Abstract
Tuberculosis (TB) is a global public health problem, with the highest burden occurring in low-income countries. In these countries, the use of more sensitive diagnostics, such as Xpert MTB/RIF (Xpert), is still limited by costs. A cost-saving strategy to diagnose other diseases is to pool samples from various individuals and test them with single tests. The samples in positive pool samples are then retested individually to identify the patients with the disease. We assessed a pooled testing strategy to optimize the affordability of Xpert for the diagnosis of TB. Adults with presumptive TB attending hospitals or identified by canvassing of households in Abuja, Nigeria, were asked to provide sputum for individual and pooled (4 per pool) testing. The agreement of the results of testing of individual and pooled samples and costs were assessed. A total of 738 individuals submitted samples, with 115 (16%) being Mycobacterium tuberculosis positive. Valid Xpert results for individual and pooled samples were available for 718 specimens. Of these, testing of pooled samples detected 109 (96%) of 114 individual M. tuberculosis-positive samples, with the overall agreement being 99%. Xpert semiquantitative M. tuberculosis levels had a positive correlation with the smear grades, and the individual sample-positive/pooled sample-negative results were likely due to the M. tuberculosis concentration being below the detection limit. The strategy reduced cartridge costs by 31%. Savings were higher with samples from individuals recruited in the community, where the proportion of positive specimens was low. The results of testing of pooled samples had a high level of agreement with the results of testing of individual samples, and use of the pooled testing strategy reduced costs and has the potential to increase the affordability of Xpert in countries with limited resources.
INTRODUCTION
Tuberculosis (TB) is a significant global public health problem (1). Despite the availability of curative treatment, TB sits behind only human immunodeficiency virus (HIV) as the major cause of mortality associated with infectious disease worldwide (1). In 2013 there were an estimated 9 million new cases and 1.5 million deaths from TB, most of which occurred in low- and middle-income countries (LMICs) (1). The highest rates of TB per capita and the highest proportion of cases with HIV coinfection occur in sub-Saharan Africa (1).
In most low-income countries, direct sputum smear microscopy is the mainstay of TB diagnostics (2), as this test is inexpensive and highly specific, but it has a low to moderate sensitivity (2). The sensitivity of direct sputum smear microscopy is lower in patients with paucibacillary disease associated with HIV coinfection and in children, due to lower bacillary loads (3), and it cannot provide information on drug susceptibility (4). Conversely, sputum culture, in particular, automated liquid culture, is the most sensitive and specific diagnostic tool available for TB and facilitates drug susceptibility testing (2). However, culture requires a laboratory infrastructure, including biosafety equipment, not widely available in low-resource settings, and results typically take 2 to 6 weeks and, therefore, are rarely helpful for initial treatment decisions (2, 4).
The Xpert MTB/RIF (Xpert) assay (Cepheid Inc., Sunnyvale, CA, USA) is a self-contained, fully automated, real-time PCR assay that facilitates rapid semiquantitative detection of Mycobacterium tuberculosis and rifampin (RIF) resistance with minimal laboratory requirements compared to those needed for culture and other manually operated nucleic acid amplification tests (NAATs) (4). Xpert is highly specific (99%) and substantially more sensitive than smear microscopy (4). The assay's turnaround time is less than 2 h, greatly shortening the time to TB diagnosis in locations where the machine is available, and it detects markers of RIF resistance (4). For low-income countries, the single-use cartridges cost $9.98 (FIND, 2013). However, despite this concessionary pricing, the cost involved to purchase and run the tests is still a limiting factor for widespread sustainable adoption of Xpert by TB control programs in LMICs (4, 5).
The high costs of diagnostics are not confined to TB, and the more cost-effective use of diagnostic tests for other infectious diseases has been explored. One approach that can reduce costs is to pool (put together) specimens from several patients and test them using a single test (6, 7). If a pool tests positive, then each specimen is tested individually to detect the positive sample(s), whereas if the pooled specimens test negative, all individuals are considered infection free (6, 7). A pooling strategy appears to be cost-effective and accurate when NAATs are used to screen blood for HIV (8) and blood-borne hepatitis viruses (9), detect Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens (7), and identify influenza virus in nasopharyngeal swab samples (4). A potential disadvantage of pooled testing, however, is a decrease in test sensitivity through dilution of positive specimens beyond an assay limit of detection (10). The cost savings of pooled testing are determined by the prevalence of disease in the tested population, the number of samples per pool, and the degree of clustering of positive individuals in the tested population (6, 11).
