Summary
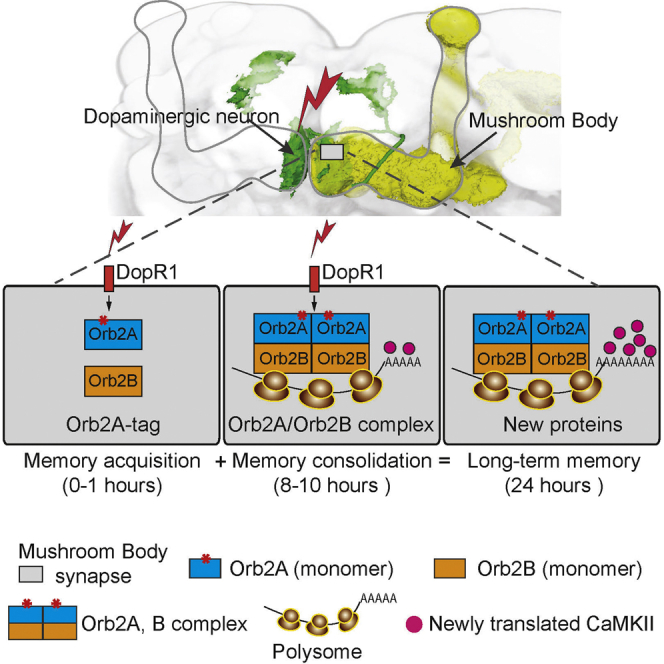
To adapt to an ever-changing environment, animals consolidate some, but not all, learning experiences to long-term memory. In mammals, long-term memory consolidation often involves neural pathway reactivation hours after memory acquisition. It is not known whether this delayed-reactivation schema is common across the animal kingdom or how information is stored during the delay period. Here, we show that, during courtship suppression learning, Drosophila exhibits delayed long-term memory consolidation. We also show that the same class of dopaminergic neurons engaged earlier in memory acquisition is also both necessary and sufficient for delayed long-term memory consolidation. Furthermore, we present evidence that, during learning, the translational regulator Orb2A tags specific synapses of mushroom body neurons for later consolidation. Consolidation involves the subsequent recruitment of Orb2B and the activity-dependent synthesis of CaMKII. Thus, our results provide evidence for the role of a neuromodulated, synapse-restricted molecule bridging memory acquisition and long-term memory consolidation in a learning animal.
Graphical Abstract
Highlights
-
•
Drosophila exhibits late long-term memory consolidation
-
•
DA-aSP13 neurons mediate both memory acquisition and memory consolidation
-
•
Drosophila CPEB, Orb2, acts downstream of DopR1 in the MB γ neurons
-
•
Synaptic Orb2A is required during memory acquisition and Orb2B during consolidation
Krüttner et al. demonstrate that, similar to mammals, invertebrates exhibit late memory consolidation mediated by neural pathways engaged earlier during memory acquisition. Late reactivation of a dopaminergic neuron class in Drosophila recruits Orb2B to form complexes in synapses tagged by Orb2A upon memory acquisition; these complexes regulate CaMKII translation.
Introduction
The brain rapidly learns environmental associations and behavioral contingencies but is selective about which lessons it commits to long-term memory. Evidence from multiple approaches has provided clues to how something learned becomes something remembered as well as how and where memories are stored. Neuromodulatory systems are thought to exert substantial control over whether a learning experience is memorable. Neuromodulatory pathways are activated during and/or after “important” behavioral moments for memory acquisition and memory consolidation (Plaçais et al., 2012; Rossato et al., 2009; Schwaerzel et al., 2003; Wise, 2004), and blocking them inhibits the formation of memories (Schwaerzel et al., 2003; Wise, 2004). Intriguingly, the process of memory consolidation often involves a critical period, sometimes many hours after the initial learning period, during which a reactivation of brain activity is required. In some cases, this reactivation involves a literal replay of a learning experience, either during the awake (Carr et al., 2011) or sleeping (Wilson and McNaughton, 1994) states.
Adult Drosophila flies exhibit remarkable behavioral complexity that can be modified by experience. They can learn to avoid or approach odors that were previously associated with an electric shock (Quinn et al., 1974) or with sugar reward (Tempel et al., 1984), respectively. Flies can also learn visual, tactile, and even spatial cues (Guo et al., 1996; Ofstad et al., 2011; Wustmann et al., 1996). A robust form of memory in flies is courtship conditioning, whereby naive males learn to preferentially court receptive virgin females after experiencing unsuccessful courtship of already mated (and therefore unreceptive) females. Depending on the learning experience, flies can form memories lasting from minutes to hours to several days (McBride et al., 1999; Siegel and Hall, 1979). However, the mechanisms that trigger production of long-term memory are not clear. Recently, it has been found that orchestrated activity of three clusters of dopaminergic neurons positively affect long-term memory formation during olfactory learning (Plaçais et al., 2012) and delayed activity of the specific dopaminergic neurons is critical for consolidation of the long-term appetitive memory (Musso et al., 2015).
Short-term memory can be mediated by a variety of protein-synthesis-independent mechanisms (Kandel, 2001). Long-term memories are thought to reflect protein-synthesis-dependent morphological and biochemical changes taking place in specific synapses within neuronal networks (Sutton and Schuman, 2006). Because more synapses in the brain are activated during memory acquisition than eventually might become consolidated, there must be mechanisms for determining which particular synapses will ultimately encode a given long-term memory. Furthermore, these mechanisms must be capable of maintaining such specificity during the interval between memory acquisition and its consolidation.
The synaptic tag and capture hypothesis (Frey and Morris, 1997; Martin et al., 2000) proposes how specific synapses come to store a given memory. The original experimental evidence in support of this hypothesis came from in vitro electrophysiological studies in hippocampal slices. Conversion of early long-term potentiation (E-LTP, an in vitro correlate of short-term memory) into L-LTP (a physiological correlate of long-term memory) at synapses activated by a strong stimulation after a weak one suggested that synapses activated during behavioral memory acquisition might be tagged for a protein-synthesis-dependent long-term consolidation. Behavioral studies in rodents demonstrated that training that elicits short-term memory can be consolidated into long-term memory by a novel experience capable of inducing protein synthesis (Moncada and Viola, 2007). Moreover, activation of the dopaminergic ventral tegmental area in rats after learning induces protein synthesis, which is required for long-term memory consolidation (Rossato et al., 2009). Nonetheless, the molecular mechanisms of synaptic tagging have not been identified within the contexts of specific neuronal pathways and learning animals.
Candidates for such a synaptic tag are members of the cytoplasmic polyadenylation element binding family of proteins (CPEB) (Si et al., 2003a). CPEB proteins can be divided into two subfamilies. The CPEB-I subfamily includes the Xenopus CPEB1 and its Drosophila ortholog Orb1, which both regulate mRNA translation during oogenesis (Mendez and Richter, 2001). Members of the CPEB-II subfamily have been found to function in synaptic plasticity (mCPEB2–4; Richter, 2001) or long-term memory formation (Drosophila Orb2; Keleman et al., 2007; Majumdar et al., 2012). Almost all CPEBs exist in multiple variants generated by alternative mRNA splicing (Theis et al., 2003; Wang and Cooper, 2009). Orb2 has two isoforms, which contain a glutamine-rich domain (Q domain), present also in some, but not all, CPEBs in other species (Hafer et al., 2011; Si et al., 2003a). Orb2A recruits Orb2B into complexes, essential for memory persistence, through its Q domain upon neuronal activation. After being recruited into complexes, Orb2B regulates translation through its RNA-binding domain (Krüttner et al., 2012; Majumdar et al., 2012). A protein network has been recently identified that links neuronal activity and the reactivity of Orb2A (White-Grindley et al., 2014). Together, these data suggest that Orb2 and its CPEB homologs are promising candidates to serve as a molecular bridge between memory acquisition and consolidation in a spatio-temporally specific manner upon dopaminergic modulation. However, this hypothesis has not been directly tested in behaving animals.
