Abstract
Purpose
To evaluate safety (primary endpoint), tolerability, pharmacokinetics, pharmacodynamic profile, and preliminary activity of the intravenous, pan-class I isoform PI3K/mTOR inhibitor PF-05212384 in patients with advanced solid tumors.
Experimental Design
Part 1 of this open-label phase I study was designed to estimate the maximum tolerated dose (MTD) in patients with non-selected solid tumors, using a modified continual reassessment method to guide dose escalation. Objectives of Part 2 were MTD confirmation and assessment of preliminary activity in patients with selected tumor types and PI3K pathway dysregulation.
Results
Seventy-seven of the 78 enrolled patients received treatment. The MTD for PF-05212384, administered intravenously once weekly, was estimated to be 154 mg. The most common treatment-related adverse events (AEs) were mucosal inflammation/stomatitis (58.4%), nausea (42.9%), hyperglycemia (26%), decreased appetite (24.7%), fatigue (24.7%), and vomiting (24.7%). The majority of patients treated at the MTD experienced only grade 1 treatment-related AEs. Grade 3 treatment-related AEs occurred in 23.8% of patients at the MTD. No treatment-related grade 4–5 AEs were reported at any dose level. Antitumor activity was noted in this heavily pretreated patient population, with two partial responses (PR) and an unconfirmed PR. Eight patients had long-lasting stable disease (>6 months). Pharmacokinetic analyses showed a biphasic concentration-time profile for PF-05212384 (half-life, 30–37 hours after multiple dosing). PF-05212384 inhibited downstream effectors of the PI3K pathway in paired tumor biopsies.
Conclusions
These findings demonstrate the manageable safety profile and antitumor activity of the PI3K/mTOR inhibitor PF-05212384, supporting further clinical development for patients with advanced solid malignancies.
Keywords: PF-05212384, PI3K, mTOR, solid tumors, PI3K/TORC1/2, AKT
Introduction
The PI3K-AKT-mTOR pathway plays a central role in regulating tumor cell metabolism and survival (1–3). Among the three PI3K classes, class I PI3K-mediated signaling contributes to cancer cell growth and homeostasis. Class I PI3K consists of a regulatory subunit and a catalytic subunit, with four different catalytic isoforms (p110α, p110β, p110γ, and p110δ) (3–4). Activated PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3). This reaction induces a signaling cascade that leads to phosphorylation of intracellular mediators such as AKT and mTORC1. The PI3K pathway is negatively regulated by the phosphatase and tensin homologue deleted on chromosome 10 (PTEN). PTEN converts PIP3 into PIP2 and thereby decreases activation of the PI3K pathway (1–4).
The PI3K pathway is commonly deregulated in cancer. Several alterations may lead to pathway activation, including PIK3CA mutation or amplification, PTEN mutation and loss of function, AKT mutation, and receptor tyrosine kinase overexpression or mutation. Activation of the PI3K pathway may represent a mechanism of resistance to treatment with tyrosine kinase inhibitors (TKIs) or chemotherapeutic agents (1–3).
PF-05212384 is an intravenous (IV), ATP-competitive, highly selective and potent pan-class I isoform PI3K and mTOR inhibitor (5) with an IC50 of 0.4nM for p110α, 6 nM for p110β, 6 nM for p110γ, 8 nM for p110δ, and 1 nM for mTOR. Preclinical studies have demonstrated activity of PF-05212384 in in vitro cell assays and in vivo xenograft models (5). Preclinical safety and pharmacologic evaluation of PF-05212384 did not show any significant effect on cardiac, central nervous system or respiratory function.
Here we report the safety, tolerability, pharmacokinetics (PK), pharmacodynamic (PD) profile, and preliminary activity of PF-05212384 in patients with advanced solid tumors.
Methods and Patients
Study design and treatment
This open-label phase I study of PF-05212384 was conducted at eight centers (one in Spain, one in the United Kingdom, six in the United States) and divided into two parts. Part 1 estimated the maximum tolerated dose (MTD) in patients with unselected solid tumors (MTD estimation phase). The starting dose of PF-05212384 was 10 mg, administered once weekly as an IV infusion over 30 minutes in 28-day cycles. No premedication was required. Additional doses initially ranged from 21 mg to 154 mg once weekly, with further escalation in 20% increments over 154 mg if the lower doses appeared tolerable. A modified continual reassessment method (CRM) was used to guide dose escalation for each cohort, with the final choice of dose being determined based on the CRM guidance, as well as other safety considerations. Treatment was continued until disease progression, if tolerated by the patient and deemed of clinical benefit by the investigator.
Patients were assessed for dose-limiting toxicity (DLT) during the first 28 days of treatment. DLTs, defined by investigator assessment as potentially related to study treatment, included a ≥ grade 3 non-hematologic adverse event (AE) despite optimal treatment, including fasting glucose >250 mg/dL or ≥ grade 3 asthenia >2 days; ≥ grade 4 thrombocytopenia, grade 3 thrombocytopenia with bleeding, grade 4 neutropenia for >7 days, febrile neutropenia, or a delay of treatment for more than 2 consecutive weeks due to treatment-related toxicity.
In Part 2 (MTD confirmation phase), the MTD was confirmed in two distinct patient cohorts. The Molecular Selection cohort (MTD1) enrolled patients to further define tolerability of PF-05212384 at the MTD, and to assess preliminary activity in patients with selected tumor types and documented evidence of dysregulation of the PI3K pathway (PIK3CA mutation, PIK3CA amplification or PTEN deficiency). The Tumor Biopsy cohort (MTD2) included at least five evaluable patients with baseline and on-treatment tumor biopsies to evaluate the effect of PF-05212384 at the MTD on the PI3K pathway.
