Abstract
Dermatitis, pyrexia, and hemorrhagic syndrome (DPHS) is a rare bovine syndrome of unclear etiology. We describe two DPHS cases, the first to occur in Italy, with clinicopathological findings suggesting a potential pathogenetic role of bovine herpesvirus-4 (BoHV-4).
CASE REPORT
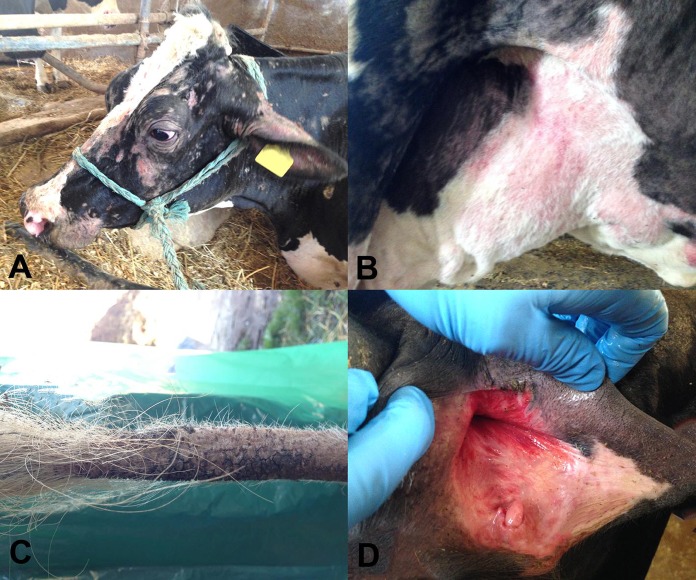
Two Italian Holstein-Friesian cows, 5 and 6 years old, from the same herd were referred to the Teaching Hospital of the Department of Veterinary Science of Turin University because of acute onset of pyrexia, pruritus, and skin lesions. One animal (cow A) was referred 1 week after the onset of clinical signs and displayed forced decubitus; the other animal (cow B) was referred 36 h after the onset of clinical signs. Both animals were anorectic, showed signs of depressed mental status, with decreased/absent milk production, pyrexia (rectal temperature, >41.0°C), and disseminated pruritic papulocrustous dermatitis of the head, neck, tail, perineum, and udder (Fig. 1A to C). In addition, cow B presented hemorrhagic suffusion of the vulvar mucosal membrane (Fig. 1D) and bloody diarrhea.
FIG 1.
Clinical features observed in two dairy cows with dermatitis, pyrexia, and hemorrhagic syndrome. (A) Severe alopecic erythematous crusty skin lesions disseminated on the head; (B) severe alopecic and erythematous skin lesions on the ventral abdomen and udder; (C) severe alopecia of the tail; (D) hemorrhagic suffusion involving the vulvar mucosa.
In cow A, the complete blood cell count (ADVIA 120 hematology system; Bayer) was unremarkable. In cow B, the hematological parameters were significantly altered: severe leukopenia (white blood cell count, 2,680 cells/ml [reference range, 6,200 to 13,600 cells/ml]), lymphopenia (2,004 cells/ml [reference range, 4,000 to 9,800 cells/ml]), neutropenia (390 cells/ml [reference range, 1,100 to 3,600 cells/ml]), monocytopenia (160 cells/ml [reference range, 200 to 1,300 cells/ml]), and thrombocytopenia (190,000 cells/ml [reference range, 412,000 to 1,000,030 cells/ml]). The biochemical profile (Cobas Mira; ABX Diagnostics) was consistent with muscular damage subsequent to forced decubitus in cow A (aspartate aminotransferase [AST], 500 IU [reference range, 47 to 127 IU]; creatine kinase [CK], 15,810 IU [reference range, 105 to 409 IU]; and lactate dehydrogenase [LDH], 8,910 IU [reference range, 2,866 to 3,600 IU]). The biochemical profile in cow B was unremarkable.
Serological investigations showed antibody (Ab) titers of 3+ and 2+ for bovine herpesvirus 4 (BoHV-4) (PrimaCheck bovine respiratory syncytial virus [BRSV] Ab; Agrolabo) in cow A and cow B, respectively. No serum antibodies for BoHV-1, bovine BoHV-2, BoHV-5, bovine virus diarrhea/mucosal disease (BVD/MD), parainfluenza 3 virus (PI3), BRSV, pseudorabies virus (PrV), Mycoplasma spp., or Mycobacterium avium subsp. paratuberculosis were detected in either animal.
