Abstract
The diagnosis of encephalitis is particularly challenging in immunocompromised patients. We report here a case of fatal West Nile virus encephalitis confounded by the presence of budding yeast in the cerebrospinal fluid (CSF) from a patient who had undergone heart transplantation for dilated cardiomyopathy 11 months prior to presentation of neurologic symptoms.
CASE REPORT
The patient was a 20-year-old young man from rural California with a history of dilated cardiomyopathy who underwent heart transplantation in September 2013. He was maintained on an immunosuppressive regimen consisting of cyclosporine and steroids, with no evidence of rejection on his most recent cardiac biopsy. In mid-August, 11 months after his transplant, he presented to the Emergency Department with 10 days of nausea, vomiting, watery diarrhea, malaise, and fever to 103°F. His history was notable for exposure to farm animals and birds. He began to have moments of confusion 1 day after admission, and within 2 days of admission, he demonstrated an acute decline in mental status consisting of worsening confusion, loss of coordination, and no response to questions or commands. An acute desaturation event resulted in emergent intubation. Brain magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) demonstrated foci of restricted diffusion within the brainstem and thalami consistent with encephalitis.
Lumbar puncture (LP) performed 3 days after admission demonstrated rare budding yeast with collarettes on Gram stain with a prominent inflammatory infiltrate composed of neutrophils and monocytes (Fig. 1 and Table 1, cerebrospinal fluid [CSF] specimen A); the patient was subsequently placed on empirical antifungal therapy consisting of amphotericin B and fluconazole. Fungal cultures of the blood and CSF were negative. Cryptococcal antigen was negative in serum and CSF, Histoplasma antigen was negative in serum, urine, and CSF, Histoplasma antibodies were negative in serum, and Coccidiodes antibodies were negative by immunodiffusion in serum and CSF. Amplification and sequencing of the rRNA internal transcribed spacer (ITS) (1) from CSF was performed at our CLIA (Clinical Laboratory Improvement Amendments)-certified laboratory and identified the yeast as Malassezia restricta. Although this result was not available until over 1 week later, empirical antifungal therapy was predicted to provide adequate M. restricta coverage. The following additional infectious disease testing was performed at this time, and the following results were negative: bacterial and mycobacterial cultures of the CSF; bacterial cultures of the blood and urine; CSF PCRs for enterovirus, herpes simplex virus (HSV), varicella-zoster virus (VZV), cytomegalovirus (CMV), adenovirus, and Mycobacterium tuberculosis; plasma PCRs for adenovirus, CMV, Epstein-Barr virus (EBV), and HIV-1; stool adenovirus antigen; and an HIV-1/2 antibody screen.
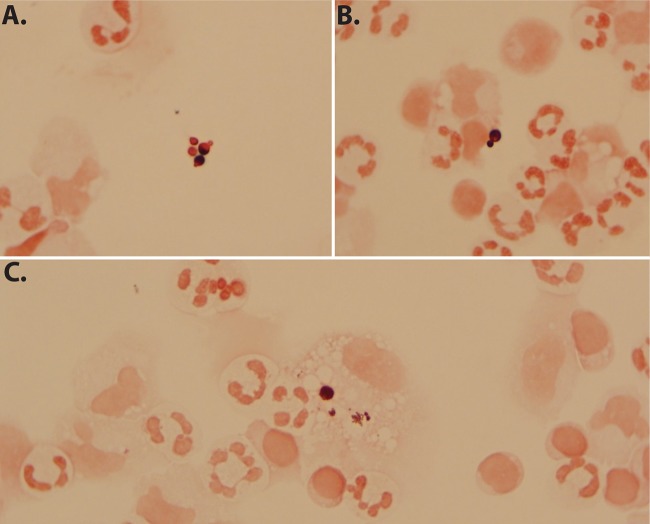
FIG 1.
Budding yeast identified in CSF specimen A by Gram stain and later confirmed as Malassezia restricta by fungal ITS rRNA PCR amplicon sequencing. (A and B) Extracellular Gram-variable spherical budding yeast forms, some demonstrating collarettes in a background of abundant polymorphonuclear cells. (C) Intracellular yeast within a mononuclear cell.
TABLE 1.