All patients in the dose escalation should have had disease evaluable for response. All the patients in the MTD cohorts were required to have at least one measurable lesion at baseline. CT scans and MRI were the preferred method for following tumor burden, and it was recommended that the same modality be used throughout the duration of the trial. Tumor assessments were performed in all patients at screening, every eight weeks during treatment, and at the end-of-treatment visit.
The primary endpoint of the study was the overall safety profile of single-agent PF-05212384 as characterized by AEs including DLTs. Secondary endpoints included single- and multiple-dose PK parameters of PF-05212384, corrected QT (QTc) interval (Fridericia correction), changes in serum glucose, C-peptide, and insulin blood levels, effects of PF-05212384 on PI3K-related pathways (e.g., phosphorylated AKT [pAKT], pS6, pS6K, pmTOR, p4EBP-1, and pPRAS40) in tumor biopsies, and objective tumor response. QTc was included among endpoints as a standard assessment.
Approval was obtained from the ethics committees at the participating institutions and regulatory authorities. All patients gave written informed consent. The study followed the Declaration of Helsinki and good clinical practice guidelines. The trial was sponsored by Pfizer and registered at ClinicalTrials.gov (NCT 00940498).
Patient selection
Patients were included in the study if they were >18 years of age and had histologically or cytologically confirmed malignant solid tumors unresponsive to approved therapies or without further treatment options. Diagnosis of any solid tumor was allowed for patients enrolled in Part 1 of the study. For inclusion in the MTD1 cohort, patients were required to have a diagnosis of breast, non–small-cell lung, ovarian, endometrial, renal, colorectal cancer or glioblastoma, and measurable disease by RECIST 1.1. Tumors had to harbor features suggestive of PI3K pathway activation (including mutation and/or amplification of PIK3CA and/or PTEN deficiency) in fresh or archived tumor biopsy samples. Availability of baseline and after-dosing tumor biopsies was required for inclusion of patients with any solid tumor in the MTD2 cohort. Patients had to have Eastern Cooperative Oncology Group (ECOG) performance score (PS) 0 to 1, adequate bone marrow, renal, and liver function, fasting glucose <126 mg/dL, and hemoglobin A1c (HbA1c) <7%.
Key patient exclusion criteria included prior chemotherapy, radiation therapy, investigational or other antineoplastic therapy within 2 weeks of first PF-05212384 infusion (6 weeks for mitomycin C or nitrosureas), high-dose chemotherapy requiring stem cell transplant, prior treatment with a PI3K and/or mTOR targeting agent, diabetes, a QTc interval greater than 470 ms, pregnancy or breastfeeding, or any significant medical illness that would increase the patient’s risk associated with study participation.
Assessments
Safety
Assessments included physical examination, vital signs measurements, 12-lead electrocardiograms (ECG), and laboratory evaluations. AEs were graded for severity using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Pharmacokinetics
PK analyses were performed in both the MTD estimation phase (Part 1) and the MTD confirmation phase (Part 2) of the study. Plasma samples were analyzed for PF-05212384 concentrations at Bioanalytical Laboratory PharmaNet USA, Inc (Princeton, NJ). PF-05212384 samples were assayed using a validated liquid chromatography electrospray ionization tandem mass spectrometric method. Calibration standard responses were linear over the range of 2 ng/mL to 2000 ng/mL by linear square regression. Plasma PF-05212384 PK parameters were calculated for each patient and treatment dose using noncompartmental analysis of plasma concentration-time data.
Antitumor activity
Objective tumor responses were determined using Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1). Complete response (CR) or partial response (PR) status was confirmed no less than 4 weeks after first determination. Objective tumor response and clinical benefit response (CBR) were included in the efficacy analysis. CBR was defined as CR, PR, or stable disease (SD) lasting ≥6 months.
Pharmacodynamics
Serum levels of fasting insulin, C-peptide, and glucose were measured at screening, on day 2 of cycle 1, and day 1 of all subsequent cycles, and analyzed by PF-05212384 dose level. Pre-treatment formalin-fixed, paraffin-embedded (FFPE) tumor tissue or unstained slides were collected at screening for assessment of exploratory biomarkers. A second biopsy (FFPE) was taken on day 22 of cycle 1 in patients enrolled to MTD2. Tumor tissue samples were analyzed for markers of PI3K pathway activation as well as gene abnormalities, such as PI3KCA mutation and amplification. For determination of PI3K/mTOR inhibition biomarkers, biopsies were analyzed using reverse phase protein microarrays (RPMA). Fluorescent signals were normalized to cytokeratin (NFC) to account for varying percentages of malignant cells in the matched biopsies. Biomarkers tested at baseline and at day 22 of cycle 1 in paired tumor biopsies included the phosphorylated and total forms of AKT S473, AKT T308, FKHR T24, FKHRL1 T32, and STAT3. Evaluation of PIK3CA dysregulation included amplification and mutation. PTEN protein levels were measured by immunohistochemistry (IHC).
Statistical analysis
A modified CRM (6) targeting a 25% DLT rate was used for estimation of the MTD in Part 1 of the study. A one-parameter hyperbolic tangent model was used for the modified CRM. The recommended dose by the modified CRM was the one closest to, but not above the estimated MTD, subject to the restriction that the maximum allowed dose increase was 107%. The planned cohort size was two to four patients. The MTD estimation phase continued until at least nine patients had been treated at a dose level that was estimated to be the MTD or the maximum sample size of 50 patients was reached for Part 1. The safety analysis set included all patients who had received at least one dose of PF-05212384. This analysis set was used for summaries of demographic, safety, and efficacy data. Geometric mean and coefficient of variation were determined for area under the curve (AUC)tau, AUCinf, AUClast, maximum plasma concentration, systemic clearance, and volume of distribution at steady state; median and range were determined for time to maximum plasma concentration; and arithmetic mean and standard deviation for terminal half-life and for changes from baseline in PD biomarkers.