Parasitological and mycological investigations of the skin and hair samples were negative in both animals.
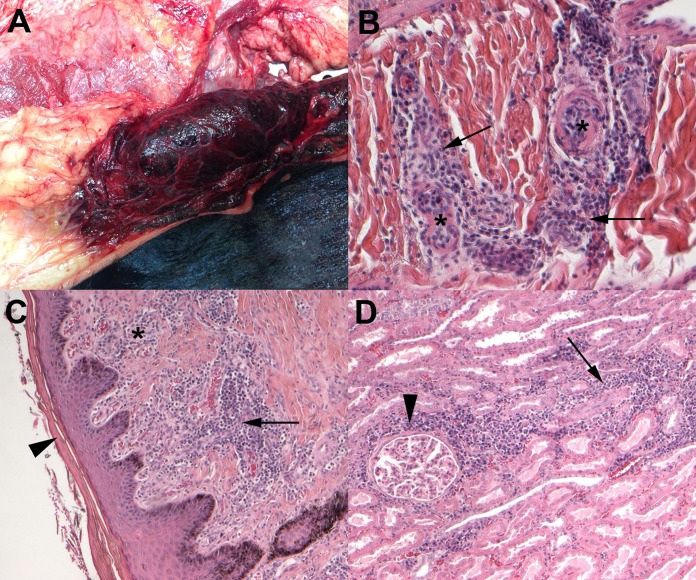
Cow A was euthanized for animal welfare reasons; cow B died spontaneously after 8 days despite supportive treatment. Gross examination revealed multifocal whitish areas involving the renal cortex in both animals; subcutaneous hematomas and severe multifocal hemorrhages of the intercostal muscles were noted in cow B (Fig. 2A). The major histopathological findings for both animals were cutaneous leukocytoclastic vasculitis (Fig. 2B) associated with superficial and deep dermatitis, hyperkeratosis (Fig. 2C), and subepidermal separations and ulcerations, in addition to chronic interstitial nephritis associated with vasculitis (Fig. 2D). Severe and diffuse splenic depletion associated with histiocytic giant cells and neutrophilic infiltrates and minimal multifocal interstitial pneumonia were observed in cow B.
FIG 2.
Gross and histopathological lesions observed in the two dairy cows with clinical signs of dermatitis, pyrexia, and hemorrhagic syndrome. (A) Severe multifocal hemorrhages of the intercostal muscles. (B) Skin sample showing acute leukocytoclastic vasculitis characterized by neutrophilic infiltrates (arrows) also involving the wall of small vessels (asterisks). Magnification of hematoxylin-eosin (HE) stain, 20×. (C) Skin sample showing chronic dermatitis characterized by heterogeneous inflammation involving the dermis (asterisk) and vessels (arrow) accompanied by hyperkeratosis (arrowhead). Magnification of HE stain, 10×. (D) Kidney sample showing chronic interstitial nephritis characterized by lymphoplasmacytic infiltrates around glomeruli (arrowhead) and tubules (arrow). Magnification of HE stain, 10×.
Bacteriological examination and indirect immunofluorescence testing (IIFT) for anti-bovine IgG in skin, lung, kidney, liver, spleen, and heart tissue samples were negative. Toxicological investigations (enzyme-linked immunosorbent assay [ELISA] and high-performance liquid chromatography) of feed and rumen contents were negative for mycotoxins (citrinin; total fumonisins; aflatoxins B1, B2, G1, and G2; zearalenone; and deoxynivalenol); herd history excluded the use of di-ureido isobutane (DUIB) and other potential toxic feed additives.
