Abstract
The laboratory diagnosis of tuberculosis usually relies on culture-based isolation of the causative Mycobacterium tuberculosis bacteria. We developed and evaluated the performance of MOD9, a new blood-free derivative of the MOD4 solid medium on which we previously reported for the isolation and culture of mycobacteria. First, inoculation of Lowenstein-Jensen medium with 21 M. tuberculosis isolates at 105, 103, and 10 CFU yielded colonies in 5.7 ± 1.5 days, 7.6 ± 0.8 days, and 10.8 ± 1.7 days versus 1.5 ± 0.4 days, 3.5 ± 0.6 days, and 4.9 ± 1 days in MOD9 (P < 0.05, Student's t test). Further, the time to detectable growth of M. tuberculosis was measured on MOD9 and Lowenstein-Jensen media after duplicate inoculation of 250 clinical specimens decontaminated with 0.7% chlorhexidine. The contamination rate was 1.6% (4/250) on MOD9 versus 4.4% (11/250) on Lowenstein-Jensen medium (P = 0.11, Fisher's exact test). Chlorhexidine-MOD9 yielded 38/250 (15.2%) isolates versus 32/250 (12.8%) isolates for the chlorhexidine-LJ (P = 0.5195, Fisher's exact test). All together, eight M. tuberculosis isolates were cultured solely from chlorhexidine-MOD9, and two M. tuberculosis isolates were cultured solely from chlorhexidine-LJ. The time to detection was 9.8 ± 3.9 (range, 5 to 18) days for chlorhexidine-MOD9 versus 17.4 ± 5.9 (range, 10 to 35) days for chlorhexidine-LJ (P < 0.05, Student's t test). These data indicate the superiority of the MOD9 medium over the standard LJ medium following chlorhexidine decontamination for the recovery of M. tuberculosis.
INTRODUCTION
Pulmonary tuberculosis caused by Mycobacterium tuberculosis complex (MTC) mycobacteria remains a global public health problem, causing on average 170 deaths every hour worldwide (1). Despite significant advances in the molecular diagnosis of tuberculosis over the past 2 decades (2), culture is still the universal gold standard for the laboratory diagnosis of tuberculosis, enabling complete postculture antimicrobial susceptibility testing and genotyping (3).
MTC mycobacteria are fastidious organisms whose growth is routinely detected only after 7 to 12 days using advanced commercially available automated systems (4). These robots use various formulations of the Middlebrook liquid culture medium and indeed reduce the delay in growth detection compared to that with inoculation of standard solid culture media, such as Lowenstein-Jensen (LJ) medium (4). The World Health Organization (WHO) still recommends inoculating specimens in parallel into a liquid medium for accelerated diagnosis of high-titer specimens and onto a solid culture medium to increase the sensitivity of laboratory diagnosis of pulmonary tuberculosis (5).
In an effort to conciliate speed and sensitivity of the culture-based diagnosis of tuberculosis, we developed new formulations of solid culture medium and new protocols for incubating and detecting colonies of M. tuberculosis. Recently, we showed that the MOD4 culture medium incorporating blood and egg lecithin, combined with an improved laboratory workflow, significantly reduced the time to detection of cultured M. tuberculosis (6).
Capitalizing on this knowledge, we developed a new formulation of the solid culture medium that we named MOD9. The MOD9 culture medium was developed in order to further enhance the sensitivity of the culture of MTC mycobacteria while eliminating the need for blood in this type of culture medium.
Here, we evaluated the performance of MOD9 culture medium in comparison with a commercially available LJ medium (bioMérieux) for the recovery of mycobacteria from clinical specimens.
MATERIALS AND METHODS
M. tuberculosis strains.
The M. tuberculosis H37Rv CIP104475T reference strain and 20 independent clinical isolates, including two Beijing family strains obtained during the routine activity of the Mycobacteria Reference Laboratory, Institut Méditerranée Infection, Marseille, France, were used in this study. All strains were subcultured on LJ medium (bioMérieux, Craponne, France), suspended in phosphate-buffered saline (PBS), rigorously vortexed after adding 3-mm glass beads (Sigma-Aldrich, Saint-Quentin-Fallavier, France), and then passed several times through a 25-G needle to disperse the remaining bacterial clumps. Homogenized suspensions were calibrated using a 580-nm cell densitometer (cell density meter; Fisher Scientific, Illkirch, France) in order to reproducibly obtain a 107 CFU/ml stock suspension.