To investigate the mechanisms of long-term memory consolidation in Drosophila, we employed a courtship memory consolidation paradigm. Courtship learning can induce either short- or long-lived courtship memories, depending on the duration of the learning experience (McBride et al., 1999; Siegel and Hall, 1979). For example, exposing a Drosophila male to an unreceptive mated female for 5–7 hr leads to a long-term suppression of his courtship preferentially toward a mated, but not a virgin, female for at least 24 hr. We were able to prevent the expression of this long-term memory by blocking the aSP13 dopaminergic neurons several hours after their initial involvement during learning experience with a mated female (Keleman et al., 2012). Further, exposure to a mated female for only 1 hr results in short-term memory, but not long-term memory; however, by stimulating the same class of dopaminergic neurons, aSP13, many hours after this exposure, we were able to transform short-term memory into long-term memory. Having revealed that long-term memory consolidation requires late activation of the same class of dopaminergic neuron, aSP13, that hours earlier is necessary for memory acquisition, we explored it further using genetic, molecular, and behavioral analyses. We established that the DopR1 type of receptor, shown previously to be necessary for memory acquisition, is also required for late long-term memory consolidation in the mushroom body (MB) γ neurons. We determined that Orb2A is localized at synapses in the MB neurons and functions during memory acquisition to mark potentially specific MB neurons and specific synapses for eventual long-term memory consolidation. Upon subsequent late dopaminergic pathway activation, Orb2A recruits Orb2B into complex to regulate translation of CaMKII, a key protein involved in triggering memory persistence (Ashraf et al., 2006; Coultrap and Bayer, 2012).
Results
Dopaminergic Stimulation after Learning Is Sufficient to Consolidate Short-Term Memory into Long-Term Memory
We employed a paradigm for courtship memory consolidation to investigate the molecular basis and spatiotemporal relationships between the two key processes of long-term memory formation, memory acquisition and its consolidation, in the Drosophila male. We combined a learning experience sufficient to establish courtship short-term memory, but not long-term memory, with a subsequent stimulation of the dopaminergic pathways, which is thought to induce local protein synthesis at synapses (Smith et al., 2005). We starved naive males for 16 hr, trained them for 1 hr with mated female, and then activated dopamine pathways globally by feeding the animals with dopamine for 23 hr (Riemensperger et al., 2011). This resulted in robust long-term memory, in the form of a strong suppression of their courtship toward mated females in comparison to virgin females when tested 24 hr later. This memory was quantified as a learning index (LI), which measures the extent of the courtship suppression (Figure 1; Table S1). By contrast, training for 1 hr alone induced normal short-term memory, but not long-term memory, and dopaminergic activation alone did not induce long-term memory. Thus post-acquisition dopamine stimulation consolidates short-term memory into long-term memory.
Figure 1.
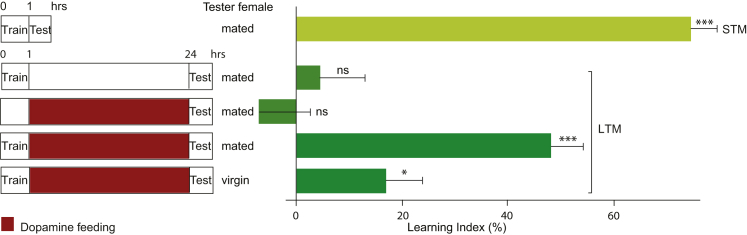
Post-learning Global Activation of Dopamine Pathways Is Sufficient to Consolidate Short-Term Memory into Long-Term Memory
The plots indicate mean learning indices ± SEM of the wild-type Canton-S males tested in single-pair assays with mated or virgin females, either 24 hr after being starved for 16 hr, trained with mated female for 1 hr, and fed with dopamine for 23 hr (if indicated; LTM; dark green bars) or immediately after training (STM; light green bar). p values are for H0 LI = 0; ∗p < 0.05; ∗∗∗p < 0.001. See Table S1.
Subset of PAM-DA Neurons, aSP13, Mediates Late Long-Term Memory Consolidation in a Protein-Synthesis-Dependent Manner
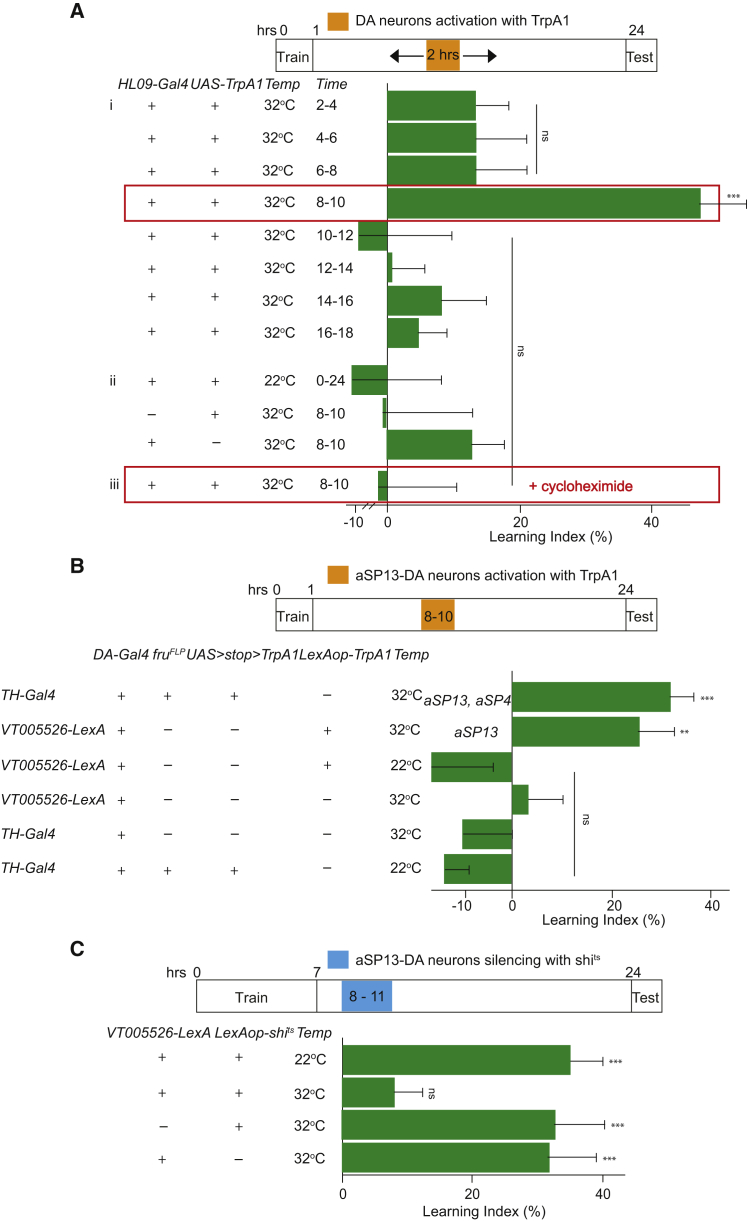
DA neurons are organized in the fly brain into 15 clusters, with the PPL1-DA and PAM-DA cluster innervating the MB lobes, a neuropil consisting of the axonal projections of the intrinsic MB cells called Kenyon cells (KC) (Mao and Davis, 2009). A subset of the PAM-DA neurons was previously implicated in courtship memory encoding (Keleman et al., 2012). Given that global post-acquisition stimulation of dopamine pathways was sufficient to consolidate short-term courtship memory into a long-lasting one, we asked whether delayed activation of PAM-DA neurons might be sufficient for consolidation of courtship short-term memory into long-term memory. To test whether and when activity of the PAM-DA neurons is sufficient for long-term memory consolidation, we thermogenetically manipulated their activity by a temperature-gated cation channel, TrpA1 (Rosenzweig et al., 2005). We expressed UAS-TrpA1 with HL09-Gal4 (Claridge-Chang et al., 2009) in a large population of DA neurons, including PAM-DA cluster and few neurons of PPL1-DA cluster, in combination with training for short-term memory.
Males expressing TrpA1 were incubated at 32°C for 2 hr at various time points after 1-hr training with a mated female. Flies, which were incubated at 32°C 8–10 hr after training fully consolidated short-term memory into long-term memory, in contrast to appropriate genetic control flies or flies that were switched to 32°C at other time points or control flies that remained at 22°C throughout (Figures 2Ai and 2Aii; Table S2). Importantly, this consolidated form of memory was dependent on de novo protein synthesis, because feeding the males with the protein synthesis inhibitor cycloheximide prevented the PAM-DA-stimulation-induced long-lasting memory (Figure 2Aiii; Table S2). Given our previous results showing that PAM-DA neurons are necessary for short-term memory acquisition, these results suggest that delayed activation of the same PAM-DA neurons between 8 and 10 hr after training is sufficient to consolidate short-term memory into a protein-synthesis-dependent long-term memory.
Figure 2.
