Abstract
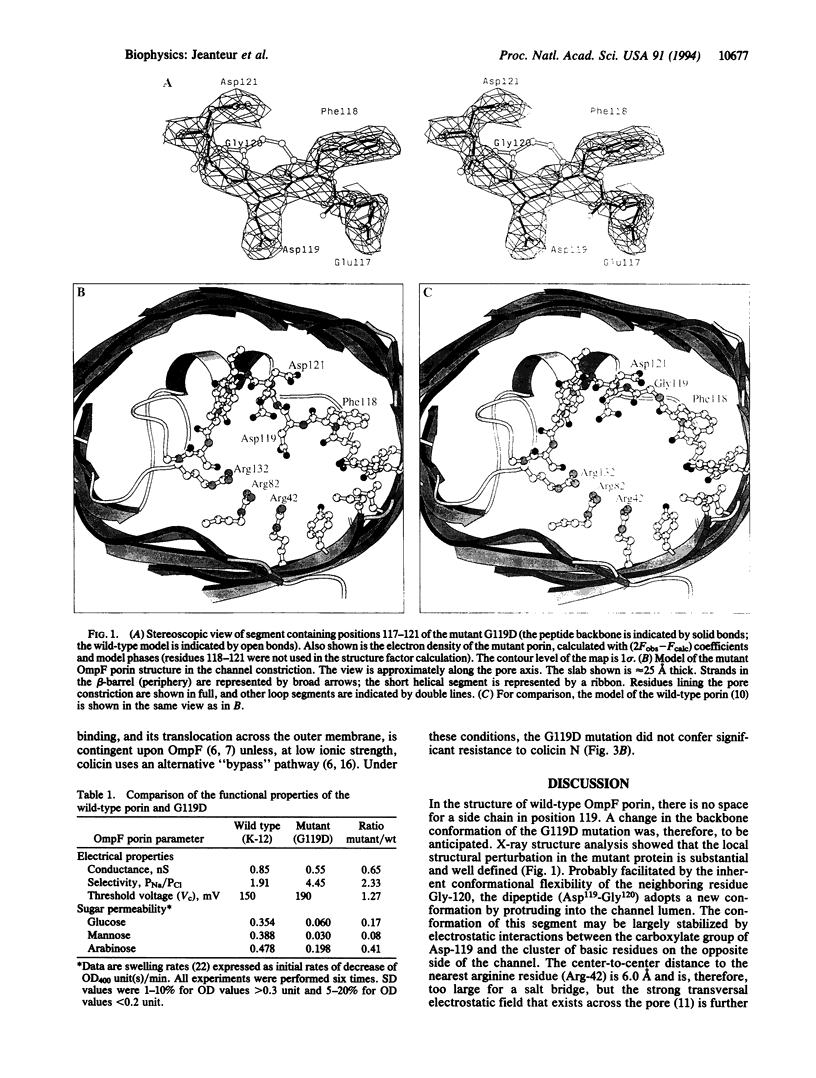
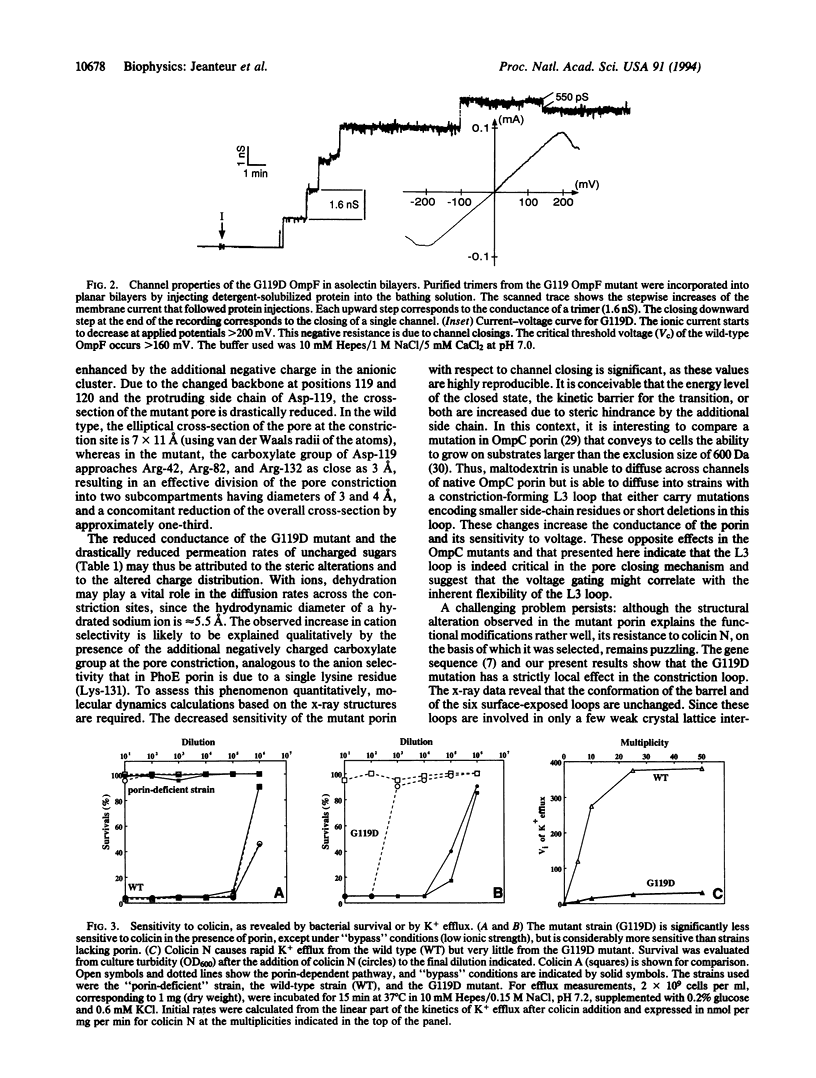
A strain of Escherichia coli, selected on the basis of its resistance to colicin N, reveals distinct structural and functional alterations in unspecific OmpF porin. A single mutation [Gly-119-->Asp (G119D)] was identified in the internal loop L3 that contributes critically to the formation of the construction inside the lumen of the pore. X-ray structure analysis to a resolution of 3.0 A reveals a locally altered peptide backbone, with the side chain of residue Asp-119 protruding into the channel, causing the area of the constriction (7 x 11 A in the wild type) to be subdivided into two intercommunicating subcompartments of 3-4 A in diameter. The functional consequences of this structural modification consist of a reduction of the channel conductance by about one-third, of altered ion selectivity and voltage gating, and of a decrease of permeation rates of various sugars by factors of 2-12. The structural modification of the mutant protein affects neither the beta-barrel structure nor those regions of the molecule that are exposed at the cell surface. Considering the colicin resistance of the mutant, it is inferred that in vivo, colicin N traverses the outer membrane through the porin channel or that the dynamics of the exposed loops are affected in the mutant such that these may impede the binding of the toxin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baty D., Frenette M., Lloubès R., Geli V., Howard S. P., Pattus F., Lazdunski C. Functional domains of colicin A. Mol Microbiol. 1988 Nov;2(6):807–811. doi: 10.1111/j.1365-2958.1988.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Benson S. A., Occi J. L., Sampson B. A. Mutations that alter the pore function of the OmpF porin of Escherichia coli K12. J Mol Biol. 1988 Oct 20;203(4):961–970. doi: 10.1016/0022-2836(88)90121-0. [DOI] [PubMed] [Google Scholar]
- Benz R., Bauer K. Permeation of hydrophilic molecules through the outer membrane of gram-negative bacteria. Review on bacterial porins. Eur J Biochem. 1988 Sep 1;176(1):1–19. doi: 10.1111/j.1432-1033.1988.tb14245.x. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Boos W., Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978 Aug 17;511(3):305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- Boulanger P., Letellier L. Characterization of ion channels involved in the penetration of phage T4 DNA into Escherichia coli cells. J Biol Chem. 1988 Jul 15;263(20):9767–9775. [PubMed] [Google Scholar]