Therefore, as a means to optimize the use of Xpert cartridges, we explored whether a pooling strategy could be applied to sputum samples from individuals being screened for TB in a low-income, high-HIV-prevalence setting. This study evaluated the agreement and cost savings of a two-stage pooled testing approach, whereby sputum samples from four consecutive patients were tested using a single Xpert cartridge with follow-on individual testing of positive pools and the results were compared to those obtained by individual Xpert testing of each sample. We also evaluated whether the rate of detection of positive samples varied with smear microscopy grade and assessed the impact of specimen dilution and the relationship between smear grade and the Xpert semiquantitative M. tuberculosis level.
MATERIALS AND METHODS
The study took place in the Federal Capital Territory (FCT) of Nigeria. New adult patients with suspected pulmonary TB (PTB), presumed on the basis of a cough for more than 2 weeks, were recruited consecutively using two strategies. First, all adults with suspected PTB who presented to five outpatient departments of district hospitals in the FCT (Wuse, Bwari, Kwali, Kuje, and the university teaching hospital) were asked to participate. Second, patients with suspected PTB (symptomatic individuals) were recruited by community health extension workers canvassing consecutive households in slum areas and rural villages of the five FCT local government area councils (Abaji, Bwari, Kuje, Kwali, and Gwagwalada). These two recruitment strategies were used, as it was expected that the TB prevalence would be higher among hospital patients than those identified in the community. Each participating individual provided at least two spot sputum samples for standard diagnostic practice, and the first one was also used for the evaluation in this study. Patients were asked to provide at least 5 ml of sputum in sputum cups with a wide mouth and a line to mark the amount. However, some patients had difficulty producing this amount of sputum. Patients submitting specimens with less than 2 ml were asked to produce further specimens because it would not have allowed testing of specimens in duplicate (Xpert requires a minimum of 2 ml per test).
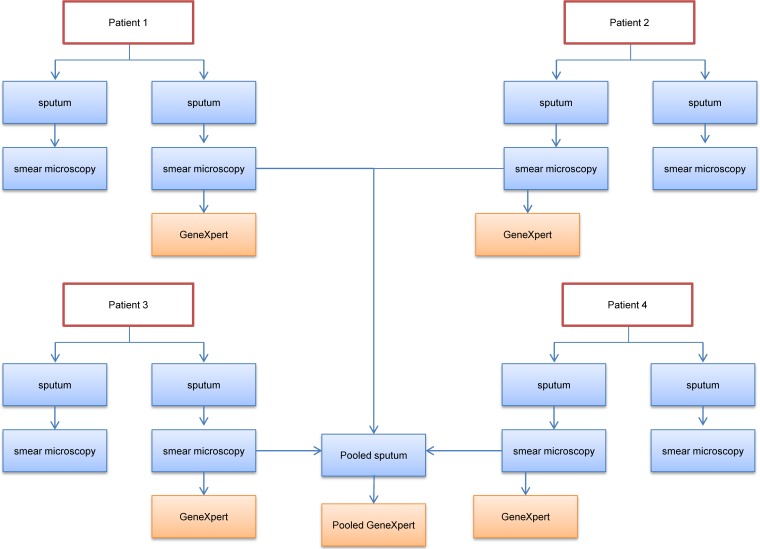
The two sputum samples were tested using Ziehl-Neelsen staining and smear microscopy and were graded per the World Health Organization (WHO) criteria (12). After smear preparation, the first sputum specimen was mixed with the Xpert MTB/RIF sample reagent (SR) in equal amounts per the manufacturer's guidelines. Two milliliters of this volume was transferred into a separate container with three other specimens. Each container of pooled sputa was manually shaken for approximately 1 min, and 2 ml of the pool was transferred to an Xpert MTB/RIF cartridge. Two milliliters of each remaining specimen processed with the SR was added to an individual Xpert cartridge. The individual and pooled samples were then tested simultaneously (Fig. 1). Xpert tests producing nonvalid results (error, an invalid result, or no result) were retested, if sufficient sample was available.
FIG 1.
Flow diagram of the sputum processing scheme used.