Subset of the PAM-DA Neurons, aSP13, Consolidates Short-Term Memory into Long-Term Memory in a Protein-Synthesis-Dependent Manner
(A) Post-acquisition activation of the DA neurons mediates protein-synthesis-dependent memory consolidation. Males of the indicated genotypes were tested in single-pair assays with mated females 24 hr after being trained for 1 hr with a mated female and warmed at 32°C for 2 hr at the indicated time points (i); experimental control males, which stayed at 22°C all the time; and the genetic control animals, which were warmed at 32°C for 2 hr between 8 and 10 hr after learning (ii); and males fed with the cycloheximide during activation with TrpA1 between 8 and 10 hr after 1 hr training (iii). p values are for H0 LI = 0; ∗∗∗p < 0.001. See Table S2.
(B) Subset of the aSP13-DA neurons is sufficient for the long-term memory consolidation. Males of the indicated genotypes were tested in single-pair assays with mated females 24 hr after training for 1 hr with a mated female and being warmed at 32°C (except control males which stayed at 22°C) between 8 and 10 hr after training. p values are for H0 LI = 0; ∗∗p < 0.01; ∗∗∗p < 0.001. See Table S3.
(C) Post-acquisition silencing of the aSP13-DA neurons prevents long-term memory formation. Males of the indicated genotypes were tested in single-pair assays with mated females 24 hr after training for 7 hr with a mated female and being warmed at 32°C (except control males, which stayed at 22°C) between 8 and 11 hr after training. p values are for H0 LI = 0; ∗∗∗p < 0.001. See Table S4.
The plots indicate mean learning indices ± SEM.
The PAM-DA cluster consists of over 100 neurons, a subset of which expresses fruitless (fru), a gene causally linked with multiple aspects of male courtship behavior (Dickson, 2008). The specific fru+ class of PAM-DA neurons innervating MB γ neuropil, aSP13, was implicated recently in courtship memory encoding (Keleman et al., 2012). To investigate whether activity of the fru+ set of PAM-DA neurons is sufficient for memory consolidation after learning, we restricted expression of UAS-TrpA1 to two fru+ neurons using TH-Gal4, UAS > stop > TrpA1, and fruFLP (where “>” represents an FRT site, the target of the Flp recombinase; Yu et al., 2010). Males expressing TrpA1 in fru+ TH-Gal4 neurons displayed suppressed courtship toward mated females at 24 hr post-training when incubated at 32°C between 8 and 10 hr after learning, indicating that they robustly formed long-term memory (Figure 2B; Table S3). When we restricted expression of TrpA1 exclusively to a single class of aSP13 neurons, using VT5526-LexA, LexAop-TrpA1 (Figure S1; B.J. Dickson, personal communication) males incubated at 32°C from 8 to 10 hr post-training fully consolidated long-term memory in contrast to genetic control under the same conditions or control flies that remained at 22°C throughout (Figure 2B; Table S3). These results suggest that post-training activation of the dopaminergic neurons, aSP13, is sufficient for consolidating short-term memory into long-term memory.
To test whether late post-acquisition activity of the aSP13-DA neurons is also necessary for formation of courtship long-term memory, we performed a complementary set of experiments. We trained males for 7 hr with recently mated females (sufficient to result in long-term memory) and silenced the same aSP13 neurons with a temperature-sensitive shibire mutant, LexAop-shits, between 8 and 11 hr after onset of training. Males expressing shits under the control of VT5526-LexA and incubated at 32°C continued to court mated females vigorously, thus failing to display long-term memory at the 24-hr test point. In contrast, genetic control animals incubated at 32°C and males that remained at 22°C displayed normal long-term memory (Figure 2C; Table S4). These results indicate that activity of the aSP13-DA neurons is required between 8 and 11 hr after initial learning for long-term memory consolidation. Thus, delayed activation of a specific neural subset is both necessary and sufficient for long-term memory consolidation of courtship learning in Drosophila.
DopR1 Is Necessary for Long-Term Memory Consolidation
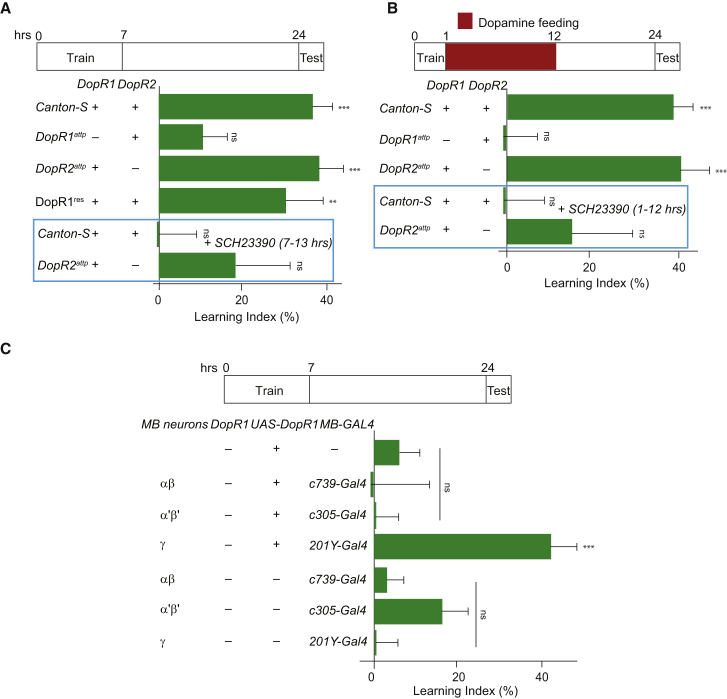
In rodents, dopamine-mediated memory persistence requires the adenylyl cyclase stimulatory D1-like type of dopamine receptors (Rossato et al., 2009). There are four dopamine receptors in Drosophila: two D1-like dopamine receptors, DopR1 and DopR2 (Hearn et al., 2002; Kim et al., 2003); one D2-like type receptor, DD2R (Draper et al., 2007); and recently identified DopEcR (Srivastava et al., 2005). To determine which dopamine receptor has a role in long-term courtship memory consolidation, we focused our analysis on DopR1 and DopR2 because DD2R is not expressed in the MB and DopEcR does not act as a dopamine receptor in courtship-suppression learning (Ishimoto et al., 2009). We tested null mutants for either type of receptor, DopR1attp or DopR2attp, in long-term memory and memory-consolidation paradigms (Keleman et al., 2012).
Males lacking DopR1 were unable to form long-term courtship memory after 7 hr of training. In contrast, DopR2 mutants displayed normal long-term memory (Figure 3A; Table S5). To confirm that long-term memory deficit in the DopR1 mutants is indeed due to loss of DopR1 function, we analyzed DopR1res flies where the deleted genomic region was reintegrated by site-specific transgenesis (Keleman et al., 2012). These flies performed just as well as wild-type animals, suggesting that DopR1, but not DopR2, is required for long-term memory (Figure 3A; Table S5). However, given that DopR1 receptor is required for acquisition of the courtship short-term memory (Keleman et al., 2012), the impairment of long-term memory in DopR1 mutants might be due to its involvement in this early phase of memory formation: thus, these results alone do not prove DopR1’s role in memory consolidation. To address explicitly the requirement of DopR1 in long-term memory after memory acquisition, we fed wild-type flies and DopR2attp mutants (lacking DopR2, but not DopR1) after 7-hr training for long-term memory with the antagonist (SCH23390) specific for both receptors (Gotzes et al., 1994). These flies did not form long-term memory in contrast to animals that were not fed with the antagonist, suggesting that DopR1 has a post-acquisition role in long-term memory (Figure 3A; Table S5).
Figure 3.
DopR1 Is Necessary in the MB γ Neurons for Long-Term Memory Consolidation
(A) Post-acquisition inactivation of DopR1 impairs long-term memory. Males of the indicated genotypes were tested in single-pair assays with mated females 24 hr after being trained for 7 hr with a mated female and fed with SCH23390 for 6 hr if indicated. p values are for H0 LI = 0; ∗∗∗p < 0.001; ∗∗p < 0.01. See Table S5.
(B) DopR1 is required for dopamine-mediated memory consolidation. Males of the indicated genotypes were tested in single-pair assays with mated females 24 hr after being starved for 16 hr, trained for 1 hr with a mated female, and fed with dopamine and SCH23390 as indicated between training and test. p values are for H0 LI = 0; ∗∗∗p < 0.001. See Table S6.
(C) DopR1 functions in long-term memory in the MB γ neurons. Males of the indicated genotypes were tested in single-pair assays with mated females 24 hr after being trained for 7 hr with a mated female. p values are for H0 LI = 0; ∗∗∗p < 0.001. See Table S7.