- Bourdineaud J. P., Boulanger P., Lazdunski C., Letellier L. In vivo properties of colicin A: channel activity is voltage dependent but translocation may be voltage independent. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1037–1041. doi: 10.1073/pnas.87.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehler L. K., Kusumoto S., Zhang H., Rosenbusch J. P. Plasticity of Escherichia coli porin channels. Dependence of their conductance on strain and lipid environment. J Biol Chem. 1991 Dec 25;266(36):24446–24450. [PubMed] [Google Scholar]
- Bénédetti H., Lloubès R., Lazdunski C., Letellier L. Colicin A unfolds during its translocation in Escherichia coli cells and spans the whole cell envelope when its pore has formed. EMBO J. 1992 Feb;11(2):441–447. doi: 10.1002/j.1460-2075.1992.tb05073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan S. W., Schirmer T., Rummel G., Steiert M., Ghosh R., Pauptit R. A., Jansonius J. N., Rosenbusch J. P. Crystal structures explain functional properties of two E. coli porins. Nature. 1992 Aug 27;358(6389):727–733. doi: 10.1038/358727a0. [DOI] [PubMed] [Google Scholar]
- Fourel D., Hikita C., Bolla J. M., Mizushima S., Pagès J. M. Characterization of ompF domains involved in Escherichia coli K-12 sensitivity to colicins A and N. J Bacteriol. 1990 Jul;172(7):3675–3680. doi: 10.1128/jb.172.7.3675-3680.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourel D., Mizushima S., Bernadac A., Pagès J. M. Specific regions of Escherichia coli OmpF protein involved in antigenic and colicin receptor sites and in stable trimerization. J Bacteriol. 1993 May;175(9):2754–2757. doi: 10.1128/jb.175.9.2754-2757.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavito R. M., Rosenbusch J. P. Isolation and crystallization of bacterial porin. Methods Enzymol. 1986;125:309–328. doi: 10.1016/s0076-6879(86)25027-2. [DOI] [PubMed] [Google Scholar]
- Jackson M. E., Pratt J. M., Stoker N. G., Holland I. B. An inner membrane protein N-terminal signal sequence is able to promote efficient localisation of an outer membrane protein in Escherichia coli. EMBO J. 1985 Sep;4(9):2377–2383. doi: 10.1002/j.1460-2075.1985.tb03942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanteur D., Lakey J. H., Pattus F. The bacterial porin superfamily: sequence alignment and structure prediction. Mol Microbiol. 1991 Sep;5(9):2153–2164. doi: 10.1111/j.1365-2958.1991.tb02145.x. [DOI] [PubMed] [Google Scholar]