Clinical specimens.
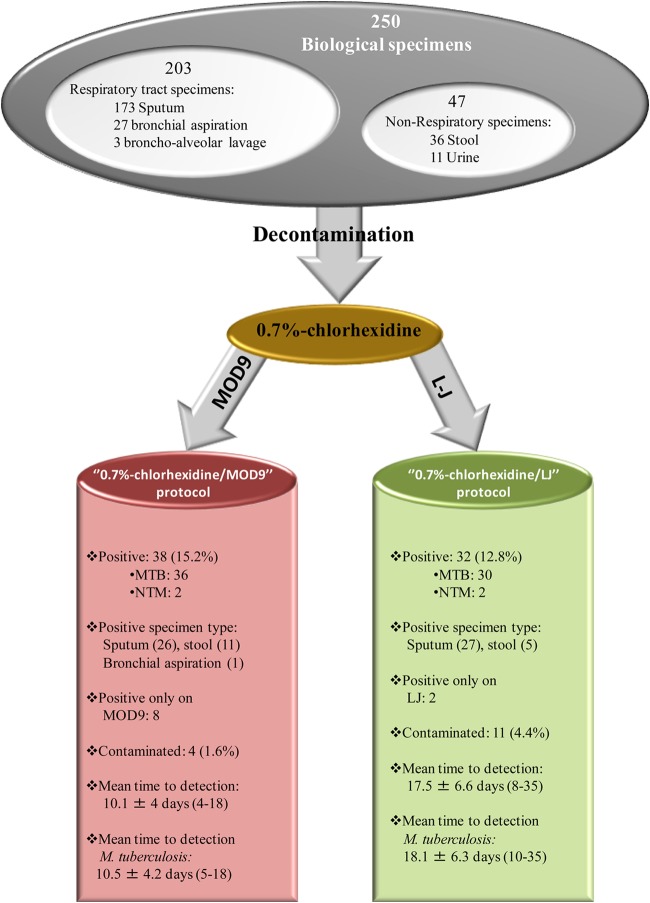
This work was studied and agreed upon by the Institut Fédératif de Recherches 48 ethics committee on 19 February 2007. A total of 250 clinical specimens, including 203 (81.2%) respiratory tract specimens (173 sputum [69.2%], 27 bronchial aspirate [10.8%], and three bronchoalveolar lavage fluid [1.2%] samples) and 47 (18.8%) non-respiratory tract specimens (36 stool samples [14.4%] and 11 urine samples [4.4%]) were collected from 145 patients suspected of tuberculosis; stool samples are routinely collected to aid in the diagnosis of pulmonary tuberculosis (7, 8). Seventy-one patients gave one specimen, 31 patients gave two specimens, 11 patients gave three specimens, five patients gave four specimens, three patients gave five, six, and seven specimens, and one patient gave 10 specimens. Samples sent to the Mycobacteria Reference Laboratory (Institut Méditerranée Infection, Marseille, France) from June to September 2014 were used in this study. The smears prepared for all respiratory tract specimens were stained by the Ziehl-Neelsen method and examined by using light microscopy at 100× magnification (9).
MOD9 medium.
The MOD9 culture medium was derived from the MOD4 culture medium (6) in which the 5% sheep blood and the 15% heat-inactivated bovine serum were eliminated and 15% heat-inactivated lamb serum (Life Technologies, Saint Aubin, France) was added. Also, 100 mg/liter ascorbic acid was added to mimic a microaerophilic atmosphere previously proven to be beneficial for M. tuberculosis growth (6). A mix of azorubine and Ponceau 4R normally used as food dye (Sainte Lucie, Gouvieux, France) was added to stain the medium red and increase the contrast with the white-beige colonies of mycobacteria. The MOD9 culture medium was poured into 55-mm sterile petri dishes (Gosselin, Borre, France).
MOD9 performances on M. tuberculosis strains.
The suspensions (106, 104, and 102 CFU/ml) made from the 21 M. tuberculosis strains under study were used to compare the growth of M. tuberculosis inoculated on MOD9 culture medium and on LJ medium. A 100-μl volume of each M. tuberculosis suspension was inoculated in triplicate on MOD9 plates and LJ tubes. The media were checked visually by the naked eye every 12 h for colonies for 15 days. Ziehl-Neelsen staining of colonies was used to confirm M. tuberculosis.