The plots indicate mean learning indices ± SEM.
To test both dopamine receptors in memory consolidation paradigm, we tested DopR1 and DopR2 mutants for courtship suppression after being trained for short-term memory in combination with dopamine feeding. Mutants for DopR1 were unable to consolidate long-term memory, whereas the DopR2 mutants performed equally well as the wild-type animals (Figure 3B; Table S6). To dissociate the role of DopR1 in memory acquisition and memory consolidation, we fed wild-type males and DopR2attp mutants with the SCH23390 in addition to dopamine. These males, in contrast to animals fed only with dopamine, did not suppress their courtship toward mated females during test, thus failing to display long-term courtship memory (Figure 3B; Table S6). These results indicate that DopR1 has a role in the consolidation of short-term memory into long-term memory upon post-acquisition dopamine stimulation.
DopR1 is required in the MB γ neurons for short-term courtship memory encoding (Keleman et al., 2012). To investigate in which MB neurons DopR1 is required for memory persistence, we expressed UAS-DopR1 transgene in the DopR1 mutant background using MB lobe-specific Gal4s (Keleman et al., 2012) and tested them for long-term memory. Memory was fully rescued when DopR1 was provided back in the γ, but not α, β and α’, β’ MB neurons, indicating MB γ neurons as a likely site of long-term memory consolidation (Figure 3C; Table S7).
Orb2 Mediates Long-Term Memory Consolidation Downstream of DopR1
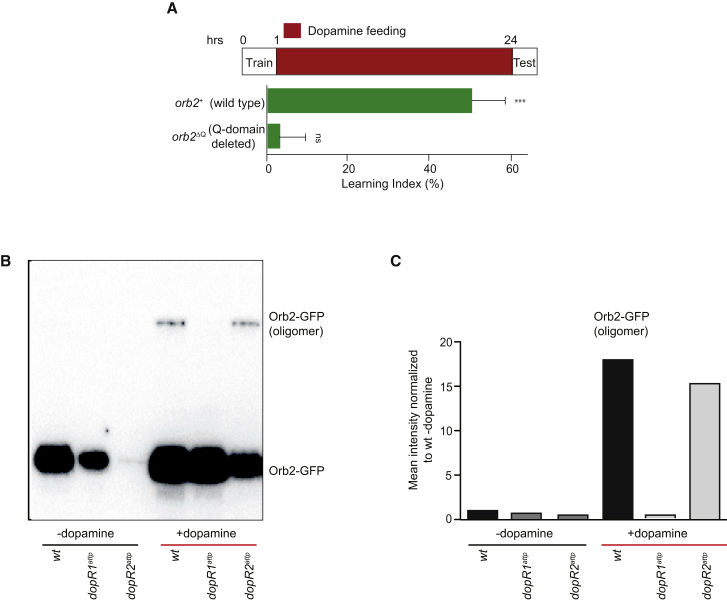
Feeding animals with dopamine activates dopaminergic pathways globally, which leads to formation of Orb2 protein complexes consisting of Orb2A and Orb2B. The Orb2 complex correlates strictly with the ability of males to form courtship long-term memory (Krüttner et al., 2012; Majumdar et al., 2012). To test whether Orb2 complexes are required for dopamine-mediated memory consolidation, we used an endogenously modified orb2 mutant allele. This mutant lacks the Q domain (orb2ΔQ) and has been previously shown to be dispensable for short-term memory but critical for both Orb2 complex formation and the maintenance of the courtship memory after 6 hr (Keleman et al., 2007; Krüttner et al., 2012). orb2ΔQ mutants were unable to consolidate short-term memory into a memory lasting 24 hr upon feeding with dopamine, in comparison to the animals bearing the wild-type allele (orb2+; Figure 4A; Table S8).
Figure 4.
Orb2 Mediates Long-Term Memory Consolidation Downstream of DopR1
(A) Orb2 is required for dopamine-mediated memory consolidation. The plots indicate mean learning indices ± SEM of males of the indicated genotypes tested in single-pair assays with mated females 24 hr after being starved for 16 hr, trained with a mated female for 1 hr, and fed with dopamine. p values are for H0 LI = 0; ∗∗∗p < 0.001. See Table S8.
(B) DopR1 mediates Orb2 oligomer formation. Head extracts from adult flies of the indicated genotypes were analyzed by IP and WB for presence of the Orb2GFP complexes, after being starved for 16 hr and fed with dopamine as indicated.
(C) WB signal (from C), corresponding to the Orb2-GFP oligomers, has been quantified using Fiji-ImageJ. The values on y axis represent the mean intensity normalized to the wild-type not treated with dopamine (wt − dopamine). See Table S9.
To investigate which type of dopamine receptor is mediating the Orb2 complex formation and hence long-term memory consolidation, we examined whether the propensity of Orb2 to form complexes depends on either DopR1 or DopR2 receptor. We investigated the endogenous Orb2 protein tagged with the GFP tag (Orb2GFP) in immunoprecipitates from brains of the DopR1 or DopR2 mutant flies upon stimulation with dopamine. As predicted, Orb2GFP complex was not detected in brain extracts from the animals that were not fed with dopamine (both the wild-type and mutants). In contrast, Orb2GFP complex was detected in brain extracts from the wild-type animals fed with dopamine, but not in DopR1 mutants. Although levels of the Orb2 protein were lower in both mutants in comparison to the wild-type animals, particularly in the animals lacking DopR2, the propensity to form Orb2 complexes seems not to be affected (Figures 4B and 4C; Table S9). These results suggest that DopR1 functions upstream of Orb2 complex formation and hence memory consolidation.
Orb2A Is Localized Mainly to Synapses in MB Neurons
Light microscopy studies using antibodies against the GFP tag fused to the endogenous Orb2 protein determined that Orb2A and Orb2B isoforms are localized in the nervous system in distinct patterns. Orb2B appears to be widely distributed throughout various regions of the nervous system, including the lobes, calyces, and soma of the MB. In neurons, Orb2B is expressed very broadly, including in ribonucleoprotein transport granules (RNPs) (Krüttner et al., 2012; Majumdar et al., 2012). In contrast, endogenous Orb2A was expressed at levels undetectable by confocal microscopy. When expressed with GFP-tagged genomic transgene rescue, Orb2A was detected at very low levels (Majumdar et al., 2012). Consistent with the genetic data that revealed a functional requirement for Orb2A in long-term memory, we hypothesized that Orb2A is expressed in the adult brain at very low levels or/and in very few cells and only in a specific cellular compartment, at the synapses, and therefore undetectable by light microscopy.
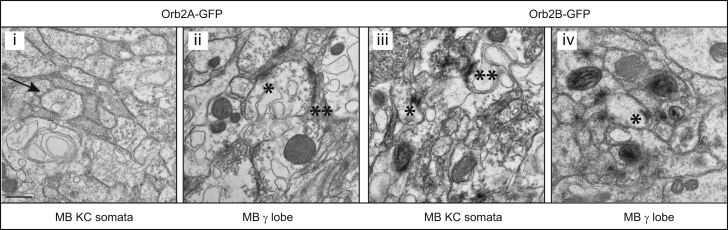
Using immuno-electron microscopy against the GFP tag on the endogenous Orb2A and Orb2B proteins encoded by orbOrb2AGFP and orbOrb2BGFP, respectively, we determined their subcellular localization (Krüttner et al., 2012). We examined KC somata and the output region of the MB, tip of the γ lobe, innervated by the aSP13 neurons in the brains of viable heterozygous orbOrb2AGFP and orbOrb2BGFP flies. We detected Orb2A protein present in a pattern distinct to that of the Orb2B protein. Orb2B is broadly expressed in the KC cell bodies and axons of the γ neurons, including synapses, whereas Orb2A is excluded from the neuronal cell bodies and is almost exclusively present in synapses of the MB γ lobe (Figure 5). These results imply that Orb2 isoforms, previously shown to function in long-term memory through distinct mechanisms (Krüttner et al., 2012), likely play distinct roles in long-term memory.
Figure 5.