- Karshikoff A., Spassov V., Cowan S. W., Ladenstein R., Schirmer T. Electrostatic properties of two porin channels from Escherichia coli. J Mol Biol. 1994 Jul 22;240(4):372–384. doi: 10.1006/jmbi.1994.1451. [DOI] [PubMed] [Google Scholar]
- Lakey J. H., Lea E. J., Pattus F. ompC mutants which allow growth on maltodextrins show increased channel size and greater voltage sensitivity. FEBS Lett. 1991 Jan 14;278(1):31–34. doi: 10.1016/0014-5793(91)80076-f. [DOI] [PubMed] [Google Scholar]
- Misra R., Benson S. A. Genetic identification of the pore domain of the OmpC porin of Escherichia coli K-12. J Bacteriol. 1988 Aug;170(8):3611–3617. doi: 10.1128/jb.170.8.3611-3617.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989 Nov;33(11):1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Porins and specific diffusion channels in bacterial outer membranes. J Biol Chem. 1994 Feb 11;269(6):3905–3908. [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983 Jan;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauptit R. A., Zhang H., Rummel G., Schirmer T., Jansonius J. N., Rosenbusch J. P. Trigonal crystals of porin from Escherichia coli. J Mol Biol. 1991 Apr 5;218(3):505–507. doi: 10.1016/0022-2836(91)90696-4. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P. The ins and outs of colicins. Part I: Production, and translocation across membranes. Microbiol Sci. 1984 Oct;1(7):168–175. [PubMed] [Google Scholar]
- Schindler H. Formation of planar bilayers from artificial or native membrane vesicles. FEBS Lett. 1980 Dec 15;122(1):77–79. doi: 10.1016/0014-5793(80)80405-4. [DOI] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3751–3755. doi: 10.1073/pnas.75.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein in planar membranes: clusters of channels in a native environment and their functional reassembly. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2302–2306. doi: 10.1073/pnas.78.4.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. E. The tol gene products and the import of macromolecules into Escherichia coli. Mol Microbiol. 1991 May;5(5):1005–1011. doi: 10.1111/j.1365-2958.1991.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Wilmsen H. U., Pugsley A. P., Pattus F. Colicin N forms voltage- and pH-dependent channels in planar lipid bilayer membranes. Eur Biophys J. 1990;18(3):149–158. doi: 10.1007/BF02427374. [DOI] [PubMed] [Google Scholar]
- el Kouhen R., Fierobe H. P., Scianimanico S., Steiert M., Pattus F., Pagès J. M. Characterization of the receptor and translocator domains of colicin N. Eur J Biochem. 1993 Jun 15;214(3):635–639. doi: 10.1111/j.1432-1033.1993.tb17963.x. [DOI] [PubMed] [Google Scholar]