Results
Patient characteristics and treatment
A total of 78 patients were enrolled in the study and 77 received treatment with PF-05212384. One enrolled patient did not receive treatment due to uncontrolled disease-related pain. Baseline demographics and clinical characteristics are presented in Table 1. The mean patient age was 53.8 years; 38 patients were male and 39 female. The vast majority of patients had ECOG PS 0 or 1 (98.7%) and measurable disease (94.8%). The most frequent primary tumor was colorectal cancer, in 19 patients (24.7%). The majority of patients had received prior surgery and radiation therapy, as well as systemic antineoplastic treatments; 58 patients (75.4%) had received three or more systemic treatment regimens.
Table 1.
Patient baseline demographics and clinical characteristics
| Parameter | PF-05212384 (N = 77) |
|---|---|
| Mean age, years (range) | 53.8 (21–75) |
| Male:female | 38:39 |
| Race, n (%) | |
| White | 74 (96.1) |
| Asian | 1 (1.3) |
| Other | 2 (2.6) |
| ECOG PS, n (%) | |
| 0 | 32 (41.6) |
| 1 | 44 (57.1) |
| 2 | 1 (1.3) |
| Measurable disease, n (%) | 73 (94.8) |
| Primary tumor, n (%) | |
| Breast cancer | 4 (5.2) |
| Endometrial cancer | 4 (5.2) |
| Glioblastoma | 3 (3.9) |
| Malignant melanoma | 2 (2.6) |
| Esophageal carcinoma | 2 (2.6) |
| Ovarian cancer | 5 (6.5) |
| Pancreatic carcinoma | 3 (3.9) |
| Colorectal cancer | 19 (24.5) |
| Non–small-cell lung cancer | 6 (7.8) |
| Salivary gland cancer | 2 (2.6) |
| Other (1 type each) | 27 (35) |
| Prior surgeries, n (%) | 70 (90.9) |
| Prior radiation therapy, n (%) | 46 (59.7) |
| Prior systemic therapy, n (%) | 76 (98.7) |
| 1 regimen | 9 (11.7) |
| 2 regimens | 9 (11.7) |
| 3 regimens | 23 (29.9) |
| >3 regimens | 35 (45.5) |
ECOG, Eastern Cooperative Oncology Group; PS, performance score.
In Part 1 of the study (MTD estimation phase), four patients each were treated with a once-weekly IV infusions of 10 mg, 21 mg, 43 mg, and 89 mg of PF-05212384. The dose was then escalated in successive cohorts (consisting of four patients) to 154 mg, 222 mg, 266 mg, and 319 mg. Once it was determined that 319 mg was above the MTD, additional patients (3–4 patient cohorts) were treated in successive de-escalating cohorts until it was determined that 154 mg was the MTD. Overall, a total of twelve, seven, eight, and four patients were treated with a 154 mg, 222 mg, 266 mg, and 319 mg, respectively. The 154 mg group (henceforth referred to as the MTD group) included a total of 42 patients; 12 patients in the MTD estimation phase and 30 patients in the MTD confirmation phase. The MTD confirmation phase included 15 patients in the molecular selection cohort (MTD1) and 15 patients in the biopsy cohort (MTD2).
Among the 12 patients experiencing DLT, grade 3 mucosal inflammation or stomatitis were the most common events, which were observed in seven patients at doses between 154 mg and 319 mg (Table 2). Two patients had grade 3 rash (266 mg and 319 mg groups) and two patients had elevations in liver enzymes (154 mg and 222 mg groups). One patient experienced grade 3 hyperglycemia (266 mg group) (Table 2). All DLTs resolved during the study. The estimated MTD and recommended phase 2 dose for PF-05212384 was 154 mg, administered as a once-weekly IV infusion.
Table 2.
Dose-limiting toxicities by dose level of PF-05212384
| Dose level (mg) |
N. of DLT- evaluable patients/N. of treated patients |
N (%) of patients with DLTs |
DLTs |
|---|---|---|---|
| 10 | 3/4 | 0 | None |
| 21 | 4/4 | 0 | None |
| 43 | 3/4 | 0 | None |
| 89 | 4/4 | 0 | None |
| 154 | 40/42 | 2 (5.0) | Mucosal inflammation (n = 1) AST/ALT levels increased (n = 1) |
| 222 | 7/7 | 5 (71.4) | Mucosal inflammation (n = 3) ALT levels increased (n = 1) Stomatitis (n = 1) |
| 266 | 8/8 | 3 (37.5) | Mucosal inflammation (n = 1) Hyperglycemia (n = 1) Rash (n = 1) |
| 319 | 4/4 | 2 (50.0) | Mucosal inflammation (n = 1) Rash (n = 1) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DLT, dose-limiting toxicity.
The median number of dosing days for this weekly regimen ranged from 7 days to 18 days (range, 1 to 48 days) across dose levels. The median, relative dose intensity was 100% for each dose level, with the exception of the 222 mg dose level with a dose intensity of 66.7%. The majority of patients (49; 63.6%) discontinued treatment because of disease progression. In general, toxicities were manageable and reversible as only three patients (3.9%) discontinued PF-05212384 due to treatment-related AEs (grade 3 mucosal inflammation at the 222 mg and 319 mg dose levels, and grade 2 ALT elevation at the 222 mg dose level).
Safety
All treated patients on study (n = 77) experienced at least 1 AE. The most common, all-cause AEs were mucosal inflammation and stomatitis (59.7%), nausea (55.8%), decreased appetite (37.7%), vomiting (35.1%), fatigue (32.5%), hyperglycemia (28.6%), asthenia (26%), diarrhea (24.7%), constipation (23.4%), cough (23.4%), pyrexia (23.4%), rash (23.4%), back pain (19.5%), dyspnea (19.5%), anemia (18.2%), and increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (16.9%). The majority of the AEs reported in the MTD group (dose escalation plus MTD cohorts; n = 42) were grades 1 and 2. Nine deaths occurred on study and within 28 days of last-dose administration; seven were due to disease progression, one to colon obstruction, and one to pneumonia.