Orb2A Is Localized in Synapses of the MB γ Lobe
Immuno-EM of the orb2Orb2AGFP and orb2Orb2BGFP heterozygous brains. The sagittal sections of the brain in the region of the KC somata and the tip of the MB γ lobe were analyzed. (i) Orb2AGFP is absent from the neuronal cell bodies (arrow) of the Kenyon cells. (ii) Orb2AGFP labeled by DAB precipitates is present in T-bars (asterisk) and active zones (double asterisk) in the MB γ lobe synapses. (iii) Orb2BGFP labeled by DAB precipitates is detected in the KC cell bodies including T-bars (asterisk) and active zones (double asterisk). (iv) Orb2BGFP labeled by DAB precipitates is present in the MB γ lobe including T-bars (asterisk). In all panels, scale bars represent 500 nm.
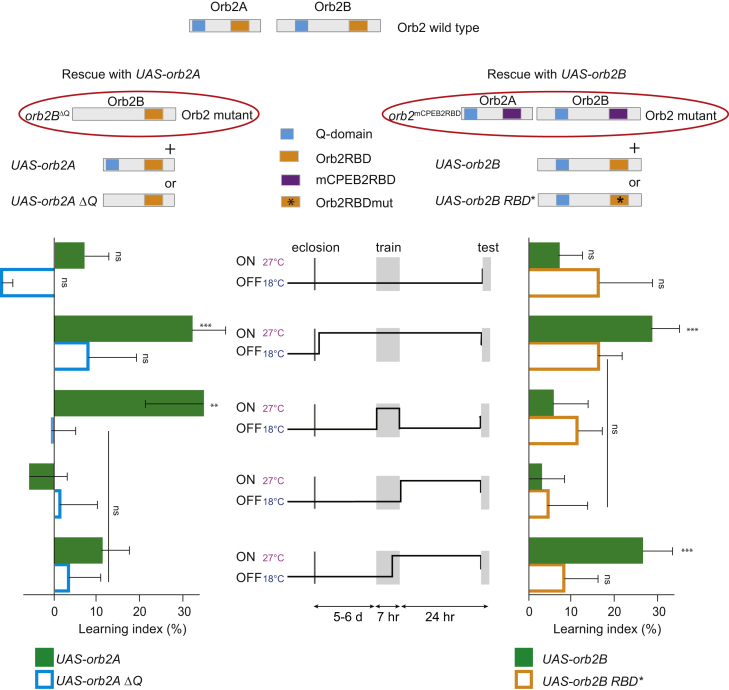
Orb2A Is Required during Memory Acquisition and Orb2B during Memory Consolidation
To investigate the temporal requirement of Orb2 isoforms in long-term memory, we manipulated in a temporal manner expression of either Orb2 isoform using TARGET expression system. TARGET uses ubiquitous expression of Gal80ts to conditionally suppress a Gal4-driven transgene; at 18°C, Gal80 inhibits Gal4 activity and expression of the transgene, but at 27°C, Gal80ts is inactive and the transgene is expressed (McGuire et al., 2004). Because it is thought that Orb2 functions in the MB γ neurons for long-term memory (Keleman et al., 2007), we used TARGET in combination with MB247-Gal4, which drives expression in the MB γ and αβ neurons, as this genetic combination resulted in the healthy progeny able to perform courtship-learning assays.
To examine temporal requirement of Orb2A and its Q domain, we expressed in the MB neurons either wild-type Orb2A (UAS-orb2AGFP) or Orb2A with the Q domain deleted (UAS-orb2ΔQGFP) under control of MB247-Gal4 and temperature-gated tub-Gal80ts in the flies lacking the A isoform and the Q domain in Orb2B (orb2BΔQ) and hence unable to form Orb2 complexes and long-term memory. We performed the IP and WB against the GFP tag to analyze the on/off kinetics of Orb2 protein expression using TARGET system. Orb2 protein is expressed at high levels within 3 or 4 hr after temperature shift to 27°C and is not detectable at the time of memory retrieval (Figure S2). Flies had fully rescued long-term memory both when kept at 27°C throughout adulthood and when shifted to 27°C for the duration of the training (Figure 6; Table S10) and only when a wild-type, but not the Q-domain-deleted, Orb2A was present. These results suggested that the Orb2A isoform and its Q domain are required in the MB neurons during memory acquisition for long-term memory formation.
Figure 6.
Orb2A Is Required during Memory Acquisition and Orb2B during Memory Consolidation
(Upper panel) Schematic of the rescue experiment with depicted domain organization of the proteins involved. (Lower panel) The plots indicate mean learning indices ± SEM of the temporal rescue of the orb2Orb2BΔQ and orb2mCPEB2RBD mutants with either UAS-orb2A/UAS-orb2AΔQ or UAS-orb2B/UAS-orb2BRBD∗ under control of MB247-Gal4 and tubGal80ts, trained and tested in single-pair assays with mated females for long-term memory and treated according to the regime outlined in the center of the panel. p values are for H0 LI = 0; ∗∗∗p < 0.001; ∗∗p < 0.01. See Table S10.
Because of the role Orb2B plays during development, animals lacking this isoform do not survive to adulthood; thus, we could not directly assess its temporal requirement in long-term memory. Therefore, we employed mutant flies in which the RNA-binding domain of Orb2B was substituted with the RNA-binding domain of the mouse homolog mCPEB2 (orb2mCPEB2RBD; Krüttner et al., 2012). These viable (but unable to form long-term memory) mutant flies allowed us to assess the temporal requirement of Orb2B and its RNA-binding domain in memory independently of its role during development. Flies expressing wild-type Orb2B (UAS-orb2BRBD) had normal long-term memory when kept at 27°C throughout adulthood and when shifted to 27°C ∼2 hr before the end of training. They did not form long-term memory when kept at 27°C during training only and when switched to 27°C right after training. Importantly, this memory was dependent on the RNA-binding domain because the males with the RNA-binding domain mutated (UAS-orb2BRBD∗) could not form long-term memory in any condition (Figure 6; Table S10). Therefore, we conclude that presence of Orb2B is dispensable during the training and shortly after but is necessary continuously about 2 hr after training. Together, these results suggest that the Orb2A isoform, which is localized to MB synapses, is necessary during memory acquisition, whereas the Orb2B isoform (recruited into complexes with Orb2A) is necessary during long-term memory consolidation.
Orb2 Regulates Translation of CaMKII in MB Neurons
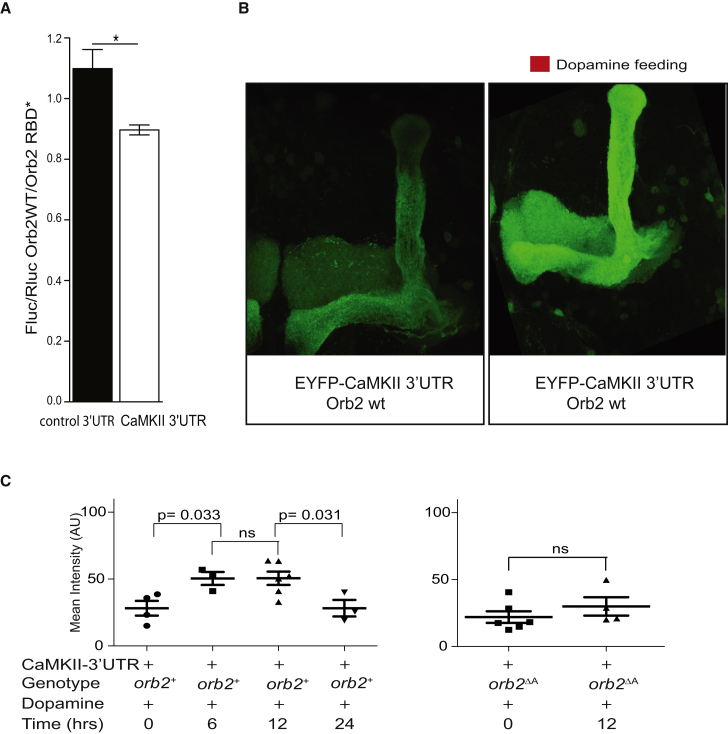
Dopamine regulates the expression of proteins essential for long-lasting memories (Berke et al., 1998), such as calcium/calmodulin-dependent kinase (CaMKII). CaMKII translation at synapses is dependent on neuronal activity both in mouse and Drosophila (Ashraf et al., 2006; Coultrap and Bayer, 2012) and is conferred by its 3′ UTR, which is recognized by CPEB proteins in mouse (Wu et al., 1998). Drosophila CaMKII has been identified as an Orb2 mRNA target (our unpublished results), and Orb2 regulates its translation by binding to the specific sequence in the 3′ UTR (Figure 7A; Table S11).
Figure 7.