Virus isolation procedures on the spleen and lung tissue samples were performed. Briefly, homogenates of fresh tissue samples were prepared and inoculated into confluent monolayers of Madin Darby bovine kidney (MDBK) (ATCC CCL-22) cells. Interestingly, a strong rapid cytopathic effect (CPE) was observed 16 h postinoculation; the slides were then stained with anti-BoHV-4 conjugate (75 μl/well) for 30 min at 37°C in a humidified chamber. In addition, 300 μl of the MDBK cell culture supernatant, previously showing CPE to virus isolation, was extracted using a PureLink genomic DNA minikit (Promega). Amplification of a region of the herpesvirus DNA polymerase gene was obtained by pan-herpesvirus-nested PCR assay using degenerate consensus primers (1). Direct immunofluorescence was negative for BVD/MD, BRSV, ovine herpesvirus 2 (OHV-2), and blue tongue virus (BTV) but showed a signal for BoHV-4. Nested PCR gave amplicons of expected sizes (from 215 to 315 bp) that were directly sequenced on an ABI 3130 genetic analyzer (Life Technologies). BLAST analysis revealed maximum identity with published BoHV-4 sequences. In addition, electron microscopy showed herpesvirus-like particles in all the cell cultures inoculated with the lung and spleen samples. The numbers of virus particles varied depending on the sample, with a higher number found in the spleen samples.
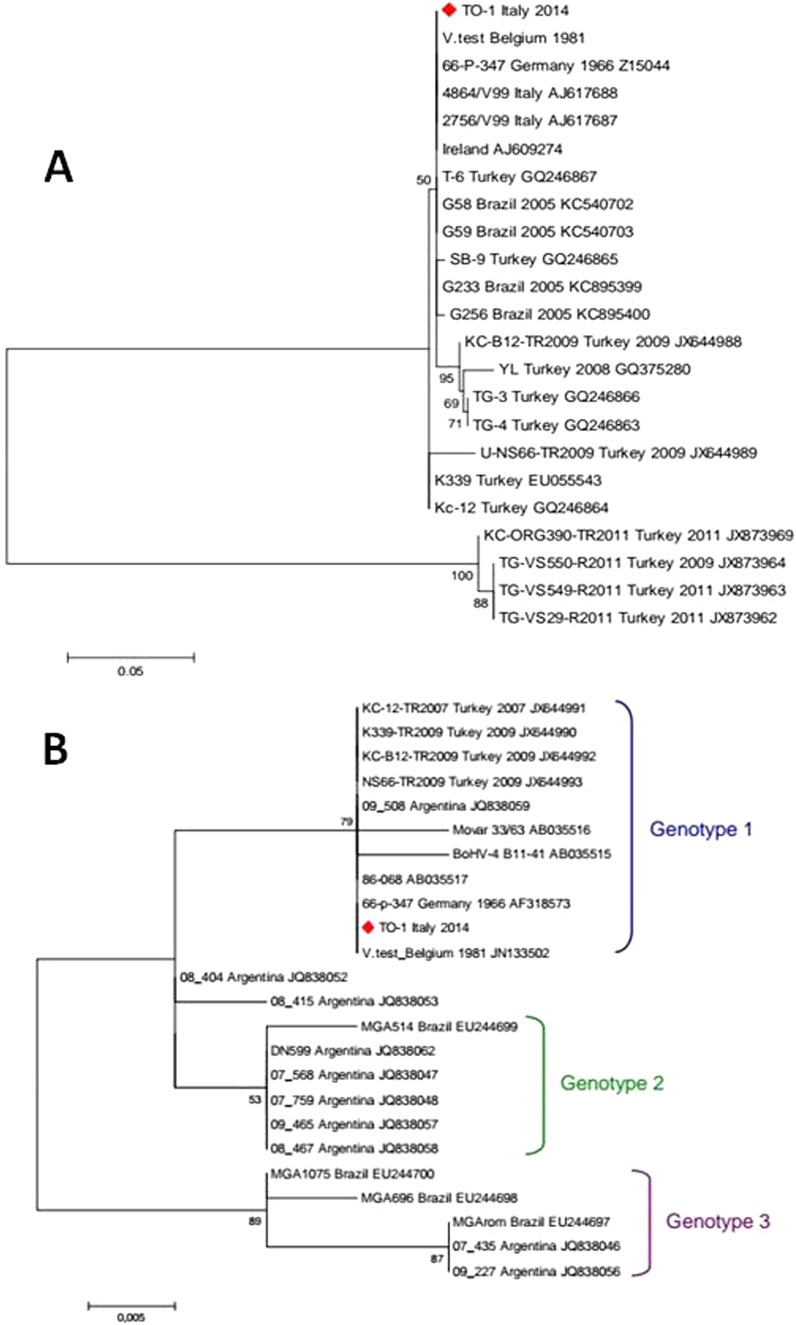
Phylogenetic analysis was performed on the glycoprotein B (gB) and thymidine kinase (TK) genes of BoHV-4. Amplification was carried out on samples that were positive by pan-herpesvirus diagnostic PCR employing the nested-PCR protocols described by Wellenberg et al. (2) for gB and Verna et al. (3) for TK. PCR products of the expected sizes were obtained during the first amplification round for both gene targets and directly sequenced on an ABI 3130 genetic analyzer (Life Technologies). The sequences retrieved from all samples were identical and used to create a representative consensus sequence. The newly generated consensus and BoHV-4 sequences available in GenBank were aligned using BioEdit. Nucleotide substitution models were evaluated using jModelTest2 (4), and the best model was selected according to Akaike information criterion analysis. Molecular Evolutionary Genetics Analysis (MEGA; version 6) was used for calculating P distance matrices, and phylogeny inference was used according to the maximum-likelihood criterion. The nucleotide substitution model was set according to jModelTest2 output, and evolutionary distances for both data sets were calculated using the Jukes-Cantor model. The robustness of the hypothesis was tested in 1,000 nonparametric bootstrap analyses. Determination of sequence similarity based on nucleotide homology revealed that the analyzed gene regions of the detected BoHV-4 isolate shared a high degree of nucleotide sequence identity with known BoHV-4 sequences, 97.9 to 100% (gB) and 96.3 to 100% (TK), except with four gB sequences detected in Turkey in 2009 to 2011, which showed much lower values (71.3%).