The most common treatment-related AEs in all patients were mucosal inflammation and stomatitis (58.4%), nausea (42.9%), hyperglycemia (26%), decreased appetite (24.7%), fatigue (24.7%), and vomiting (24.7%). The majority of patients enrolled in the MTD group experienced only grade 1 treatment-related AEs. The most common treatment-related AEs of any grade observed in the MTD group were mucosal inflammation and stomatitis (54.7%), nausea (40.5%), hyperglycemia (26.2%), vomiting (23.8%), asthenia (21.4%), decreased appetite (21.4%), and fatigue (21.4%) (Table 3). Treatment-related AEs reported in two or more patients treated at dose levels below or above the estimated MTD are listed in Supplemental Tables 1 and 2. Most of the treatment-related AEs were grade 1 and were manageable with standard medication (mouthwash and pain medication, anti-nausea and anti-diabetic treatment for mucosal inflammation, nausea/vomiting, and hyperglycemia management, respectively).
Table 3.
Incidence and severity of treatment-related adverse events in ≥5% of patients treated at the maximum tolerated dose
| AE^ (N = 42) |
Grade 1 n (%) |
Grade 2 n (%) |
Grade 3 n (%) |
All Grades n (%) |
|---|---|---|---|---|
| Mucosal inflammation and stomatitis | 19 (45.2) | 1 (2.4) | 3 (7.1) | 23 (54.7) |
| Nausea | 15 (35.7) | 1 (2.4) | 1 (2.4) | 17 (40.5) |
| Hyperglycemia | 7 (16.7) | 3 (7.1) | 1 (2.4) | 11 (26.2) |
| Vomiting | 8 (19.0) | 1 (2.4) | 1 (2.4) | 10 (23.8) |
| Asthenia | 3 (7.1) | 5 (11.9) | 1 (2.4) | 9 (21.4) |
| Decreased appetite | 6 (14.3) | 3 (7.1) | 0 | 9 (21.4) |
| Fatigue | 8 (19.0) | 1 (2.4) | 0 | 9 (21.4) |
| Dysgeusia | 8 (19.0) | 0 | 0 | 8 (19.0) |
| AST increased | 1 (2.4) | 3 (7.1) | 2 (4.8) | 6 (14.3) |
| Diarrhea | 6 (14.3) | 0 | 0 | 6 (14.3) |
| Dermatitis acneiform and rash | 6 (14.3) | 0 | 0 | 6 (14.3) |
| ALT increased | 1 (2.4) | 1 (2.4) | 3 (7.1) | 5 (11.9) |
| Pyrexia | 4 (9.5) | 0 | 1 (2.4) | 5 (11.9) |
| Dry mouth | 4 (9.5) | 0 | 0 | 4 (9.5) |
| Hypertriglyceridemia | 4 (9.5) | 0 | 0 | 4 (9.5) |
| Constipation | 3 (7.1) | 0 | 0 | 3 (7.1) |
| Dehydration | 0 | 3 (7.1) | 0 | 3 (7.1) |
| Dry skin | 3 (7.1) | 0 | 0 | 3 (7.1) |
| Hypercholesterolemia | 3 (7.1) | 0 | 0 | 3 (7.1) |
| Lymphopenia | 1 (2.4) | 2 (4.8) | 0 | 3 (7.1) |
No grade 4 and grade 5 treatment-related AEs were reported.
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
At the MTD, grade 3 treatment-related AEs were noted in 23.8% of patients, and the most frequently reported (>1 patient) included mucosal inflammation and stomatitis (n = 3; 7.1%), increased ALT (n = 3; 7.1%), and increased AST (n = 2; 4.8%) (Table 3). No treatment-related AEs of grade 4 or 5 severity were reported at any dose level (Table 3 and Supplemental Tables 1 and 2).
Laboratory abnormalities requiring dose reduction or treatment interruption included increases in blood alkaline phosphatase, AST or ALT levels, hyperbilirubinemia, hyperglycemia, hypokalemia, anemia, and hypomagnesemia.
The majority of patients treated with PF-05212384 in the MTD group had normal QTc intervals (Fridericia correction); eight (19%) patients experienced a grade 1 and one patient (2.4%) experienced a grade 3 QTc prolongation. The patient with grade 3 QTc prolongation had a medical history of hypertension and had received PF-05212384 154 mg, eight days before the event. At the time of the QTc prolongation, the patient was also experiencing grade 1 hypokalemia and grade 1 hypomagnesemia. Furthermore, in addition to QTc prolongation, the patient’s ECG showed a negative T wave, considered unrelated to study therapy and suggestive of myocardial ischemia. Since metabolic abnormalities and myocardial ischemia are known causes of QTc prolongation and because of the likely, relatively low serum concentration of PF-05212384 at the time of the event (eight days following the most recent dose), it is unlikely that the observed QTc prolongation was related to the study drug. Overall, in the 19 patients treated above the MTD, there was one patient (5%) each with grade 1 and grade 2 QTc prolongation, and there were no patients with grade 3 QTc prolongation. Based on the lack of a dose-effect relationship, PF-05212384 is likely not associated with QTc prolongation.