Orb2 Regulates Synthesis of CaMKII in MB Neurons
(A) Orb2 regulates translation of CaMKII through its 3′ UTR. Translation of the Firefly luciferase (Fluc) reporter tethered to the CaMKII 3′ UTR is suppressed by Orb2, but not when tethered to the control 3′ UTR, which does not contain Orb2-specific binding sequence. The values on y axis represent the ratio of the normalized Fluc to Rluc fluorescence in the presence of wt Orb2 to the Fluc/Rluc fluorescence in the presence of Orb2RBD∗ with RBD mutated. ∗p < 0.05. See Table S11.
(B) Representative confocal projections of the MB (lobes) expressing UAS-EYFP-CaMKII-3′UTR with the MB247-Gal4 and stained with the anti-GFP antibodies. The adult brains were either unstimulated (left panel) or stimulated (right panel) by feeding with dopamine.
(C) UAS-EYFP-CaMKII-3′UTR was expressed in the MB neurons with the MB247-Gal4. Intensity of fluorescence was measured in the MB γ lobe of the adult brains either wild-type (orb2+; left panel) or orb2 mutant (orb2ΔA; right panel) stimulated by feeding with dopamine at the indicated time intervals. p values are for H0 ftx = ft0. See Table S12.
Given that CaMKII is a key molecule implicated in memory persistence and Orb2 regulates translation of CaMKII, we investigated whether Orb2 might function in memory consolidation by regulating synthesis of CaMKII. We expressed a fluorescent reporter of CaMKII translation, CaMKII 3′ UTR appended to the EYFP coding region (UAS-EYFP3′UTR), in the α, β, γ MB neurons using MB247-Gal4 (Ashraf et al., 2006). We monitored the change in intensity of the EYFP signal in the MB γ neurons upon neuronal stimulation with dopamine in comparison to unstimulated control brains.
We observed a striking increase of the EYFP signal after stimulation with dopamine. The EYFP signal was highest at 6 and 12 hr post-dopamine stimulation in comparison to unstimulated control brains at baseline. Importantly, we did not observe a dopamine-induced EYFP increase in an Orb2 mutant lacking the Orb2A isoform (orb2ΔA; Figures 7B and 7C; Table S12), whose Q domain is required for Orb2 complex formation (Krüttner et al., 2012). Thus, these results suggest that Orb2 complexes induced upon dopamine stimulation regulate translation of CaMKII and possibly other molecules essential for synaptic plasticity.
Discussion
Our results demonstrate that the process of long-term memory consolidation in Drosophila requires activation of the same neural pathway that, hours earlier, is required for memory acquisition. Specifically, we identified a subset of PAM-DA neurons (aSP13) whose activation mediates both memory acquisition and late memory consolidation. This permitted us to examine how memory is maintained during the interval between memory acquisition and memory consolidation. First, we established that aSP13 neurons mediate both memory acquisition and memory consolidation through the activation of the DopR1 type of receptor and through Drosophila CPEB, Orb2, in the MB γ neurons. Then, we determined that the Orb2A isoform is localized mainly to synapses in the MB neurons and is required during memory acquisition, tagging the relevant neurons and potentially their synapses for subsequent memory consolidation, whereas Orb2B, recruited into complexes with Orb2A, is required during memory consolidation. Finally, we show that, together, they regulate the activity-dependent synthesis of CaMKII, a key protein involved in the molecular basis of memory persistence (Coultrap and Bayer, 2012; Lucchesi et al., 2011; Redondo et al., 2010).
Delayed post-learning reactivation of neural pathways has been shown to exist in vertebrates (Buzsáki, 1998; Foster and Wilson, 2006; Wilson and McNaughton, 1994). Spontaneous neuronal replay after learning occurs both in the awake and sleeping states (Carr et al., 2011; Wilson and McNaughton, 1994), but the causal link between replay and memory consolidation has not been firmly established. Interfering with sharp wave ripples (SWRs), which are temporally correlated with neuronal replay in awake animals (Buzsáki et al., 1992), impaired spatial memory formation in rats (Jadhav et al., 2012). These results suggested that replay might mediate memory consolidation; however, they could not rule out that other effects of SWRs may be critical for memory consolidation. Our results that courtship memory acquisition and consolidation in Drosophila are mediated by activation of the same neuronal pathway provides further evidence that reactivation might play a key role in memory consolidation. An emerging view is that multiple types of neural signals are involved in memory formation, including neural representations of the specific content to be stored, along with signals pertaining to the importance or valence of an event, which may influence whether the content becomes consolidated. The aSP13 pathway implicated in the present work may be of the latter type, given that it is a neuromodulatory pathway. Prior work in rodents has implicated both types of signals in memory formation: for example, SWRs are thought to carry the content of a spatial trajectory (Carr et al., 2011) whereas the neuromodulatory pathways are thought to convey the salience of the content (Musso et al., 2015; Waddell, 2010).
There are emerging clues regarding the molecular bases underlying delayed neuronal-reactivation-dependent memory consolidation. Recently, a requirement of NMDA receptor reactivation for memory consolidation has been explored in rodents (Wang et al., 2006). This led to the formulation of the synaptic re-entry reinforcement hypothesis (SRR), which posits that memory consolidation requires delayed reactivation of the NMDA receptor to convert short-term memory into long-term memory. Interestingly, one of the signaling molecules downstream of NMDA receptor is CaMKII, which is believed to be responsible for potentiating the synapses involved in learning (Nicoll and Malenka, 1999). Our results are consistent with the SRR but involving DopR1 instead of NMDAR, as we found that the Drosophila DopR1, which is required during memory acquisition (Keleman et al., 2012), seems to be also necessary for late memory consolidation. This is consistent with a recent finding that DopR1 expression in the MB γ neurons is sufficient to fully support both short- and consolidated long-term memory in Drosophila (Qin et al., 2012). If the SRR hypothesis is correct (i.e., synaptic re-entry leads to reinforcement), what molecular change signifies that an “initial entry” has previously occurred in a synapse, such that another synaptic event counts as “re-entry”?
The synaptic tagging and capture hypothesis has provided a conceptual framework for how relevant information might be stored in the intervening time period between memory acquisition and memory consolidation and how specific synapses eventually come to store a given memory. The ability to temporally dissociate memory acquisition and its consolidation in the courtship memory consolidation paradigm allowed us to investigate the molecular basis of this hypothesis in learning Drosophila. Our results that the synaptically localized Orb2A isoform and its Q domain are required during memory acquisition in MB neurons for subsequent long-term memory consolidation support the likelihood that synaptic Orb2A might act to tag the specific synapses for later memory consolidation. At present, we cannot distinguish between the possibilities that this tagging is an effect of a synapse-specific post-translational modification of Orb2A or its mere presence at a synapse (White-Grindley et al., 2014). Thus, during memory acquisition, Orb2A or a modification thereof might mark activated synapses as potential sites for subsequent memory storage. Only in those synapses where the delayed activation occurred would Orb2A recruit Orb2B (and possibly its associated mRNAs; Krüttner et al., 2012) into translationally active protein complexes (Si et al., 2003b) to regulate synthesis of proteins essential for the long-term memory, such as CaMKII.
In this work, we provide evidence that the late activation of the same neuronal and molecular pathways that are necessary and sufficient for early memory acquisition is also necessary and sufficient for late memory consolidation in Drosophila. These findings confirm principles that were strongly implied by work in mammals (Carr et al., 2011; Wang et al., 2006; Wilson and McNaughton, 1994) and extend this paradigm to invertebrates. Taking advantage of the tools available for the molecular and circuit analysis in Drosophila, we provide a functional link between occurrence of the delayed neural pathway activation and memory consolidation and start to identify the molecular and circuit mechanisms underlying this consolidation. The occurrence of these phenomena in evolutionarily distinct species implies that delayed activation might serve a key algorithmic role in adaptive learning. Moreover, a high degree of conservation of the involved molecules (Theis et al., 2003) suggests that the molecular mechanisms uncovered in flies might be broadly utilized in the animal kingdom.
Experimental Procedures
Fly Stocks
Flies were maintained on conventional cornmeal-agar medium under a 12 hr light/dark cycle at 25°C and 60% relative humidity. The Canton-S strain was used as the wild-type D. melanogaster flies. The following fly stocks, HL09-Gal4, TH-Gal4, c739-Gal4, c305-Gal4, Y201-Gal4, MB247-Gal4, UAS-Trp, UAS-DopR1, DopR1attp, DopR2attp, and DopR1res were previously described by Keleman et al. (2007) and Keleman et al. (2012). The Orb2DQ, Orb2+, and Orb2mCPEBRBD flies were generated by Krüttner et al. (2012). The VT5526-LexA driver and LexAop-Trp flies are unpublished reagents from B.J. Dickson (personal communication). The UAS-EYFP-CaMKII3′UTR flies were generated by Ashraf et al. (2006). All mutant flies were backcrossed to the Canton-S for four generations before being used for behavioral assays.