Results from CSF analysis
| Parametera | Result for CSF specimenb: |
|||
|---|---|---|---|---|
| A | B | C | D | |
| No. of days postadmission | 3 | 12 | 15 | 20 |
| Source | LP | EVD | EVD | LP |
| WBC count, cells/μl | 305 | 3 | 21 | 458 |
| Neutrophils, % | 65 | 53 | 85 | 1 |
| Lymphocytes, % | 29 | 36 | 5 | 52 |
| Monocytoid cells, % | 6 | 5 | 9 | 42 |
| RBC count, cells/μl | 6 | 1,995 | 5,450 | 557 |
| Glucose concn, mg/dl | 71 | 70 | 104 | ND |
| Total protein concn, mg/dl | 130 | 51 | 86 | ND |
| Gram stain | Numerous PMNs | No PMNs | Rare PMNs | Rare PMNs |
| Numerous mononuclear cells | No mononuclear cells | Rare mononuclear cells | Numerous mononuclear cells | |
| Rare budding yeast cells | No organisms seen | No organisms seen | No organisms seen | |
| Bacterial and fungal culture | Negative | Negative | Negative | Negative |
WBC, white blood cell; RBC, red blood cell.
LP, lumbar puncture; EVD, external ventricular drain; ND, not done; PMNs, polymorphonuclear cells.
Neurologic symptoms of intermittent shaking and nystagmus followed, and an electroencephalogram (EEG) showed diffuse slowing consistent with encephalopathy. During a neurologic exam off sedation, the patient continued to show decreased alertness and focal signs suggestive of brainstem injury given an inability to adduct the right eye with associated left lateral gaze (intranuclear ophthalmoplegia) and possible involvement of the corticospinal tract given decreased left-sided limb movements. Subsequent head computed tomography (CT) showed possible asymmetry of gray-white matter differentiation in the right temporal lobe without midline shift. Repeat brain MRI/MRA showed diffuse edema in the putamen, caudate, thalamus, cerebellum, and pons with mass effect on the fourth ventricle. An urgent external ventricular drain (EVD) was placed 12 days after admission given concern for increased intracranial pressure and a decline in the Glasgow coma scale (GCS) score from 9 to 6. Ventricular CSF studies performed at the time of EVD placement (Table 1, CSF specimens B and C) revealed a resolution of the CSF pleocytosis and the absence of budding yeast forms, as well as negative fungal CSF culture and rRNA ITS PCR. CSF fungal cultures with olive oil also did not allow isolation of M. restricta. However, the patient's worsening clinical appearance prompted further infectious diseases and autoimmune workup. This included negative serum tests for IgG and IgM antibodies against West Nile virus (WNV), Eastern equine encephalitis virus, Western equine encephalitis virus, California encephalitis virus, St. Louis encephalitis virus, lymphocytic choriomeningitis virus (LCMV), and Toxoplasma gondii, as well as negative serum tests for Leptospira, Treponema pallidum, and Bartonella antibodies. Negative serum tests were also obtained for anti-cardiolipin IgG and IgM, β-2 glycoprotein IgG and IgM, anti-thyroid peroxidase (anti-TPO), antithyroglobulin, anti-nuclear antibody, and anti-N-methyl-d-aspartate (anti-NMDA) receptor antibodies. Plasma was negative for parvovirus B19 and human herpesvirus 6 (HHV-6) DNA by PCR. CSF was VDRL test negative.
Despite the extensive negative testing, there was continued suspicion for an infectious etiology, and the patient received intravenous immune globulin (IVIG) therapy (2 g/kg body weight). The EVD was subsequently removed to minimize further infectious risk, and although steroids were initially administered for the presence of edema on imaging, they were then discontinued given concern for worsening infection. The patient's ability to follow commands waxed and waned, EEG showed diffuse slowing and multifocal epileptiform discharges, and the patient was placed on antiepileptic medications. No budding yeast cells were identified on an LP performed 20 days after admission (Table 1, CSF specimen D). The CSF was fungal culture negative (with and without olive oil), and additional infectious and rheumatologic studies on the CSF, including VDRL and anti-NMDA antibodies, neuromyelitis optica (NMO) and LCMV IgG and IgM antibodies, as well as PCR tests for HSV, VZV, EBV, CMV, HHV-6, parvovirus B19, JC virus, enterovirus, and parechovirus nucleic acids, were negative. Notably, the CSF was positive for WNV IgM (2.31; reference index value of <0.9) and negative for WNV IgG (reference index value of <1.3) (Focus Diagnostics, Cypress, CA). To confirm infection with WNV, CSF (specimen A on day 3 and D on day 20) and plasma (days 3 and 18) samples were tested using a laboratory-developed reverse transcription (RT)-PCR that detects and distinguishes between WNV and Japanese encephalitis virus (JEV). WNV RNA was detected in CSF specimen A, and very weak detection of WNV RNA was observed in CSF specimen D. WNV RNA was not detected in either plasma sample. JEV RNA was not detected in any sample.