Pharmacokinetics
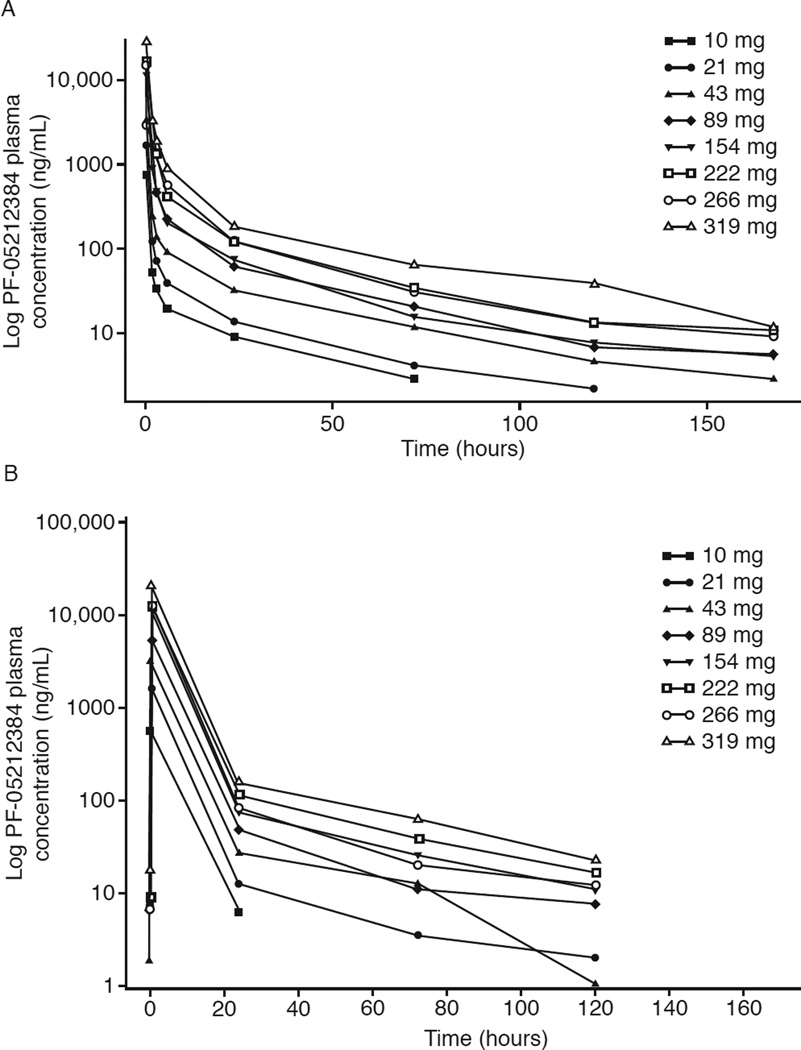
Median plasma concentrations of PF-05212384 after single-dose administration in cycle 1 declined rapidly in the first 4 to 24 hours and more slowly in the subsequent 25 to 168 hours (Fig. 1A). Maximum concentrations (Cmax) and exposure (AUC) appeared to increase proportionally with increasing dose across most treatment groups. In the MTD group, interpatient variability in Cmax and AUC after single-dose administration was low to moderate. Terminal T1/2 was similar across all dose levels with mean values of 33 to 41 hours, and a mean value of approximately 36 hours in the MTD group (Supplemental Table 3).
Figure 1.
A. Plasma mean concentration-time profiles of PF-05212384 after a single intravenous dose
B. Plasma mean concentration-time profiles of PF-05212384 after multiple intravenous dosing
Determination of PF-05212384 plasma levels in cycle 2, after multiple-dose administration, showed a similar, biphasic concentration-time profile, with a slower decline in concentrations starting 24 hours post-infusion (Fig. 1B). Cmax and AUC showed dose-proportional increases over the 10 to 319 mg dose range. At the MTD dose level, interpatient variability was 54% for AUC and 64% for Cmax. Terminal T1/2 had mean values of 30 to 37 hours across treatment groups, as previously noted with single-dose treatment, and of approximately 36 hours in the MTD group. Evaluation of accumulation ratios for Cmax showed no accumulation with repeated, weekly dosing of PF-05212384 (Supplemental Table 4).
Antitumor activity of PF-05212384
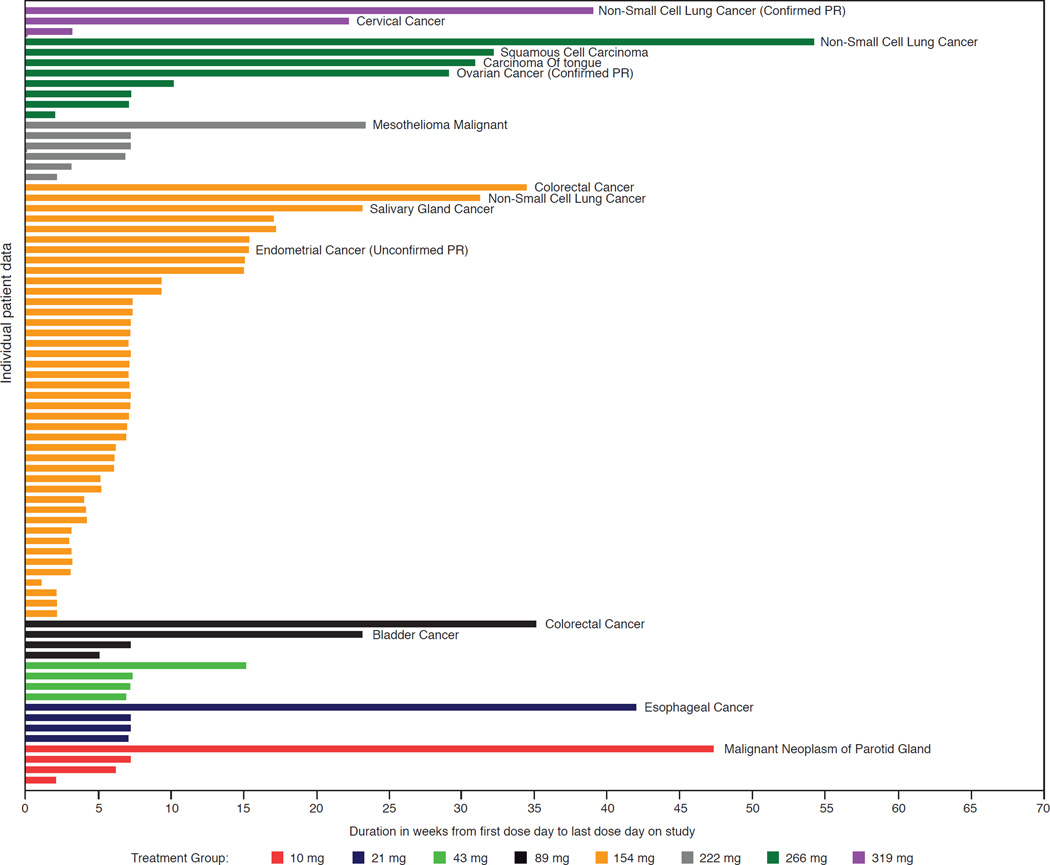
Single-agent treatment with PF-05212384 demonstrated activity in the patients with advanced and extensively pretreated solid malignancies evaluated in this study. Two patients had a PR, leading to an objective tumor response rate of 2.6% in the overall study population. A third patient had an unconfirmed PR (not included in the final analysis as a PR) (Supplemental Table 5). One of the responders had a diagnosis of non-small-cell lung cancer (NSCLC) with liver and lung metastases, and PTEN-null, mutant PIK3CA-negative status at baseline. Treatment was reduced from the initial 319-mg dose level to 239 mg on day 21 of cycle 1 and to 160 mg on day 22 of cycle 2; duration of treatment in this patient was 10 cycles (Fig. 2). The second responder had a diagnosis of granulosa cell tumor of the ovary with peritoneal and pleural metastases and PTEN-null status at baseline. The patient started treatment at the 266 mg dose level which was reduced to 197 mg on day 15 of cycle 1. The PIK3CA gene status was not evaluable in this patient, who maintained a partial response at the cycle 8 assessment (Fig. 2). The patient with unconfirmed PR had endometrial cancer with pulmonary and soft tissue metastases, and low PTEN expression. This patient experienced an 80% reduction in target lesions (cycle 4) following initial dosing at 222 mg and subsequent dose reduction to 115 mg. The patient went off study treatment for surgical resection of the symptomatic pelvic mass and the tumor response was not further confirmed. Overall, SD was noted in 27 patients (35.1%). Eight of these patients (10.4%) had SD lasting more than six months. The CBR was 13%.
Figure 2.
