and EGCG, exerted neuroprotective effects through activation of AMPK (58, 59). Although reports showed that sestrin2 modulated AMPK subunit expression (55) and was connected to regulation of Nox4 expression and ROS production by AMPK (60), the interplay between sestrin2/AMPK and autophagy was not fully elucidated. Here, we showed that rotenone induced an increase in phosphorylation of AMPK with a time course similar to that of sestrin2 upregulation. This study further proved, for the first time, that AMPK is required for the enhancement of autophagy, as pharmacological or genetic inhibition of AMPK blocked autophagy activation by sestrin2. We propose that sestrin2 is a positive upstream regulator of AMPK. However, the AMPK activator metformin slightly upregulated sestrin2 protein levels. This might be interpreted as a feedback regulatory mechanism. AMPK might receive signal from sestrin2 and pass it to downstream targets, including AKT, and finally back to sestrin2 (61). There are two separate pathways that contribute to regulation of mTOR by sestrins, the AMPK-TSC2 (tumor suppressor proteins that are associated with an autosomal dominant disorder known as tuberous sclerosis complex) axis and Rag GTPase (62). The AMPK-TSC2 pathway has been shown to function in ionizing radiation in breast cancer, hyperglycemia-induced glomerular injury, and quercetin-induced apoptosis, while the latter pathway recently was elucidated to function in sensing amino acid and mTOR lysosomal localization (54, 55). Although both pathways might function simultaneously, the present study showed that, in an in vitro rotenone PD model, sestrin2 could regulate autophagy through activation of AMPK. This study, however, did not exclude the possible contribution of other signaling pathways, including Rag GTPase, to autophagy activity in the rotenone PD model.
Sestrin2 was involved in degradation of α-synuclein.
It has been reported that α-synuclein is degraded by autophagy and the ubiquitin-proteasome system (UPS) (63–65). Our results proved a role of sestrin2 in α-synuclein degradation for the first time. It is evidenced that there are both mono- and oligosynucleins in SN pars compacta (SNPc) in PD patients, which may be a pathological cause of neuronal death in Parkinsonism (66). Genetic and nongenetic approaches showed that α-synuclein is toxic, and genetic overexpression of wide-type or mutant α-synuclein caused PD in both in vivo and in vitro models (3, 4). Many studies suggest that there is an impairment of autophagy flux in cellular and in vivo PD models (48, 67, 68). The failure in maintaining proper autophagy activity causes accumulation of misfolded proteins, including α-synuclein and damaged mitochondria (69, 70), and finally causes dysfunction and degeneration of dopaminergic neurons. The present study demonstrated that upregulation of sestrin2 in response to rotenone was responsible for maintenance of autophagy activity to a certain level. Thus, sestrin2 helps dopaminergic neurons to degrade aggregate-prone proteins and remove damaged cellular organelles. For further support of a prosurvival role of sestrin2, the present study demonstrated that overexpression of sestrin2 or pharmacological activation of AMPK inhibited caspase 3 activation and rotenone-induced cytotoxicity.
In summary, our present study demonstrated that sestrin2 was elevated and was involved in the maintenance of autophagy activity after rotenone treatment; sestrin2 knockdown or AMPK inhibition decreased autophagy activity and aggravated rotenone-induced α-synuclein accumulation and cell death. Further uncovering the role of sestrin2 in α-synuclein degradation and cell viability may lead to a novel strategy for PD treatment.
ACKNOWLEDGMENTS
This study was funded by the State Key Basic Science Project (973 Project; 2011CB510003), the Natural Science Foundation of China (no. 31300888), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PADA), Jiangsu Innovation Fund for Aging Research, and the Jiangsu Science Innovation Fund (CXLX12_0852).
We have no actual or potential conflicts of interest to declare.