For the purposes of this analysis, the individual Xpert MTB/RIF result was considered an individual's definitive TB status. Categorical data were summarized using frequency counts and percentages, with the chi-square test being used to test for significant differences where appropriate. Continuous data were summarized using the median and range. The agreement of the results obtained by use of the pooled and individual testing strategies was determined, with tests of agreement being performed (the kappa statistic was calculated). The relationship between the smear grade and the individual Xpert M. tuberculosis concentration and between the individual and pooled Xpert M. tuberculosis concentrations was evaluated using the Spearman rank order correlation. Cost differences were calculated on the basis of the number of cartridges that would have been required to test all specimens when using either a pooled or an individual testing strategy at a cartridge price of $9.98. Theoretical cost savings for pools of different sizes were also calculated using the formula proposed by Raboud et al. that estimates the number of positive pools for a given disease prevalence (13).
Ethical approval was obtained from the Liverpool School of Tropical Medicine Research Ethics Committee and the FCT Health Research Ethics Committee. All participants gave informed consent.
RESULTS
A total of 738 individuals with suspected PTB were recruited and supplied spot sputum samples for the study. Of the 738 individuals, 488 (66%) were recruited in the community and 250 (34%) were from district hospitals. The participants' baseline characteristics are described in Table 1. The 738 sputum samples were tested individually, and 183 pools of four samples plus 2 pools that contained only three samples (185 pools) were tested with the Xpert MTB/RIF assay.
TABLE 1.
Baseline characteristics of participantsa
| Characteristic | Community | District hospital | Total |
|---|---|---|---|
| No. of participants | 488 | 250 | 738 |
| Median (range) age (yr) | 38 (12–85) | 35 (15–90) | 37 (12–90) |
| No. (%) participants | |||
| Femaleb | 230 (54) | 99 (47) | 329 (51) |
| GeneXpert-confirmed PTB cases | 51 (11) | 64 (26) | 115 (16) |
| Confirmed PTB cases that were smear positive | 20 (45) | 37 (65) | 57 (56) |
Only 101 of 115 Xpert M. tuberculosis-positive PTB cases had a valid smear result. For both Xpert M. tuberculosis-positive and smear-positive cases, the percentages given use the number of cases with available data as the denominator.
Gender data were available for only 641 participants.
One hundred fifteen (16%) of 738 samples were individual Xpert M. tuberculosis positive, 614 (83%) were negative, and 9 (1%) had failed results (error, invalid result, no result). Thirty-two (4%) samples had an initial failed result, and 23 of these were successfully retested. Four (3%) of 115 M. tuberculosis-positive specimens were resistant to rifampin (Xpert RIF positive). As expected, there was a strong positive correlation between smear microscopy grade and Xpert semiquantitative M. tuberculosis level (rho = 0.694, P < 0.001) (Table 2). Only 2 (6%) of 31 samples with M. tuberculosis levels that were very low (cycle threshold [CT] value, >28) or low (CT value, 22 to 28) were smear positive, 20 (63%) of 32 samples with a medium M. tuberculosis level (CT value, 16 to 22) were smear positive, and 33 (92%) of 36 samples with a high M. tuberculosis level (CT value, <16) were smear positive.
TABLE 2.
Individual Xpert M. tuberculosis results by smear gradea
| Smear grade | No. (%) of participants with the following Xpert semiquantitative M. tuberculosis level: |
|||||
|---|---|---|---|---|---|---|
| Negative | Very low | Low | Medium | High | Total | |
| Negative | 540 (98) | 14 (88) | 15 (100) | 12 (38) | 3 (8) | 584 (90) |
| Scanty | 5 (1) | 1 (6) | 0 (0) | 3 (9) | 4 (11) | 13 (2) |
| 1+ | 4 (1) | 0 (0) | 0 (0) | 7 (22) | 8 (22) | 19 (3) |
| 2+ | 0 (0) | 0 (0) | 0 (0) | 8 (25) | 11 (31) | 19 (3) |
| 3+ | 0 (0) | 1 (6) | 0 (0) | 2 (6) | 10 (28) | 13 (2) |
| Total | 549 (100) | 16 (100) | 15 (100) | 32 (100) | 36 (100) | 648 (100) |
Only cases that had both a valid Xpert M. tuberculosis result and a smear microscopy result are included. Specifically, data for only 99 of 115 individual Xpert M. tuberculosis-positive cases in the study are included here, as 14 did not have an available smear result, and 2 individuals with smear-positive/Xpert-positive results were missing Xpert semiquantitative M. tuberculosis level data. Smear grading was as follows: scanty, 1 to 9 acid-fast bacilli per 100 immersion fields; 1+, 10 to 99 acid-fast bacilli per 100 immersion fields; 2+, 1 to 10 acid-fast bacilli per immersion field; and 3+, >10 acid-fast bacilli per immersion field. Xpert semiquantitative M. tuberculosis levels were classified as follows: very low, CT value of >28; low, CT value of 22 to 28; medium, CT value of 16 to 22; and high, CT value of <16.