We detected no unique amino acid changes in our isolate for both the predicted partial gB and TK proteins in a comparison with sequences in GenBank. Phylogenetic trees based on the aligned nucleotide sequences of BoHV-4 gB and TK genes are shown in Fig. 3.
FIG 3.
Phylogeny inferred by maximum-likelihood analysis obtained by alignment of 335 nucleotides covering the partial glycoprotein B (gB) gene region of BoHV-4 (A) and 200 nucleotides covering the partial thymidine kinase (TK) gene region of BoHV-4 (B). The phylogenetic tree includes the novel BoHV-4 isolate (TO-1, red rhombus) and sequences available in GenBank. Country of origin and collection date, when available, are indicated together with the accession number for each sequence. Bootstrap (1,000 replicates) values of >50 are shown at the internal nodes. The length of each pair of branches represents the distance between sequence pairs. The scale bar represents the percentage of nucleotide differences.
To the best of our knowledge, this is the first report of dermatitis, pyrexia, and hemorrhagic syndrome (DPHS) in Italy. The clinical signs and histopathological lesions in both cows resembled those previously described in dairy cows affected by the syndrome (5–10). Although its etiology is still unclear, citrinin mycotoxicosis (5, 11) or di-ureido isobutane (DUIB) poisoning (6) has been suggested as a potential cause. Toxic causes were excluded in the present cases. The clinicopathological findings suggested a potential pathogenetic role of BoHV-4. Indeed, published data, the complete blood cell count of cow B, electron microscopy, and viral isolation results from the lungs and spleens of both animals all suggested an active viral infection (12–15).
Moreover, vasculitis has been reported as a consequence of systemic herpesvirus infections caused by malignant catarrhal fever (MCF) (16) and BoHV-4 (17), and bovine endothelial cell cultures were found to be susceptible to BoHV-4 infection (18). Although the pathogenesis of vasculitis remains unsolved, it is usually assumed that a small fraction of infected lymphocytes induces the proliferation and deregulation of uninfected cells with a nonspecific cytotoxic effect (19). In the present cases, both electron microscopy and viral culture of skin samples were negative, consistent with previous observations in cases of cutaneous MCF, in which viral particle detection and viral antigen expression in skin samples may be limited (19). Furthermore, BoHV-4 has been isolated from cattle with a variety of inflammatory lesions, such as mammary pustular dermatitis, metritis, pneumonia, diarrhea, respiratory infections, interdigital dermatitis, and vaginitis (20–23).