With progressive autonomic instability and loss of all brainstem function, supportive measures were discontinued following confirmation of brain death and a multidisciplinary meeting with the patient's family. The patient was pronounced dead 35 days after admission and 45 days after initial symptomatic presentation. The family declined autopsy.
In summary, the patient was an immunocompromised 20-year-old man who underwent heart transplant in September 2013 and experienced a rapid decline in mental status with eventual death. The initial morphological analysis of CSF demonstrated budding yeast confirmed to be Malassezia restricta by sequence analysis, but subsequent serologic and molecular analysis confirmed the presence of WNV, likely acquired through natural infection.
WNV is a member of the Flavivirus genus, part of the Japanese encephalitis antigenic complex of the family Flaviviridae. First described in a Ugandan patient in 1937 and subsequently identified as the cause of an outbreak in North America in 1999, WNV is now considered endemic in the United States as the most commonly implicated cause of arboviral encephalitis (2, 3). Consistent with the patient reported here, who presented in mid-August, the highest incidence of infection occurs in the summer and early fall months when the birds that function as primary amplifiers are most viremic (4, 5). Transmission usually occurs via the bite of Culex sp. mosquitoes followed by a 2- to 14-day incubation period. Although no specific history of mosquito exposure was noted in this case, the patient lived in an area of California where surveillance activities have documented WNV infection of the mosquito vectors (6). In immunocompetent individuals, 70 to 80% of infections are subclinical or asymptomatic, and those that develop symptoms typically experience an undifferentiated acute systemic febrile illness lasting 3 to 6 days (7). Neuroinvasive disease, characterized by encephalitis, meningitis, or polio-like acute flaccid paralysis, is rare in immunocompetent individuals (<1%) (8–10) but is observed more frequently in immunocompromised individuals following both mosquito-borne transmission (40%) and donor-derived transmission (50 to 75%) (7, 11, 12). While immunosuppression was the primary risk factor for neuroinvasive disease in this case, other risk factors include an age of 50 years or older, alcohol abuse, and diabetes (9). For those who develop neuroinvasive disease, mortality ranges from 6 to 16% (13).
WNV cannot be distinguished from other causes of encephalitis based on clinical or radiologic findings. As such, specific laboratory testing is required for the definitive diagnosis of WNV encephalitis. The primary means by which to screen for WNV infection is via IgM enzyme immunoassay (EIA) (5). Because infection with other flaviviruses may result in false-positive IgM reactions (14), confirmation of antibody specificity by plaque reduction neutralization testing (PRNT) may be required in the absence of virus isolation or RNA detection (5). In this case, PRNT was not performed; however, in addition to the results from Focus Diagnostics, the California State Department of Public Health (CDPH) Viral and Rickettsial Disease Laboratory reproducibly detected anti-WNV IgM antibodies by EIA in CSF specimen D, with index values of 2.87 and 2.72, respectively. Furthermore, WNV RNA was detected by RT-PCR in CSF, thus meeting the criteria required to fulfill the definition of confirmed WNV neuroinvasive disease (5).
Interestingly, the patient did not develop a peripheral anti-WNV IgM or IgG antibody response, as determined by EIA testing of serum specimens collected on hospital day 11 (Focus Diagnostics), as well as hospital days 24 and 35 (CDPH Viral and Rickettsial Disease Laboratory). Anti-WNV IgM antibodies are usually detectable in serum 3 to 8 days after the onset of illness, with IgG production following ∼3 to 4 days later (12, 15). However, a peripheral antibody response is not required for the diagnosis of WNV neuroinvasive disease, and in these cases, anti-WNV IgM antibodies often appear in CSF prior to their detection in serum (9, 16). Additionally, the lack of peripheral anti-WNV antibodies has been described in transplant recipients with neuroinvasive WNV disease, and it may be that immunosuppression blunted or delayed this patient's response to infection (12, 17).
While WNV RNA was detected in the CSF, no evidence of viremia was obtained in concurrently collected plasma specimens. It is possible that the patient was viremic prior to collection of these specimens or that the virus was present at levels below the limit of detection of the assay, although the virus dynamics during natural human infection and the timing and mechanism of neuroinvasion are incompletely understood (15, 18).