Eighty-one (44%) of the 185 pools were Xpert positive for M. tuberculosis, 101 (55%) were negative, and 3 (2%) had a failed result reported. Six (3%) pools had failed results reported initially; however, three were successfully retested. There was no significant difference (P = 0.47) in the number of failed Xpert results reported (before retesting) with testing of pooled (6 of 185, 3%) and individual (32 of 738, 4%) samples. Ninety-eight of 185 pools contained only samples collected in the community, and of these, 33 (34%) were positive; 42 pools contained only samples collected from district hospitals, and of these, 27 (64%) were positive; and 45 pools contained a mixture of community and hospital samples, and of these, 21 (47%) were positive.
Eighty (99%) of 81 M. tuberculosis-positive pools had at least one M. tuberculosis-positive sample from individual testing (true positives), with 1 M. tuberculosis-positive pool (1%) containing only negative samples (false positive). Conversely, 96 (95%) of the 101 M. tuberculosis-negative pools contained only M. tuberculosis-negative samples from individual testing (true negatives); 5 M. tuberculosis-negative pools (5%) had one sample that was M. tuberculosis positive upon individual testing (false negatives). Fifty-six (70%) of the 80 true-positive pools had one M. tuberculosis-positive sample, 20 (25%) pools had two M. tuberculosis-positive samples, 3 (4%) pools had three M. tuberculosis-positive samples, and 1 (1%) pool had four M. tuberculosis-positive samples. Resistance to rifampin was detected in 3 (4%) of the 81 M. tuberculosis-positive pools. All three (100%) pools had one or more RIF-resistant samples upon individual testing. Seventy-seven (99%) of the 78 RIF-negative pools contained only rifampin-sensitive samples, with 1 RIF-sensitive pool (1%) containing one RIF-resistant sample.
A cross tabulation of 61 pools containing only one M. tuberculosis-positive sample is shown in Table 3. As expected, there was a strong positive correlation between the Xpert M. tuberculosis concentration in the individual and pooled tests (rho = 0.799, P < 0.001). Five (50%) of 10 samples with very low individual M. tuberculosis levels were negative when tested in a pool. Likewise, samples with low, medium, and high individual M. tuberculosis levels frequently had lower concentrations reported in the pooled assay.
TABLE 3.
Cross tabulation of Xpert M. tuberculosis individual and pooled test concentrationsa
| Xpert semiquantitative M. tuberculosis level in pooled test | No. (%) of participants with the following individual Xpert semiquantitative M. tuberculosis level: |
||||
|---|---|---|---|---|---|
| Very low | Low | Medium | High | Total | |
| Negative | 5 (50) | 0 (0) | 0 (0) | 0 (0) | 5 (8) |
| Very low | 4 (40) | 7 (70) | 2 (8) | 0 (0) | 13 (21) |
| Low | 0 (0) | 3 (30) | 9 (38) | 0 (0) | 12 (20) |
| Medium | 1 (10) | 0 (0) | 9 (38) | 8 (47) | 18 (30) |
| High | 0 (0) | 0 (0) | 4 (17) | 9 (53) | 13 (21) |
| Total | 10 (100) | 10 (100) | 24 (100) | 17 (100) | 61 (100) |
Only results for pools containing one GeneXpert-positive sample are included. Xpert semiquantitative M. tuberculosis levels were classified as follows: very low, CT value of >28; low, CT value of 22 to 28; medium, CT value of 16 to 22; high, CT value of <16.