Duration of treatment with PF-05212384 in patients with advanced solid malignancies
In the 154 mg dose-escalation and expansion cohorts combined there were three out of 20, one out of 33, and 16 out of 34 patients with evaluable tumor data who had tumors harboring PIK3CA mutation, PIK3CA amplification or PTEN loss, respectively. There was no clear relationship between PIK3CA gene alterations and clinical benefit, although only four PIK3CA alterations were noted in the study. Although the two patients with PR treated at higher doses both had PTEN-null tumors, there was no overall relationship between PTEN protein status (IHC) and clinical outcome: among the 13 patients with tumors with PTEN loss in the 154-mg expansion cohort, 7/13 (54%) experienced SD and 6/13 (46%) experienced progressive disease as best response. Across all dose levels, of the eight patients with SD lasting > 6 months, four had tumors with a PIK3CA and/or PTEN alteration, whereas alterations were not identified in the remaining four patients with available FFPE specimens. Finally, within the 154 mg expansion cohort, there were nine patients who started at least three cycles of treatment, three of whom had tumors with no PI3K pathway abnormality among those that were assayed (Table 4).
Table 4.
PI3K pathway alterations in patients treated at the maximum tolerated dose who remained on-study for > 2 cycles
| Patient | PI3K amplification |
PI3K mutation |
PTEN status | Response |
|---|---|---|---|---|
| 1^ | No | Not evaluable | Absent | SD |
| 2 | No | Yes | Absent | SD |
| 3 | No | No | Present | SD |
| 4 | Not evaluable | No | Absent | SD |
| 5 | No | No | Present | SD |
| 6 | No | No | Absent | SD |
| 7 | Not evaluable | Not evaluable | Absent | SD |
| 8 | No | No | Present | SD |
| 9* | No | No | Absent | SD |
Last dose: day 8 of cycle 3;
last dose: day 7 of cycle 3.
Pharmacodynamic markers of PF-05212384 activity
PD biomarkers were measured in order to ascertain whether PF-05212384 blocked PI3K/mTOR signaling in human tumors. PF-05212384 induced a dose-dependent increase in blood glucose levels, with corresponding changes in C-peptide and insulin levels. Changes in blood glucose levels after single-dose administration of PF-05212384 at the MTD were maximal on day 2 of cycle 1 (Supplemental Table 6). Consistently, changes in insulin and C-peptide levels were observed following administration of PF-05212384, reaching maximal levels on day 2 of cycle 1. At the MTD, mean change in insulin levels versus baseline was an increase of 22.8 (standard deviation [SD], 25.68) milliunits (mU)/mL on day 2 of treatment. Increases in cholesterol and triglycerides levels were also reported, consistent with inhibition of the mTOR kinase by PF-05212384 (Table 3).
Analysis of the phosphorylated forms of AKT S473, AKT T308, FKHR T24, FKHRL1 T32, and STAT3 at baseline and at day 22 of cycle 1 in paired tumor biopsies from patients in the MTD group revealed a 10.6 reduction in pAKT S473 compared with baseline, as measured by fluorescence units normalized to cytokeratin (NFC) (Supplemental Table 7). Varying degrees of inhibition of the other activation biomarkers (decreased 3.0–56.9) were also noted in this analysis, indicating PF-05212384 treatment-induced inhibitory effects on the PI3K/mTOR signaling pathway.