We carried out phylogenetic analyses on partial gB and TK nucleotide sequences of BoHV-4. The gB analysis (Fig. 3A) showed that the BoHV-4 sequence locates within a major cluster with other GenBank sequences from Belgium, Germany, Italy, Ireland, Brazil, and Turkey, confirming that this region of gB is quite conserved among BoHV-4 strains (24) and, therefore, not helpful in providing clues about their geographical origin. Of note, four Turkish isolates published in GenBank diverge from the major cluster into a separate branch, though they do not carry differences at the amino acid level. Phylogeny based on TK sequences revealed that the BoHV-4 sequence identified in this study clustered within genotype 1, without significant divergence from other known strains. Figure 3B illustrates the main clusters corresponding to the proposed genotypes, 1, 2, and 3, that include European (Mover-like, genotype 1) and American strains isolated from bison and cat, the African strain isolated from buffalo (DN599-like, genotype 2), and Argentinean and Brazilian strains (genotype 3) from bovine (3, 25).
The clinicopathological findings were suggestive of an active BoHV-4 infection; however, molecular characterization of other field isolates and broadening of the spectrum of BoHV-4 phylogenetic targets are needed to clarify BoHV-4's role in the pathogenesis of DPHS syndrome.
Nucleotide sequence accession numbers.
Sequences reported in this study were submitted to GenBank with accession numbers KT166423 and KT166424.
REFERENCES
- 1.VanDevanter DR, Warrener P, Bennett L, Schultz ER, Coulter S, Garber RL, Rose TM. 1996. Detection and analysis of diverse herpesviral species by consensus primer PCR. J Clin Microbiol 34:1666–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wellenberg GJ, Verstraten ER, Belák S, Verschuren SB, Rijsewijk FA, Peshev R, Van Oirschot JT. 2001. Detection of bovine herpesvirus 4 glycoprotein B and thymidine kinase DNA by PCR assays in bovine milk. J Virol Methods 97:101–112. doi: 10.1016/S0166-0934(01)00341-X. [DOI] [PubMed] [Google Scholar]
- 3.Verna AE, Manrique JM, Pérez SE, Leunda MR, Pereyra SB, Jones LR, Odeón AC. 2012. Genomic analysis of bovine herpesvirus type 4 (BoHV-4) from Argentina: high genetic variability and novel phylogenetic groups. Vet Microbiol 160:1–8. doi: 10.1016/j.vetmic.2012.04.039. [DOI] [PubMed] [Google Scholar]
- 4.Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyson DA, Reed JB. 1977. Haemorrhagic syndrome of cattle of suspected mycotoxic origin. Vet Rec 100:400–402. doi: 10.1136/vr.100.19.400. [DOI] [PubMed] [Google Scholar]
- 6.Breukink HJ, Gruys E, Holzhauer C, Westenbroek AC. 1978. Pyrexia with dermatitis in dairy cows. Vet Rec 103:221–222. doi: 10.1136/vr.103.10.221. [DOI] [PubMed] [Google Scholar]
- 7.Matthews JG, Shreeve BJ. 1978. Pyrexia/pruritus/haemorrhagic syndrome in dairy cows. Vet Rec 103:408–409. [DOI] [PubMed] [Google Scholar]
- 8.Thomas GW. 1978. Pyrexia with dermatitis in dairy cows. Vet Rec 102:368. doi: 10.1136/vr.102.16.368. [DOI] [PubMed] [Google Scholar]
- 9.Turner SJ, Kelly DF, Spackman D. 1978. Pyrexia with dermatitis in dairy cows. Vet Rec 102:488–489. [DOI] [PubMed] [Google Scholar]
- 10.Holden AR. 1980. Two outbreaks of pyrexia with dermatitis in dairy cows. Vet Rec 106:413–414. doi: 10.1136/vr.106.18-20.413. [DOI] [PubMed] [Google Scholar]