Given the complexities of virus dynamics and antibody response to infection in the serum and CSF, particularly in immunocompromised patients, the concomitant testing of paired serum and CSF for WNV IgG and IgM, as well as WNV RNA by RT-PCR, may improve detection of WNV infection. Furthermore, serologic and molecular testing of additional paired specimens later in the course of illness may serve to identify infections that initially presented in the eclipse period (prior to detectable RNA) or window period (between detectable RNA and detectable antibody).
Initial CSF evaluation detected budding yeast cells on Gram stain that were subsequently identified as Malassezia restricta by ITS rRNA PCR amplicon sequencing. Although M. restricta is a commensal forehead and scalp organism most often implicated in seborrheic dermatitis (19, 20), there have been rare case reports of central nervous system disease caused by this organism in immunocompromised patients (21). The lipophilic nature of M. restricta and the need for specialized media to permit growth may explain the failure to isolate this organism from CSF specimen A by routine fungal culture. The supplementation of subsequent CSF fungal cultures with olive oil did not allow isolation, although these specimens were collected while the patient was on antifungal therapy.
An initial response to antifungal therapy, as evidenced by the absence of budding yeast in CSF, provided support for the pathogenicity of M. restricta. While the resolution of CSF pleocytosis was also interpreted as suggestive of therapeutic response, the observed decrease in cerebrospinal fluid white blood cell (WBC) count may have been the result of the location of CSF collection, as total WBC counts in CSF from the lumbar cistern (CSF specimen A) are known to exceed those of ventricular samples (CSF specimen B) (22). Ultimately, the lack of definitive clinical improvement as demonstrated by the patient's decline in neurologic status prompted a broader differential to include viral etiologies. Following the exclusion of other infectious and autoimmune etiologies, the overall findings in this patient were most consistent with fulminant encephalitis secondary to acute WNV infection. Supportive care is the mainstay of patient management, as potential antiviral therapies, including ribavirin, interferon alpha, and IVIG have not shown clinical efficacy (23, 24). The initial identification of M. restricta in the CSF was interpreted as a concurrent pathogen, although the possibility of contamination introduced during specimen collection or processing could not be excluded.
Delineation of the causative agent in immunocompromised patients who present with encephalitis may be extremely challenging given the variety of possible etiologies. The identification of one potential pathogen early in the patient's medical course should not exclude the presence of additional infectious agents. Serologic and molecular evaluation for the most commonly implicated entities ensures appropriate management of the immunocompromised patient who presents with clinical symptoms of encephalitis (25).
REFERENCES
- 1.Moncada PA, Budvytiene I, Ho DY, Deresinski SC, Montoya JG, Banaei N. 2013. Utility of DNA sequencing for direct identification of invasive fungi from fresh and formalin-fixed specimens. Am J Clin Pathol 140:203–208. doi: 10.1309/AJCPNSU2SDZD9WPW. [DOI] [PubMed] [Google Scholar]
- 2.Petersen LR, Fischer M. 2012. Unpredictable and difficult to control—the adolescence of West Nile virus. N Engl J Med 367:1281–1284. doi: 10.1056/NEJMp1210537. [DOI] [PubMed] [Google Scholar]
- 3.Petersen LR, Carson PJ, Biggerstaff BJ, Custer B, Borchardt SM, Busch MP. 2013. Estimated cumulative incidence of West Nile virus infection in US adults, 1999-2010. Epidemiol Infect 141:591–595. doi: 10.1017/S0950268812001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindsey NP, Lehman JA, Staples JE, Fischer M. 2014. West Nile virus and other arboviral diseases—United States, 2013. MMWR Morb Mortal Wkly Rep 63:521–526. [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2013. West Nile Virus in the United States: guidelines for surveillance, prevention, and control. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 6.United States Geological Survey. 13 February 2015, posting date. West Nile virus maps—mosquito—California United States Geological Survey, Reston, VA: http://diseasemaps.usgs.gov/wnv_ca_mosquito.html. [Google Scholar]