Behavioral Assays
Behavioral assays were executed at variable phases of the circadian clock of the flies. Courtship conditioning assays were carried out as described previously (Keleman et al., 2007; Siwicki and Ladewski, 2003). Details can be found in the Supplemental Information.
Memory-consolidation assay by dopamine feeding was performed as follows. Freshly hatched males were collected and aged individually in food chambers as for courtship conditioning. Prior to training, flies were starved on a wet filter paper for 16 hr. After short-term memory training (1 hr), at indicated time points, flies were transferred to chambers containing filter paper soaked with 80 μl of 2% sucrose solution supplemented with either dopamine, cyclohexamide, or SCH23390 as indicated (concentrations used: 20 mM dopamine, 35 mM cyclohexamide, or 1 μM SCH23390). Memory-consolidation assay by thermogenetic activation with TrpA1 was performed as follows. Freshly hatched males were collected and aged individually in food chambers at 22°C for 6 or 7 days. First, they were trained for 1 hr at 22°C, shifted to 32°C at indicated time points for 2 hr, and thereafter placed at 22°C until the test at 25°C (24 hr after training).
For silencing with Shits, males were collected and aged as described above. They were trained for 7 hr at 22°C, shifted to 32°C at indicated time points, and thereafter placed at 22°C until the test at 25°C (24 hr after training).
TARGET experiments were conducted as described (McGuire et al., 2003). For the experiment, all flies were raised and kept at 18°C and shifted to 27°C at indicated time intervals. Test was performed at 25°C. Genotypes of the experimental flies were: w+: tub-GAL80ts, UAS-orb2A (UAS-orb2AΔQ); orb2BΔQ, MB247-Gal4 and w+: tub-GAL80ts, UAS-orb2B (UAS-orb2B∗); orb2mCPEB2RBD, MB247-Gal4.
Immunohistochemistry
Immunohistochemistry on adult brains was performed as described (Yu et al., 2010). Details on antibodies used can be found in the Supplemental Information.
Immunoprecipitation and Western Blot
IP and WB were carried out as described previously (Krüttner et al., 2012) on adult brains of w+;+; orb2GFP, DopR1attp and w+;+; orb2GFP, DopR2attp to investigate Orb2GFP complex formation. To determine on/off kinetics of Orb2 expression in TARGET experiment, IP and WB were performed on brain extract from w+;tubGal80, UAS-orb2B;Orb2mCPEBRBD,MB247-Gal4. Details can be found in the Supplemental Information.
Immuno-EM
The heads of heterozygous viable orb2Orb2AGFP and orb2Orb2BGFP 6- or 7-day-old flies were fixed in 4% paraformalde, 0.1% glutaraldehyde, and 0.07 M phosphate buffer (pH 7.3) for 3 hr at 40°C and prepared for immuno-EM as described (Krüttner et al., 2012). Details can be found in the Supplemental Information.
Statistical Analysis
LIs were calculated using a custom MATLAB script based on the algorithm described in Kamyshev et al. (1999) and implemented in Keleman et al. (2007). Briefly, the entire set of courtship indices for both the naive and trained flies was pooled and then randomly assorted into simulated naive and trained sets of the same size as in the original data. A LIp was calculated for each of 100,000 randomly permuted data sets, and p values were estimated as the fraction for which LIp > LI (to test H0, LI = 0) or LIp > LI − LI0 (to test H0, LI = LI0). p values are for H0: LI = LI1 (permutation test) and ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 for H0, LI = 0 (permutation test). Figures in the main text show LIs ± SEM calculated using the propagation of error formula and p values calculated from mean CIs; supplemental tables show values derived from both mean ± SD and median CIs.
Author Contributions
S.K. and K.K. conceived the project and designed the experiments. S.K. and L.T. performed all the experiments with the exception of the memory-consolidation assay using virgin females as testers, silencing of the aSP13 neurons after long-term memory training, and post-acquisition inactivation of DopRs in memory consolidation and long-term memory assays, which were performed by U.D. S.K. and L.T. performed data analysis with help from U.D. K.J. helped S.K. and L.T. with behavioral experiments. B.S. performed CaMKII translation-repression assay. N.I. performed EM analysis. J.N.N. and L.G.F. made an initial observation of Orb2A localization. K.K. supervised the project and wrote the manuscript with help of B.D.M.
Acknowledgments
We thank U. Heberlein, B. Dickson, and V. Jayaraman for critical comments on the manuscript; R. Fetter for supervising the EM studies; C. Kent for advice on statistics; and B. Koster, Anja de Jong, Jos Onderwater, Erik Bos, and Christina Avramut for help with the initial EM experiments. The work was supported by the grants from Austrian Science Funds to K.K. as well as FWF P21854-B09 and WWTF P21854.
Published: June 18, 2015
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, two figures, and twelve tables and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2015.05.037.
Supplemental Information
References
- Ashraf S.I., McLoon A.L., Sclarsic S.M., Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. doi: 10.1016/j.cell.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Berke J.D., Paletzki R.F., Aronson G.J., Hyman S.E., Gerfen C.R. A complex program of striatal gene expression induced by dopaminergic stimulation. J. Neurosci. 1998;18:5301–5310. doi: 10.1523/JNEUROSCI.18-14-05301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Memory consolidation during sleep: a neurophysiological perspective. J. Sleep Res. 1998;7(1):17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Horváth Z., Urioste R., Hetke J., Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Carr M.F., Jadhav S.P., Frank L.M. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat. Neurosci. 2011;14:147–153. doi: 10.1038/nn.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claridge-Chang A., Roorda R.D., Vrontou E., Sjulson L., Li H., Hirsh J., Miesenböck G. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap S.J., Bayer K.U. CaMKII regulation in information processing and storage. Trends Neurosci. 2012;35:607–618. doi: 10.1016/j.tins.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson B.J. Wired for sex: the neurobiology of Drosophila mating decisions. Science. 2008;322:904–909. doi: 10.1126/science.1159276. [DOI] [PubMed] [Google Scholar]
- Draper I., Kurshan P.T., McBride E., Jackson F.R., Kopin A.S. Locomotor activity is regulated by D2-like receptors in Drosophila: an anatomic and functional analysis. Dev. Neurobiol. 2007;67:378–393. doi: 10.1002/dneu.20355. [DOI] [PubMed] [Google Scholar]
- Foster D.J., Wilson M.A. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Frey U., Morris R.G. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Gotzes F., Balfanz S., Baumann A. Primary structure and functional characterization of a Drosophila dopamine receptor with high homology to human D1/5 receptors. Receptors Channels. 1994;2:131–141. [PubMed] [Google Scholar]
- Guo A., Li L., Xia S.Z., Feng C.H., Wolf R., Heisenberg M. Conditioned visual flight orientation in Drosophila: dependence on age, practice, and diet. Learn. Mem. 1996;3:49–59. doi: 10.1101/lm.3.1.49. [DOI] [PubMed] [Google Scholar]
- Hafer N., Xu S., Bhat K.M., Schedl P. The Drosophila CPEB protein Orb2 has a novel expression pattern and is important for asymmetric cell division and nervous system function. Genetics. 2011;189:907–921. doi: 10.1534/genetics.110.123646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearn M.G., Ren Y., McBride E.W., Reveillaud I., Beinborn M., Kopin A.S. A Drosophila dopamine 2-like receptor: Molecular characterization and identification of multiple alternatively spliced variants. Proc. Natl. Acad. Sci. USA. 2002;99:14554–14559. doi: 10.1073/pnas.202498299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto H., Sakai T., Kitamoto T. Ecdysone signaling regulates the formation of long-term courtship memory in adult Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2009;106:6381–6386. doi: 10.1073/pnas.0810213106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav S.P., Kemere C., German P.W., Frank L.M. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamyshev N.G., Iliadi K.G., Bragina J.V. Drosophila conditioned courtship: two ways of testing memory. Learn. Mem. 1999;6:1–20. [PMC free article] [PubMed] [Google Scholar]
- Kandel E.R. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Keleman K., Krüttner S., Alenius M., Dickson B.J. Function of the Drosophila CPEB protein Orb2 in long-term courtship memory. Nat. Neurosci. 2007;10:1587–1593. doi: 10.1038/nn1996. [DOI] [PubMed] [Google Scholar]