- 11.Griffiths IB, Done SH. 1991. Citrinin as a possible cause of the pruritus, pyrexia, haemorrhagic syndrome in cattle. Vet Rec 129:113–117. doi: 10.1136/vr.129.6.113. [DOI] [PubMed] [Google Scholar]
- 12.Osorio FA, Redd DE. 1983. Experimental inoculation of cattle with bovine herpesvirus-4: evidence for a lymphoid-associated persistent infection. Am J Vet Res 44:975–980. [PubMed] [Google Scholar]
- 13.Egyed L, Berencsi G, Bartha A. 1999. Periodic reappearance of bovine herpesvirus type 4 DNA in the sera of naturally and experimentally infected rabbits and calves. Comp Immunol Microbiol Infect Dis 22:199–206. doi: 10.1016/S0147-9571(98)00137-4. [DOI] [PubMed] [Google Scholar]
- 14.Egyed L, Bartha A. 1998. PCR studies on the potential sites for latency of BHV-4 in calves. Vet Res Commun 22:209–216. doi: 10.1023/A:1006029523226. [DOI] [PubMed] [Google Scholar]
- 15.Morris DD. 2009. Alteration in the leukogram, p 405–410. In Smith BP. (ed), Large animals internal medicine, 4th ed Mosby Elsevier, St. Louis, MO. [Google Scholar]
- 16.Munday JS, French AF, Smith A, Wang J, Squires RA. 2008. Probable malignant catarrhal fever presented as transient generalised crusting dermatitis in a cow. N Z Vet J 56:89–93. doi: 10.1080/00480169.2008.36815. [DOI] [PubMed] [Google Scholar]
- 17.Egyed L, Baska F. 2003. Histological lesions in vascular tissues of bovine herpes virus type 4-infected rabbits. Vet Microbiol 91:1–10. doi: 10.1016/S0378-1135(02)00261-4. [DOI] [PubMed] [Google Scholar]
- 18.Lin TM, Shi GY, Jiang SJ, Tsai CF, Hwang BJ, Hsieh CT, Wu HL. 1999. Persistent infection of bovine herpesvirus type 4 in bovine endothelial cell cultures. Vet Microbiol 70:41–53. doi: 10.1016/S0378-1135(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 19.O'Toole D, Li H. 2014. The pathology of malignant catarrhal fever, with an emphasis on ovine herpesvirus 2. Vet Pathol 51:437–452. doi: 10.1177/0300985813520435. [DOI] [PubMed] [Google Scholar]
- 20.Thiry E, Bublot M, Dubuisson J, Pastoret PP. 1989. Bovine herpesvirus-4 (BHV-4) infection in cattle, p 96–115. In Wittmann G. (ed), Herpesvirus diseases of cattle, horses and pigs. Kluwer, Boston, MA. [Google Scholar]
- 21.Bartha A, Juhasz M, Liebermann H. 1966. Isolation of a bovineherpesvirus from calves with respiratory disease and keratoconjunctivitis. Acta Vet Acad Sci Hung 16:357–358. [PubMed] [Google Scholar]
- 22.Nikolin VM, Donofrio G, Milosevic B, Taddei S, Radosavljevic V, Milicevic V. 2007. First Serbian isolates of bovine herpesvirus 4 (BoHV 4) from a herd with a history of postpartum metritis. New Microbiol 30:53–57. [PubMed] [Google Scholar]
- 23.Miyano H, Haritani M, Sentsui H, Tsuboi T, Tanimura N, Kimura KM, Kobayashi M, Obara N, Akimoto Y. 2004. Mammary lesions associated with bovine herpesvirus type 4 in a cow with clinical mastitis. J Vet Med Sci 66:457–460. doi: 10.1292/jvms.66.457. [DOI] [PubMed] [Google Scholar]
- 24.Campos FS, Franco AC, Oliveira MT, Firpo R, Strelczuk G, Fontoura FE, Kulmann MI, Maidana S, Romera SA, Spilki FR, Silva AD, Hübner SO, Roehe PM. 2014. Detection of bovine herpesvirus 2 and bovine herpesvirus 4 DNA in trigeminal ganglia of naturally infected cattle by polymerase chain reaction. Vet Microbiol 171:182–188. doi: 10.1016/j.vetmic.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Thiry E, Dubuisson J, Bublot M, Van Bressem MF, Pastoret PP. 1990. The biology of bovine herpesvirus-4 infection of cattle. Dtsch Tierarztl Wochenschr 97:72–77. [PubMed] [Google Scholar]