REFERENCES
- 1.Dauer W, Przedborski S. 2003. Parkinson's disease: mechanisms and models. Neuron 39:889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 2.Kahle PJ, Haass C, Kretzschmar HA, Neumann M. 2002. Structure/function of alpha-synuclein in health and disease: rational development of animal models for Parkinson's and related diseases. J Neurochem 82:449–457. doi: 10.1046/j.1471-4159.2002.01020.x. [DOI] [PubMed] [Google Scholar]
- 3.Decressac M, Mattsson B, Lundblad M, Weikop P, Bjorklund A. 2012. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of alpha-synuclein in midbrain dopamine neurons. Neurobiol Dis 45:939–953. doi: 10.1016/j.nbd.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Xilouri M, Vogiatzi T, Vekrellis K, Park D, Stefanis L. 2009. Aberrant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PLoS One 4:e5515. doi: 10.1371/journal.pone.0005515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood SJ, Wypych J, Steavenson S, Louis JC, Citron M, Biere AL. 1999. Alpha-synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson's disease. J Biol Chem 274:19509–19512. [DOI] [PubMed] [Google Scholar]
- 6.Luk KC, Song C, O'Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM. 2009. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A 106:20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shacka JJ, Roth KA, Zhang J. 2008. The autophagy-lysosomal degradation pathway: role in neurodegenerative disease and therapy. Front Biosci 13:718–736. doi: 10.2741/2714. [DOI] [PubMed] [Google Scholar]
- 8.Nixon RA. 2013. The role of autophagy in neurodegenerative disease. Nat Med 19:983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- 9.Dehay B, Martinez-Vicente M, Caldwell GA, Caldwell KA, Yue Z, Cookson MR, Klein C, Vila M, Bezard E. 2013. Lysosomal impairment in Parkinson's disease. Mov Dis 28:725–732. doi: 10.1002/mds.25462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E. 2009. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. J Neurosci 29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. 2006. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 12.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. 2004. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 13.Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. 2006. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci U S A 103:5805–5810. doi: 10.1073/pnas.0507436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Zhang X, Le W. 2008. Altered macroautophagy in the spinal cord of SOD1 mutant mice. Autophagy 4:290–293. doi: 10.4161/auto.5524. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto N, Nagai M, Ohta Y, Miyazaki K, Kurata T, Morimoto M, Murakami T, Takehisa Y, Ikeda Y, Kamiya T, Abe K. 2007. Increased autophagy in transgenic mice with a G93A mutant SOD1 gene. Brain Res 1167:112–117. doi: 10.1016/j.brainres.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 16.Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Bjorklund A. 2013. TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proc Natl Acad Sci U S A 110:E1817–E1826. doi: 10.1073/pnas.1305623110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, Rubinsztein DC. 2004. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 18.Lafay-Chebassier C, Paccalin M, Page G, Barc-Pain S, Perault-Pochat MC, Gil R, Pradier L, Hugon J. 2005. mTOR/p70S6k signalling alteration by Abeta exposure as well as in APP-PS1 transgenic models and in patients with Alzheimer's disease. J Neurochem 94:215–225. doi: 10.1111/j.1471-4159.2005.03187.x. [DOI] [PubMed] [Google Scholar]
- 19.Malagelada C, Ryu EJ, Biswas SC, Jackson-Lewis V, Greene LA. 2006. RTP801 is elevated in Parkinson brain substantia nigral neurons and mediates death in cellular models of Parkinson's disease by a mechanism involving mammalian target of rapamycin inactivation. J Neurosci 26:9996–10005. doi: 10.1523/JNEUROSCI.3292-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santini E, Heiman M, Greengard P, Valjent E, Fisone G. 2009. Inhibition of mTOR signaling in Parkinson's disease prevents L-DOPA-induced dyskinesia. Sci Signal 2:ra36. [DOI] [PubMed] [Google Scholar]
- 21.Tain LS, Mortiboys H, Tao RN, Ziviani E, Bandmann O, Whitworth AJ. 2009. Rapamycin activation of 4E-BP prevents Parkinsonian dopaminergic neuron loss. Nat Neurosci 12:1129–1135. doi: 10.1038/nn.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Kundu M, Viollet B, Guan KL. 2011. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL. 2013. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol 15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. 2011. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egan D, Kim J, Shaw RJ, Guan KL. 2011. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 7:643–644. doi: 10.4161/auto.7.6.15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan T, Rawal P, Wu Y, Xie W, Jankovic J, Le W. 2009. Rapamycin protects against rotenone-induced apoptosis through autophagy induction. Neuroscience 164:541–551. doi: 10.1016/j.neuroscience.