MOD9 performance on clinical specimens.
Respiratory tract specimens (sputum, bronchial aspirate, and bronchoalveolar lavage fluid specimens) and urine specimens were decontaminated using 0.7% chlorhexidine. Briefly, 3-ml of 0.7% chlorhexidine (MP Biomedicals, Illkirch, France) was mixed with the clinical specimen (adjusted to a volume of 1 ml with sterile PBS) and incubated for 15 min at room temperature (20 to 25°C) with continuous agitation. The volume was then completed to 40 ml with PBS, the suspension was centrifuged at 1,700 × g for 15 min, the supernatant was discarded, and the pellet was suspended in 0.5 ml of sterile PBS and inoculated on either LJ culture medium (chlorhexidine/LJ protocol) or MOD9 culture medium (chlorhexidine/MOD9 protocol) (Fig. 1). As for stool specimens, 10 μl was recovered using a sterile loop and suspended in 2 ml of sterile PBS, and then a triple volume of 0.7% chlorhexidine was added and the rest of the procedure was performed as described above. The culture media were checked visually by the naked eye every 24 h for the presence of colonies for 4 weeks on MOD9 and 8 weeks on LJ. All isolates were identified by Ziehl-Neelsen staining and real-time quantitative PCR (qPCR) for the detection of M. tuberculosis and Mycobacterium avium, as previously described (S. Asmar and M. Drancourt, unpublished data).
FIG 1.
Diagnostic results of 0.7% chlorhexidine/MOD9 and 0.7% chlorhexidine/Lowenstein-Jensen. NTM, nontuberculous mycobacteria.
Statistical analyses.
Fisher's exact test was used to assess the significance of the differences in the contamination and mycobacterial isolation rates and sensitivity. Student's t test was used to compare the detection times. The difference was considered significant when the P value was <0.05.
RESULTS
MOD9 performance on strains.
The mean time to detection of M. tuberculosis colonies was 1.5 ± 0.4 days, 3.5 ± 0.6 days, and 4.9 ± 1 days for 106, 104, and 102 CFU/ml pure suspension, respectively, on MOD9 culture medium versus 5.7 ± 1.5 days (P < 0.05, Student's t test), 7.6 ± 0.8 days (P < 0.05, Student's t test), and 10.8 ± 1.7 days (P < 0.05, Student's t test), respectively, on LJ culture medium. All isolates were positively confirmed by Ziehl-Neelsen staining.
MOD9 performance of clinical specimens.
The contamination rate was 4/250 (1.6%) on MOD9 culture medium (three stool and one sputum sample), significantly lower than 11/250 (4.4%) for LJ (P = 0.1129, Fisher's exact text) (six stool, four sputum, and one bronchial aspirate sample). Three specimens (two stool and one sputum sample) grew contaminants on both media (Burkholderia multivorans, Burkholderia cenocepacia, and Streptococcus oralis); two isolates were not recovered from LJ due to contamination.
A total of 40/250 (16%) specimens collected from 13 patients (9%) yielded positive Mycobacterium species cultures on at least one medium, including 38 M. tuberculosis isolates and two M. abscessus isolates identified by real-time PCR. A total of 18/40 (45%) of these culture-positive specimens were smear-positive specimens, whereas 210 culture-negative specimens were smear negative. Six patients had one positive specimen, two patients had two positive specimens, one patient had three positive specimens, two patients had six positive specimens, one patient had seven positive specimens, and one had eight positive specimens. Thirty out of the 40 (75%) positive specimens from nine patients grew on both media; the LJ medium yielded 32 mycobacterial isolates (80%) (27 from sputum and five from stools) identified as 30 M. tuberculosis isolates (in 25 sputum and five stools) and two M. abscessus isolates from two sputum samples. The MOD9 medium yielded 38 mycobacterial isolates (95%) (26 from sputum, 11 from stool, and one from a bronchial aspiration sample) identified as 36 M. tuberculosis isolates (in 24 sputum, 11 stool, and one bronchial aspirate sample) and two M. abscessus isolates (P = 0.0872, Fisher's exact test). Two M. tuberculosis isolates from sputum specimens were recovered by the LJ protocol only, while eight M. tuberculosis isolates were recovered from six stool, one sputum, and one bronchial aspirate sample by the MOD9 protocol only. The mean time to detection of all mycobacteria grown in common on both media (n = 30) and M. tuberculosis isolates grown on both media (n = 28) was significantly lower for 0.7% chlorhexidine/MOD9 than that for to 0.7% chlorhexidine-LJ (P < 0.05, Student's t test), as shown in Table 1.