Discussion
We report here the safety, tolerability, PK, PD, and preliminary efficacy results obtained with PF-05212384 in patients with advanced solid malignancies.
The MTD for PF-05212384, administered intravenously once weekly, was estimated to be 154 mg. PF-05212384 demonstrated a manageable toxicity profile. At the 154 mg dose level, most of the treatment-related AEs were mild to moderate. Some of the most common AEs observed, such as mucosal inflammation, hyperglycemia, and liver enzyme elevations, are known class-related effects (7–12). Hyperglycemia could be managed with anti-diabetes therapy. The nausea associated with the once-weekly IV drug administration was acute, rather than chronic as observed with oral agents, and manageable with pre-medication. Stomatitis was reversible with temporary treatment interruptions or dose reduction, and symptomatic treatment.
Analysis of PF-05212384 PK showed a biphasic concentration-time profile; the half-life ranged from 30 to 37 hours after multiple dosing. The concentrations achieved were above those leading to growth inhibition in preclinical models. Evaluation of the PF-05212384 PD profile showed hyperglycemia, as reported for other PI3K inhibitors (7). PF-05212384 treatment also induced increases in insulin and C-peptide levels, as observed with other PI3K inhibitors; furthermore, increases in cholesterol and triglyceride levels were consistent with mTOR kinase inhibition. Further analyses showed that PF-05212384 inhibited downstream mediators of the PI3K-pathway in tumor biopsies, specifically resulting in decreased levels of phosphorylated AKT and other effectors.
PF-05212384 has demonstrated antitumor activity in this patient population with heavily pretreated, solid malignancies. Both pre-clinical (13–16) and clinical (17–22) data support patient selection based on alterations in the PI3K pathway. Somatic mutations in PIK3CA may confer tumor susceptibility to selective PI3Kα inhibitors and to pan-isoform PI3K inhibitors (13–23). However, it is still unknown whether different mutations in the PI3K pathway would lead to different responses to treatment with PI3K inhibitors. Similarly, it is still unknown whether there is a threshold for PTEN expression that correlates with sensitivity to these agents or whether different mechanisms causing loss of PTEN expression would modulate or interfere with sensitivity to pan-isoform PI3K inhibitors, although it has been reported that PTEN deficiency may determine susceptibility to PI3Kβ inhibitors (3). Of note, both patients with a partial response to treatment with the pan-class I isoform PI3K/mTOR inhibitor PF-05212384 in this study had PTEN-null status and the patient with an unconfirmed partial response had low PTEN expression. The patient selection criteria in the MTD2 cohort did not incorporate other factors that could potentially represent mechanisms of resistance, such as the presence of KRAS mutations (19–20).
One of the key advantages of a dual, pan-isoform PI3K and mTOR inhibitor, such as PF-05212384, over other isoform-specific PI3K inhibitors, is the potential to treat tumors harboring oncogenic abnormalities that signal through different PI3K isoforms (3). The results of this study are comparable to those reported with other PI3K and PI3K/mTOR inhibitors in terms of toxicity and pathway inhibition (7, 8, 10, 24). IV once-weekly administration may be more convenient, as it may minimize gastrointestinal toxicity associated with chronic oral administration. It may be useful in patients with tumors causing dysphagia or interfering with intestinal absorption or bowel activity (e.g., patients with peritoneal carcinomatosis). In addition, it may be more readily combined with chemotherapy or targeted agents than oral, continuously dosed PI3K inhibitors. It is still being evaluated whether IV administration of PF-05212384 may achieve greater PI3K pathway inhibition, compared with oral daily treatment with other investigational agents. Further, it is still not known whether higher, intermittent PI3K inhibition, in combination with other targeted therapies, could achieve optimal pathway inhibition with minimal toxicity.
In conclusion, PF-05212384 demonstrated antitumor activity and a manageable safety profile, as well as modulation of PI3K/mTOR signaling biomarkers, in this population of heavily pretreated patients with solid malignancies. Clinical benefit was noted in patients with metastatic NSCLC, granulosa cell tumor of the ovary, and endometrial cancer. PF-05212384 is currently being further evaluated as a single agent in a phase II study in patients with endometrial cancer (NCT01420081). In addition, it is being investigated in phase I or II clinical trials in combination with chemotherapeutic agents, such as irinotecan, cisplatin, or docetaxel, and with targeted agents, such as the mitogen-activated/extracellular signal-regulated kinase (MEK) inhibitor PD-0325901 (NCT01925274; NCT01347866) or the pan-human epidermal growth factor receptor (HER) inhibitor dacomitinib (NCT01920061) (25, 26).
Supplementary Material
Statement of Translational Relevance.
The PI3K-AKT-mTOR pathway is frequently dysregulated in cancer cells and may underlie tumor resistance to targeted and cytotoxic agents. PF-05212384 is a potent, catalytic, pan-class I PI3K and mTORC1/2 inhibitor with broad antitumor activity in several preclinical models. In this first-in-human study we have determined the recommended dose for weekly administration of intravenous PF-05212384, based on its safety profile and pharmacodynamic activity. Treatment was associated with modulation of insulin and C-peptide levels in plasma, as well as evidence of PI3K pathway inhibition in paired tumor biopsies. Clinical activity was observed following treatment with PF-05212384, including two partial responses and long-lasting (>6 months) stable disease in eight of the 77 treated patients. These findings support the ongoing clinical development of PF-05212384 for patients with advanced solid malignancies.
Acknowledgments
Grant Support
J. Spicer acknowledges financial support from the UK Department of Health via the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust (and NIHR Clinical Research Facility). This study was sponsored by Pfizer. Medical writing support was provided by S. Mariani, MD, PhD, of Engage Scientific, Envision Pharma, and was funded by Pfizer.