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Budanov AV, Karin M. 2008. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JH, Budanov AV, Talukdar S, Park EJ, Park HL, Park HW, Bandyopadhyay G, Li N, Aghajan M, Jang I, Wolfe AM, Perkins GA, Ellisman MH, Bier E, Scadeng M, Foretz M, Viollet B, Olefsky J, Karin M. 2012. Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell Metab 16:311–321. doi: 10.1016/j.cmet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bae SH, Sung SH, Oh SY, Lim JM, Lee SK, Park YN, Lee HE, Kang D, Rhee SG. 2013. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab 17:73–84. doi: 10.1016/j.cmet.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Soloway SB. 1976. Naturally occurring insecticides. Environ Health Perspect 14:109–117. doi: 10.1289/ehp.7614109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherer TB, Kim JH, Betarbet R, Greenamyre JT. 2003. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol 179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- 32.Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudiere E, Foretz M, Viollet B, Diolez P, Bastin J, Benit P, Rustin P, Carling D, Sandri M, Ventura-Clapier R, Pende M. 2007. S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metab 5:476–487. doi: 10.1016/j.cmet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Budanov AV, Lee JH, Karin M. 2010. Stressin' Sestrins take an aging fight. EMBO Mol Med 2:388–400. doi: 10.1002/emmm.201000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maiuri MC, Malik SA, Morselli E, Kepp O, Criollo A, Mouchel PL, Carnuccio R, Kroemer G. 2009. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle 8:1571–1576. doi: 10.4161/cc.8.10.8498. [DOI] [PubMed] [Google Scholar]
- 35.Choi JS, Park C, Jeong JW. 2010. AMP-activated protein kinase is activated in Parkinson's disease models mediated by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Biochem Biophys Res Commun 391:147–151. doi: 10.1016/j.bbrc.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Spasic MR, Callaerts P, Norga KK. 2009. AMP-activated protein kinase (AMPK) molecular crossroad for metabolic control and survival of neurons. Neuroscientist 15:309–316. doi: 10.1177/1073858408327805. [DOI] [PubMed] [Google Scholar]
- 37.Klucken J, Poehler AM, Ebrahimi-Fakhari D, Schneider J, Nuber S, Rockenstein E, Schlotzer-Schrehardt U, Hyman BT, McLean PJ, Masliah E, Winkler J. 2012. Alpha-synuclein aggregation involves a bafilomycin A 1-sensitive autophagy pathway. Autophagy 8:754–766. doi: 10.4161/auto.19371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filomeni G, Graziani I, De Zio D, Dini L, Centonze D, Rotilio G, Ciriolo MR. 2012. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: possible implications for Parkinson's disease. Neurobiol Aging 33:767–785. doi: 10.1016/j.neurobiolaging.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. 1999. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976-1990. Neurology 52:1214–1220. doi: 10.1212/WNL.52.6.1214. [DOI] [PubMed] [Google Scholar]
- 40.de Rijk MC, Breteler MM, Graveland GA, Ott A, Grobbee DE, van der Meche FG, Hofman A. 1995. Prevalence of Parkinson's disease in the elderly: the Rotterdam study. Neurology 45:2143–2146. doi: 10.1212/WNL.45.12.2143. [DOI] [PubMed] [Google Scholar]
- 41.Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. 2004. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 42.Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. 2009. The TOR pathway comes of age. Biochim Biophys Acta 1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460:392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JH, Bodmer R, Bier E, Karin M. 2010. Sestrins at the crossroad between stress and aging. Aging 2:369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergamini E. 2006. Autophagy: a cell repair mechanism that retards ageing and age-associated diseases and can be intensified pharmacologically. Mol Aspects Med 27:403–410. doi: 10.1016/j.mam.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y. 1997. Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease. Histol Histopathol 12:25–31. [PubMed] [Google Scholar]
- 47.Yu WH, Dorado B, Figueroa HY, Wang L, Planel E, Cookson MR, Clark LN, Duff KE. 2009. Metabolic activity determines efficacy of macroautophagic clearance of pathological oligomeric alpha-synuclein. Am J Pathol 175:736–747. doi: 10.2353/ajpath.2009.080928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dehay B, Bove J, Rodriguez-Muela N, Perier C, Recasens A, Boya P, Vila M. 2010. Pathogenic lysosomal depletion in Parkinson's disease. J Neurosci 30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janda E, Isidoro C, Carresi C, Mollace V. 2012. Defective autophagy in Parkinson's disease: role of oxidative stress. Mol Neurobiol 46:639–661. doi: 10.1007/s12035-012-8318-1. [DOI] [PubMed] [Google Scholar]