- Keleman K., Vrontou E., Krüttner S., Yu J.Y., Kurtovic-Kozaric A., Dickson B.J. Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature. 2012;489:145–149. doi: 10.1038/nature11345. [DOI] [PubMed] [Google Scholar]
- Kim Y.C., Lee H.G., Seong C.S., Han K.A. Expression of a D1 dopamine receptor dDA1/DmDOP1 in the central nervous system of Drosophila melanogaster. Gene Expr. Patterns. 2003;3:237–245. doi: 10.1016/s1567-133x(02)00098-4. [DOI] [PubMed] [Google Scholar]
- Krüttner S., Stepien B., Noordermeer J.N., Mommaas M.A., Mechtler K., Dickson B.J., Keleman K. Drosophila CPEB Orb2A mediates memory independent of Its RNA-binding domain. Neuron. 2012;76:383–395. doi: 10.1016/j.neuron.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchesi W., Mizuno K., Giese K.P. Novel insights into CaMKII function and regulation during memory formation. Brain Res. Bull. 2011;85:2–8. doi: 10.1016/j.brainresbull.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Majumdar A., Cesario W.C., White-Grindley E., Jiang H., Ren F., Khan M.R., Li L., Choi E.M., Kannan K., Guo F. Critical role of amyloid-like oligomers of Drosophila Orb2 in the persistence of memory. Cell. 2012;148:515–529. doi: 10.1016/j.cell.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Mao Z., Davis R.L. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S.J., Grimwood P.D., Morris R.G. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- McBride S.M., Giuliani G., Choi C., Krause P., Correale D., Watson K., Baker G., Siwicki K.K. Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron. 1999;24:967–977. doi: 10.1016/s0896-6273(00)81043-0. [DOI] [PubMed] [Google Scholar]
- McGuire S.E., Le P.T., Osborn A.J., Matsumoto K., Davis R.L. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- McGuire S.E., Mao Z., Davis R.L. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- Mendez R., Richter J.D. Translational control by CPEB: a means to the end. Nat. Rev. Mol. Cell Biol. 2001;2:521–529. doi: 10.1038/35080081. [DOI] [PubMed] [Google Scholar]
- Moncada D., Viola H. Induction of long-term memory by exposure to novelty requires protein synthesis: evidence for a behavioral tagging. J. Neurosci. 2007;27:7476–7481. doi: 10.1523/JNEUROSCI.1083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso P.Y., Tchenio P., Preat T. Delayed dopamine signaling of energy level builds appetitive long-term memory in Drosophila. Cell Rep. 2015;10:1023–1031. doi: 10.1016/j.celrep.2015.01.036. [DOI] [PubMed] [Google Scholar]
- Nicoll R.A., Malenka R.C. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann. N Y Acad. Sci. 1999;868:515–525. doi: 10.1111/j.1749-6632.1999.tb11320.x. [DOI] [PubMed] [Google Scholar]
- Ofstad T.A., Zuker C.S., Reiser M.B. Visual place learning in Drosophila melanogaster. Nature. 2011;474:204–207. doi: 10.1038/nature10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaçais P.Y., Trannoy S., Isabel G., Aso Y., Siwanowicz I., Belliart-Guérin G., Vernier P., Birman S., Tanimoto H., Preat T. Slow oscillations in two pairs of dopaminergic neurons gate long-term memory formation in Drosophila. Nat. Neurosci. 2012;15:592–599. doi: 10.1038/nn.3055. [DOI] [PubMed] [Google Scholar]
- Qin H., Cressy M., Li W., Coravos J.S., Izzi S.A., Dubnau J. Gamma neurons mediate dopaminergic input during aversive olfactory memory formation in Drosophila. Curr. Biol. 2012;22:608–614. doi: 10.1016/j.cub.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn W.G., Harris W.A., Benzer S. Conditioned behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo R.L., Okuno H., Spooner P.A., Frenguelli B.G., Bito H., Morris R.G. Synaptic tagging and capture: differential role of distinct calcium/calmodulin kinases in protein synthesis-dependent long-term potentiation. J. Neurosci. 2010;30:4981–4989. doi: 10.1523/JNEUROSCI.3140-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J.D. Think globally, translate locally: what mitotic spindles and neuronal synapses have in common. Proc. Natl. Acad. Sci. USA. 2001;98:7069–7071. doi: 10.1073/pnas.111146498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemensperger T., Isabel G., Coulom H., Neuser K., Seugnet L., Kume K., Iché-Torres M., Cassar M., Strauss R., Preat T. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl. Acad. Sci. USA. 2011;108:834–839. doi: 10.1073/pnas.1010930108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig M., Brennan K.M., Tayler T.D., Phelps P.O., Patapoutian A., Garrity P.A. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato J.I., Bevilaqua L.R., Izquierdo I., Medina J.H., Cammarota M. Dopamine controls persistence of long-term memory storage. Science. 2009;325:1017–1020. doi: 10.1126/science.1172545. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M., Monastirioti M., Scholz H., Friggi-Grelin F., Birman S., Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si K., Giustetto M., Etkin A., Hsu R., Janisiewicz A.M., Miniaci M.C., Kim J.H., Zhu H., Kandel E.R. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in aplysia. Cell. 2003;115:893–904. doi: 10.1016/s0092-8674(03)01021-3. [DOI] [PubMed] [Google Scholar]
- Si K., Lindquist S., Kandel E.R. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell. 2003;115:879–891. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- Siegel R.W., Hall J.C. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc. Natl. Acad. Sci. USA. 1979;76:3430–3434. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwicki K.K., Ladewski L. Associative learning and memory in Drosophila: beyond olfactory conditioning. Behav. Processes. 2003;64:225–238. doi: 10.1016/s0376-6357(03)00137-2. [DOI] [PubMed] [Google Scholar]
- Smith W.B., Starck S.R., Roberts R.W., Schuman E.M. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron. 2005;45:765–779. doi: 10.1016/j.neuron.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Srivastava D.P., Yu E.J., Kennedy K., Chatwin H., Reale V., Hamon M., Smith T., Evans P.D. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J. Neurosci. 2005;25:6145–6155. doi: 10.1523/JNEUROSCI.1005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton M.A., Schuman E.M. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Tempel B.L., Livingstone M.S., Quinn W.G. Mutations in the dopa decarboxylase gene affect learning in Drosophila. Proc. Natl. Acad. Sci. USA. 1984;81:3577–3581. doi: 10.1073/pnas.81.11.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis M., Si K., Kandel E.R. Two previously undescribed members of the mouse CPEB family of genes and their inducible expression in the principal cell layers of the hippocampus. Proc. Natl. Acad. Sci. USA. 2003;100:9602–9607. doi: 10.1073/pnas.1133424100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell S. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 2010;33:457–464. doi: 10.1016/j.tins.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.P., Cooper N.G. Characterization of the transcripts and protein isoforms for cytoplasmic polyadenylation element binding protein-3 (CPEB3) in the mouse retina. BMC Mol. Biol. 2009;10:109. doi: 10.1186/1471-2199-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Hu Y., Tsien J.Z. Molecular and systems mechanisms of memory consolidation and storage. Prog. Neurobiol. 2006;79:123–135. doi: 10.1016/j.pneurobio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- White-Grindley E., Li L., Mohammad Khan R., Ren F., Saraf A., Florens L., Si K. Contribution of Orb2A stability in regulated amyloid-like oligomerization of Drosophila Orb2. PLoS Biol. 2014;12:e1001786. doi: 10.1371/journal.pbio.1001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.A., McNaughton B.L. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wise R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wu L., Wells D., Tay J., Mendis D., Abbott M.A., Barnitt A., Quinlan E., Heynen A., Fallon J.R., Richter J.D. CPEB-mediated cytoplasmic polyadenylation and the regulation of experience-dependent translation of alpha-CaMKII mRNA at synapses. Neuron. 1998;21:1129–1139. doi: 10.1016/s0896-6273(00)80630-3. [DOI] [PubMed] [Google Scholar]
- Wustmann G., Rein K., Wolf R., Heisenberg M. A new paradigm for operant conditioning of Drosophila melanogaster. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 1996;179:429–436. doi: 10.1007/BF00194996. [DOI] [PubMed] [Google Scholar]
- Yu J.Y., Kanai M.I., Demir E., Jefferis G.S., Dickson B.J. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr. Biol. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.