TABLE 1.
Comparison of 0.7% chlorhexidine/MOD9 and 0.7% chlorhexidine/Lowenstein-Jensen protocols for the culture of mycobacteria in 250 clinical specimens
| Parametera | Lowenstein-Jensen medium | MOD9 medium |
|---|---|---|
| Sensitivity (no. identified/total no. [%]) | 32/40 (80) | 38/40 (95) |
| Contamination rate (%) | 4.4 | 1.6 |
| Time to detection of mycobacteria (mean ± SD [range]) (days) | 17.5 ± 6.6 (8–35) | 10.1 ± 4 (4–18)b |
| ZN+ | 13.7 ± 4 (8–25) | 6.5 ± 1.7 (4–9) |
| ZN− | 21.9 ± 6.3 (11–35) | 13.4 ± 3.1 (8–18) |
| Time to detection of M. tuberculosis (mean ± SD [range]) (days) | 18.1 ± 6.3 (10–35) | 10.5 ± 4.2 (5–18)b |
ZN+ and ZN−, positive and negative by Ziehl-Neelsen staining, respectively.
P < 0.05, Fisher's exact test.
All together, a total of 229 specimens were free of any contaminant. These 229 specimens yielded a total of 32 isolates, including 30 M. tuberculosis and two M. abscessus isolates.
Considering these 229 specimens, the sensitivities of MOD9 and LJ for culturing mycobacteria were 93.8% and 78.1%, respectively. The mean time to detection of growth was significantly lower for MOD9 than that for LJ (P < 0.05), as shown in Table 1.
DISCUSSION
From the seminal isolation of M. tuberculosis by Robert Koch (10) until today, >15 different culture media have been developed in order to continuously improve the sensitivity and speed of M. tuberculosis culture, yet only a few media are routinely used today (11). This led to the development of several automated systems employing early growth indicators to accelerate growth detection (12). The main and common disadvantages of these systems are their high price and requirement for technical expertise, which have limited their use in resource-poor countries (13). This has allowed the LJ solid medium to remain the most widely used and hence gold standard culture medium (4).
We tried to optimize solid culture media and protocols in order to help speed up and simplify the routine isolation of mycobacteria, including MTC mycobacteria; these efforts yielded a new protocol and a new medium (MOD4) with good performance (6). Here, we modified the MOD4 medium formulation in order to improve its shelf life by eliminating the requirement for fresh blood.
We observed that the protocol combining the decontamination of clinical specimens with 0.7% chlorhexidine and of the use MOD9 culture medium showed clearly optimized performance compared to that with the standard LJ protocol. We validated the new protocol with 38 isolates on MOD9 culture medium versus 32 on LJ culture medium, including six stool isolates illustrating the superior sensitivity of MOD9 over Lowenstein-Jensen, as stools present a lower inoculum of M. tuberculosis than that for sputum. As for the 18 M. tuberculosis isolates that grew in both culture media evaluated, we observed that the MOD9 culture medium yielded a 45% reduction in the time to detection compared to that with the LJ protocol. In this work, it was not possible to use microscopy to read the Lowenstein-Jensen tubes; therefore, microscopy was also not used to read the MOD9 plates, to maintain a similar method in the readings. Indeed, if we had used microscopy as for the thin-layer agar and microscopic observations of drug susceptibility protocols (14, 15), this would have resulted in the rapid detection of microcolonies and thus rapid diagnosis (6).
The fact that Robert Koch reported the isolation of M. tuberculosis in <10 days by inoculating a simple serum-based medium has been neglected (10). With the MOD9 culture medium, we resurrected the Koch's serum-based medium from the late 19th century, adding growth enhancers to propose a protocol that is now routinely used at La Timone Hospital for the rapid detection of tuberculosis and nontuberculous mycobacteria. Evaluation of MOD9 medium on a larger number of tuberculosis (TB)-positive clinical specimens at La Timone Hospital and other external sites are needed for further verification and validation of the newly proposed culture protocol in the near future.