Footnotes
Previously presented at the EORTC-NCI-AACR International Conference on Molecular Targets in Cancer Therapeutics; 2010; Berlin, Germany; and at the AACR-NCI-EORTC International Conference on Molecular Targets in Cancer Therapeutics; 2011; San Francisco, CA, USA.
References
- 1.Engelman JA. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 2.Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol. 2013;10:143–153. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 3.Brana I, Siu LL. Clinical development of phosphatidylinositol 3-kinase inhibitors for cancer treatment. BMC Med. 2012;10:161. doi: 10.1186/1741-7015-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 5.Mallon R, Feldberg LR, Lucas J, Chaudhary I, Dehnhardt C, Delos Santos E, et al. Antitumor efficacy of PKI-587, a highly potent dual PI3K/mTOR kinase inhibitor. Clin Cancer Res. 2011;17:3193–3203. doi: 10.1158/1078-0432.CCR-10-1694. [DOI] [PubMed] [Google Scholar]
- 6.Goodman SN, Zahurak ML, Piantadosi S. Some practical improvements in the Continual Reassessment Method for Phase 1 studies. Stat Med. 1995;14:1149–1161. doi: 10.1002/sim.4780141102. [DOI] [PubMed] [Google Scholar]
- 7.Bendell JC, Rodon J, Burris HA, De Jonge M, Verweij J, Birle D, et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 8.Hong DS, Bowles DW, Falchook GS, Messersmith WA, George GC, O'Bryant CL, et al. A multicenter phase I trial of PX-866, an oral irreversible phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4173–4182. doi: 10.1158/1078-0432.CCR-12-0714. [DOI] [PubMed] [Google Scholar]
- 9.Juric D, Rodon J, Gonzalez-Angulo AM, Burris HA, Bendell J, Berlin JD, et al. BYL719, a next generation PI3K alpha specific inhibitor: Preliminary safety PK, efficacy results from the first-in-human study. American Association for Cancer Research 103rd Annual Meeting; March 31–April 4, 2012; Chicago, IL, USA.. [Google Scholar]
- 10.Brana I, Lorusso P, Baselga J, Heath EI, Patnaik A, Gendreau S, et al. A phase I dose-escalation study of the safety, pharmacokinetics (PK), and pharmacodynamics of XL765 (SAR245409), a PI3K/TORC1/TORC2 inhibitor administered orally to patients (pts) with advanced malignancies. American Society of Clinical Oncology 46th Annual Meeting; June 1–5, 2010; Chicago, IL, USA.. [Google Scholar]
- 11.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 12.Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 13.Huang A, Fritsch C, Wilson C, Reddy A, Liu M, Lehar J, et al. Single agent activity of PIK3CA inhibitor BYL719 in a broad cancer cell line panel. American Association for Cancer Research 103rd Annual Meeting; March 31–April 4, 2012; Chicago, IL, USA.. [Google Scholar]
- 14.Jessen K, Kessler L, Kucharski J, Guo X, Staunton J, Janes M, et al. A potent and selective PI3K inhibitor, INK1117, targets human cancers harboring oncogenic PIK3CA mutations. AACR-NCI-EORTC: 23rd Molecular Targets and Cancer Therapeutics; November 12–16, 2011; San Francisco, CA, USA.. [Google Scholar]
- 15.Ihle NT, Lemos R, Jr, Wipf P, Yacoub A, Mitchell C, Siwak D, et al. Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumor activity of the inhibitor PX-866 whereas oncogenic ras is a dominant predictor for resistance. Cancer Res. 2009;69:143–150. doi: 10.1158/0008-5472.CAN-07-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 17.Janku F, Tsimberidou AM, Garrido-Laguna I, Wang X, Luthra R, Hong DS, et al. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther. 2011;10:558–565. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janku F, Wheler JJ, Westin SN, Moulder SL, Naing A, Tsimberidou AM, et al. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol. 2012;30:777–782. doi: 10.1200/JCO.2011.36.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodon J, Juric D, Gonzalez-Angulo AM, Bendell J, Berlin J, Bootle D, et al. Towards defining the genetic framework for clinical response to treatment with BYL719, a PI3Kalpha-specific inhibitor. AACR Annual Meeting; April 6–10, 2013; Washington DC, USA.. [Google Scholar]
- 20.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabernero J, Bell-McGuinn K, Bendell J, Molina J, Kwak E, Millham R, et al. First-in-patient study of PF-05212384, a small molecule intravenous dual inhibitor of PI3K and mTOR in patients with advanced cancer: update on safety, efficacy, and pharmacology. AACR-NCI-EORTC International Conference: Molecular Cancer Therapy; November 12–16, 2011; San Francisco, CA, USA.. [Google Scholar]
- 22.Shapiro GI, Molina J, Bendell J, Brana I, Spicer J, Kwak E, et al. First-in-human study of PF-05212384, a small-molecule, intravenous, dual inhibitor of PI3K and mTOR in patients with advanced cancer: preliminary report on safety and pharmacokinetics. Eur J Cancer. 2010;8(Supplement):123. [Google Scholar]
- 23.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro GI, Rodon J, Bedel C, Kwak EL, Baselga J, Braña I, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245408 (XL147), an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2014;20:233–245. doi: 10.1158/1078-0432.CCR-13-1777. [DOI] [PubMed] [Google Scholar]
- 25.Haura EB, Ricard AD, Larson TG, Stella PG, Bazhenova L, Miller VA, et al. A phase II study of PD-0325901, an oral MEK inhibitor, in previously treated patients with advanced non-small-cell lung cancer. Clin Cancer Res. 2010;16:2450–2457. doi: 10.1158/1078-0432.CCR-09-1920. [DOI] [PubMed] [Google Scholar]
- 26.Ramalingam SS, Blackhall F, Krzakwski M, Barrios CH, Park K, Bover I, et al. Randomized phase II study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2012;30:3337–3344. doi: 10.1200/JCO.2011.40.9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.