- 50.Giordano S, Lee J, Darley-Usmar VM, Zhang J. 2012. Distinct effects of rotenone, 1-methyl-4-phenylpyridinium and 6-hydroxydopamine on cellular bioenergetics and cell death. PLoS One 7:e44610. doi: 10.1371/journal.pone.0044610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goswami P, Gupta S, Biswas J, Joshi N, Swarnkar S, Nath C, Singh S. 28 November 2014. Endoplasmic reticulum stress plays a key role in rotenone-induced apoptotic death of neurons. Mol Neurobiol doi: 10.1007/s12035-014-9001-5. [DOI] [PubMed] [Google Scholar]
- 52.Jeong EK, Kim SJ, Oh Y, Kim H, Lee YS, Kwon BS, Park S, Park KC, Yoon KS, Kim SS, Ha J, Kang I, Choe W. 2014. p53 negatively regulates Pin1 expression under ER stress. Biochem Biophys Res Commun 454:518–523. doi: 10.1016/j.bbrc.2014.10.101. [DOI] [PubMed] [Google Scholar]
- 53.Bruning A, Rahmeh M, Friese K. 2013. Nelfinavir and bortezomib inhibit mTOR activity via ATF4-mediated sestrin-2 regulation. Mol Oncol 7:1012–1018. doi: 10.1016/j.molonc.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eid AA, Lee DY, Roman LJ, Khazim K, Gorin Y. 2013. Sestrin 2 and AMPK connect hyperglycemia to Nox4-dependent endothelial nitric oxide synthase uncoupling and matrix protein expression. Mol Cell Biol 33:3439–3460. doi: 10.1128/MCB.00217-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanli T, Linher-Melville K, Tsakiridis T, Singh G. 2012. Sestrin2 modulates AMPK subunit expression and its response to ionizing radiation in breast cancer cells. PLoS One 7:e32035. doi: 10.1371/journal.pone.0032035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Canto C, Auwerx J. 2011. Calorie restriction: is AMPK a key sensor and effector? Physiology 26:214–224. doi: 10.1152/physiol.00010.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merry BJ. 2002. Molecular mechanisms linking calorie restriction and longevity. Int J Biochem Cell Biol 34:1340–1354. doi: 10.1016/S1357-2725(02)00038-9. [DOI] [PubMed] [Google Scholar]
- 58.Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T. 2011. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease. Neuro-Signals 19:163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ng CH, Guan MS, Koh C, Ouyang X, Yu F, Tan EK, O'Neill SP, Zhang X, Chung J, Lim KL. 2012. AMP kinase activation mitigates dopaminergic dysfunction and mitochondrial abnormalities in Drosophila models of Parkinson's disease. J Neurosci 32:14311–14317. doi: 10.1523/JNEUROSCI.0499-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zimmermann A, Bachmann C, Baumgartner R. 1986. Severe liver fibrosis in argininosuccinic aciduria. Arch Pathol Lab Med 110:136–140. [PubMed] [Google Scholar]
- 61.Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, Karin M. 2010. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng M, Yin N, Li MO. 2014. Sestrins function as guanine nucleotide dissociation inhibitors for Rag GTPases to control mTORC1 signaling. Cell 159:122–133. doi: 10.1016/j.cell.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. 2003. Alpha-synuclein is degraded by both autophagy and the proteasome. J Biol Chem 278:25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 64.Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, Rockenstein E, Masliah E, Hyman BT, McLean PJ, Unni VK. 2011. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of alpha-synuclein. J Neurosci 31:14508–14520. doi: 10.1523/JNEUROSCI.1560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engelender S. 2012. Alpha-synuclein fate: proteasome or autophagy? Autophagy 8:418–420. doi: 10.4161/auto.19085. [DOI] [PubMed] [Google Scholar]
- 66.Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, Hetzer C, Loher T, Vilar M, Campioni S, Tzitzilonis C, Soragni A, Jessberger S, Mira H, Consiglio A, Pham E, Masliah E, Gage FH, Riek R. 2011. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci U S A 108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dehay B, Ramirez A, Martinez-Vicente M, Perier C, Canron MH, Doudnikoff E, Vital A, Vila M, Klein C, Bezard E. 2012. Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc Natl Acad Sci U S A 109:9611–9616. doi: 10.1073/pnas.1112368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D. 2012. Deficiency of ATP13A2 leads to lysosomal dysfunction, alpha-synuclein accumulation, and neurotoxicity. J Neurosci 32:4240–4246. doi: 10.1523/JNEUROSCI.5575-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ebrahimi-Fakhari D, McLean PJ, Unni VK. 2012. Alpha-synuclein's degradation in vivo: opening a new (cranial) window on the roles of degradation pathways in Parkinson disease. Autophagy 8:281–283. doi: 10.4161/auto.8.2.18938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ. 2012. The role of autophagy in Parkinson's disease. Cold Spring Harbor Perspect Med 2:a009357. [DOI] [PMC free article] [PubMed] [Google Scholar]