- 7.Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, Cooper MJ, Katz N, Liljebjelke KA, Biggerstaff BJ, Fine AD, Layton MC, Mullin SM, Johnson AJ, Martin DA, Hayes EB, Campbell GL. 2001. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet 358:261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 8.Nash D, Mostashari F, Fine A, Miller J, O'Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, Wong S, Layton M, West Nile Outbreak Response Working Group. 2001. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med 344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 9.Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, Pergam SA, King MK, DeMasters BK, Tyler KL. 2006. West Nile virus neuroinvasive disease. Ann Neurol 60:286–300. doi: 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- 10.Bode AV, Sejvar JJ, Pape WJ, Campbell GL, Marfin AA. 2006. West Nile virus disease: a descriptive study of 228 patients hospitalized in a 4-county region of Colorado in 2003. Clin Infect Dis 42:1234–1240. doi: 10.1086/503038. [DOI] [PubMed] [Google Scholar]
- 11.Kumar D, Drebot MA, Wong SJ, Lim G, Artsob H, Buck P, Humar A. 2004. A seroprevalence study of West Nile virus infection in solid organ transplant recipients. Am J Transplant 4:1883–1888. doi: 10.1111/j.1600-6143.2004.00592.x. [DOI] [PubMed] [Google Scholar]
- 12.Levi ME. 2013. West Nile virus infection in the immunocompromised patient. Curr Infect Dis Rep 15:478–485. doi: 10.1007/s11908-013-0367-8. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. 2014. West Nile virus disease cases and deaths reported to CDC by year and clinical presentation, 1999–2013. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/westnile/resources/pdfs/cummulative/99_2013_casesanddeathsclinicalpresentationhumancases.pdf. [Google Scholar]
- 14.Hogrefe WR, Moore R, Lape-Nixon M, Wagner M, Prince HE. 2004. Performance of immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays using a West Nile virus recombinant antigen (preM/E) for detection of West Nile virus- and other flavivirus-specific antibodies. J Clin Microbiol 42:4641–4648. doi: 10.1128/JCM.42.10.4641-4648.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busch MP, Kleinman SH, Tobler LH, Kamel HT, Norris PJ, Walsh I, Matud JL, Prince HE, Lanciotti RS, Wright DJ, Linnen JM, Caglioti S. 2008. Virus and antibody dynamics in acute West Nile virus infection. J Infect Dis 198:984–993. doi: 10.1086/591467. [DOI] [PubMed] [Google Scholar]
- 16.Tardei G, Ruta S, Chitu V, Rossi C, Tsai TF, Cernescu C. 2000. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile virus infection. J Clin Microbiol 38:2232–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleinschmidt-DeMasters BK, Marder BA, Levi ME, Laird SP, McNutt JT, Escott EJ, Everson GT, Tyler KL. 2004. Naturally acquired West Nile virus encephalomyelitis in transplant recipients: clinical, laboratory, diagnostic, and neuropathological features. Arch Neurol 61:1210–1220. doi: 10.1001/archneur.61.8.1210. [DOI] [PubMed] [Google Scholar]
- 18.Suen WW, Prow NA, Hall RA, Bielefeldt-Ohmann H. 2014. Mechanism of West Nile virus neuroinvasion: a critical appraisal. Viruses 6:2796–2825. doi: 10.3390/v6072796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawson TL., Jr 2007. Malassezia globosa and restricta: breakthrough understanding of the etiology and treatment of dandruff and seborrheic dermatitis through whole-genome analysis. J Investig Dermatol Symp Proc 12:15–19. doi: 10.1038/sj.jidsymp.5650049. [DOI] [PubMed] [Google Scholar]
- 20.Hiruma M, Cho O, Hiruma M, Kurakado S, Sugita T, Ikeda S. 2014. Genotype analyses of human commensal scalp fungi, Malassezia globosa, and Malassezia restricta on the scalps of patients with dandruff and healthy subjects. Mycopathologia 177:263–269. doi: 10.1007/s11046-014-9748-2. [DOI] [PubMed] [Google Scholar]
- 21.Olar A, Shafi R, Gilger M, Stager C, Moss J, Schady D, Goodman C. 2012. Central nervous system involvement by Malassezia restricta, an unusual fungal pathogen: a report of two cases. Neurology 78(Meet Abstr 1):P01.245. [Google Scholar]
- 22.Bonadio WA. 1992. The cerebrospinal fluid: physiologic aspects and alterations associated with bacterial meningitis. Pediatr Infect Dis J 11:423–431. doi: 10.1097/00006454-199206000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Petersen LR, Brault AC, Nasci RS. 2013. West Nile virus: review of the literature. JAMA 310:308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diamond MS. 2009. Progress on the development of therapeutics against West Nile virus. Antiviral Res 83:214–227. doi: 10.1016/j.antiviral.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, Stahl JP, Mailles A, Drebot M, Rupprecht CE, Yoder J, Cope JR, Wilson MR, Whitley RJ, Sullivan J, Granerod J, Jones C, Eastwood K, Ward KN, Durrheim DN, Solbrig MV, Guo-Dong L, Glaser CA. 2013. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the International Encephalitis Consortium. Clin Infect Dis 57:1114–1128. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]