ACKNOWLEDGMENTS
S. Asmar, S. Chatellier, M. Drancourt, and D. Raoult are coinventors, with a patent owned by IHU Méditerranée Infection and bioMérieux, protecting the MOD9 culture medium reported here. C. Mirande, I. Canard, S. Chatellier, and A. van Belkum are employees of bioMérieux.
REFERENCES
- 1.World Health Organization. 2014. Global tuberculosis report 2014. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf?ua=1. [Google Scholar]
- 2.Steingart KR, Sohn H, Schiller I, Kloda LA, Boehme CC, Pai M, Dendukuri N. 2013. Xpert MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 1:CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenover FC, Crawford JT, Huebner RE, Geiter LJ, Horsburgh CR Jr, Good RC. 1993. The resurgence of tuberculosis: is your laboratory ready? J Clin Microbiol 31:767–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rageade F, Picot N, Blanc-Michaud A, Chatellier S, Mirande C, Fortin E, van Belkum A. 2014. Performance of solid and liquid culture media for the detection of Mycobacterium tuberculosis in clinical materials: meta-analysis of recent studies. Eur J Clin Microbiol Infect Dis 33:867–870. doi: 10.1007/s10096-014-2105-z. [DOI] [PubMed] [Google Scholar]
- 5.Nolte FS, Metchock B. 1999. Mycobacterium, p 400–437. In Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolkia RH, ed. 1999. Manual of clinical microbiological laboratory, 6th ed ASM Press, Washington, DC. [Google Scholar]
- 6.Ghodbane R, Raoult D, Drancourt M. 2014. Dramatic reduction of culture time of Mycobacterium tuberculosis. Sci Rep 4:4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Khéchine A, Henry M, Raoult D, Drancourt M. 2009. Detection of Mycobacterium tuberculosis complex organisms in the stools of patients with pulmonary tuberculosis. Microbiology 155:2384–2389. doi: 10.1099/mic.0.026484-0. [DOI] [PubMed] [Google Scholar]
- 8.Bonnave PE, Raoult D, Drancourt M. 2013. Gastric aspiration is not necessary for the diagnosis of pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis 32:569–571. doi: 10.1007/s10096-012-1776-6. [DOI] [PubMed] [Google Scholar]
- 9.Van Deun A, Hossain MA, Gumusboga M, Rieder HL. 2008. Ziehl-Neelsen staining: theory and practice. Int J Tuberc Lung Dis 12:108–110. [PubMed] [Google Scholar]
- 10.Cambau E, Drancourt M. 2014. Steps towards the discovery of Mycobacterium tuberculosis by Robert Koch, 1882. Clin Microbiol Infect 20:196–201. doi: 10.1111/1469-0691.12555. [DOI] [PubMed] [Google Scholar]
- 11.Soto A, Agapito J, Acuña-Villaorduña C, Solari L, Samalvides F, Gotuzzo E. 2009. Evaluation of the performance of two liquid-phase culture media for the diagnosis of pulmonary tuberculosis in a national hospital in Lima, Peru. Int J Infect Dis 13:40–45. doi: 10.1016/j.ijid.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Hillemann D, Richterand E, Rüsch-Gerdes S. 2006. Use of the Bactec Mycobacteria Growth Indicator Tube 960 automated system for recovery of mycobacteria from 9,558 extrapulmonary specimens, including urine samples. J Clin Microbiol 44:4014–4017. doi: 10.1128/JCM.00829-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perkins MD, Cunningham J. 2007. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J Infect Dis 196:S15–S27. doi: 10.1086/518656. [DOI] [PubMed] [Google Scholar]
- 14.Moore DA, Evans CA, Gilman RH, Caviedes L, Coronel J, Vivar A, Sanchez E, Piñedo Y, Saravia JC, Salazar C, Oberhelman R, Hollm-Delgado MG, LaChira D, Escombe AR, Friedland JS. 2006. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med 15:1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Battaglioli T, Rintiswati N, Martin A, Palupi KR, Bernaerts G, Dwihardiani B, Ahmad RA, Matthys F, Mahendradhata Y, Van der Stuyft P. 2013. Comparative performance of thin layer agar and Lowenstein-Jensen culture for diagnosis of tuberculosis. Clin Microbiol Infect 19:E502–E508. doi: 10.1111/1469-0691.12265. [DOI] [PubMed] [Google Scholar]