Seven hundred eighteen sputum samples had both valid individual and pooled Xpert results, while 639 samples had a valid smear result and both valid individual and pooled Xpert results. A pooled testing strategy (pooled testing plus follow-on individual testing of each specimen from positive pools) would have detected 109 (96%) of the 114 individual M. tuberculosis-positive samples and correctly identified 604 (100%) of 604 individual M. tuberculosis-negative samples. One M. tuberculosis-positive pool, however, contained only M. tuberculosis-negative samples on follow-on individual testing, thus giving an M. tuberculosis-negative result for the pooled testing strategy. Thus, the results of the pooled testing strategy agreed with those of the individual testing approach in 713 (99%) out of 718 instances (kappa value, 0.973; P < 0.001). After exclusion of samples without a smear result, the pooled testing approach would have detected 55 (98%) of 56 smear-positive samples and 42 (95%) of 44 smear-negative/M. tuberculosis-positive samples. After further exclusion of smear-negative samples pooled with smear-positive samples (which could be responsible for pool positivity), a pooled testing approach would still have detected 32 (94%) of 34 smear-negative/M. tuberculosis-positive cases.
We assessed the time that it took the investigator to perform the manual steps of the assay under different scenarios for the first 284 patients. The scenarios included processing of a single sample, simultaneous processing of a batch of 4 samples for individual testing, and processing of a pool of 4 samples. The results were used to estimate the time saved by the use of pooled testing. Testing of samples individually required 607 h, and testing of samples individually in batches of four reduced the time by 446 h (73%), assuming that all samples were available for testing and processed simultaneously. The pooled strategy, which required testing of 71 pools followed by the individual testing of 140 samples from positive pools, reduced the testing time by 377 h (62%), assuming that individual samples from positive pools were tested simultaneously in batches of 4.
The cost of the cartridges for the testing of 738 samples individually was $7.365.24. Testing of 185 pools and retesting of 323 samples individually from the 81 positive pools (80 × 4 samples and 1 × 3 samples) would cost $5,069.84. Overall, a pooled testing strategy would have saved $2,295.40 (31%, equivalent to 230 cartridges). Pooled testing of the 98 community-only samples would cost $2,295.40, whereas testing of the 392 samples individually would cost $3,912.16; thus, the savings are $1,616.76 (41%). Conversely, pooled testing of the 42 district hospital-only samples would cost $1,487.02, whereas testing of the 167 samples individually would cost $1,666.66; thus, the savings are only $179.64 (11%). The theoretical cost savings for pools of different sizes using the PTB prevalence values obtained in this study are shown in Table 4.
TABLE 4.
Theoretical cost savings of a pooled testing strategy for 738 samples using different pool sizes by study setting
| Study setting (disease prevalence) | Pool size | No of pooled tests + no. of individual tests requireda | Cost ($) of pooled testing strategy | Cost savings ($) with pooled testing strategyb |
|---|---|---|---|---|
| Community (11%) | 3 | 246 + 219 | 4,640.70 | 2,724.54 (37) |
| 4 | 185 + 276 | 4,600.78 | 2,764.46 (38) | |
| 5 | 148 + 325 | 4,720.54 | 2,644.70 (36) | |
| District hospital (26%) | 3 | 246 + 438 | 6,826.32 | 538.92 (7) |
| 4 | 185 + 520 | 7,035.90 | 329.34 (4) | |
| 5 | 148 + 575 | 7,215.54 | 149.70 (2) | |
| Total population (16%) | 3 | 246 + 300 | 5,449.08 | 1,916.16 (26) |
| 4 | 185 + 372 | 5,558.86 | 1,806.38 (25) | |
| 5 | 148 + 430 | 5,768.44 | 1,596.80 (22) |
The probability of pool testing positive is equal to 1 − (1 − P)n, where P is the prevalence of disease and n is the size of the pool.
Reduction in Xpert cartridge costs compared to individual testing at a cost of $7,365.24 for 738 samples. Values in parentheses are the percent savings compared with the cost of testing of individual samples.
DISCUSSION
New testing platforms such as Xpert MTB/RIF have a significant potential to increase the sensitivity of TB diagnostics in areas with a high prevalence of TB (4). However, their high cost relative to the cost of smear microscopy is still a limitation to their widespread use (4). This study evaluated pooled testing of sputum with the Xpert MTB/RIF assay as a way to increase its affordability and demonstrated substantial cost savings with only a limited loss of accuracy.
The overall prevalence of PTB in the study population was 16%, with 56% of confirmed cases having smear-positive disease. Not unexpectedly, individuals recruited from the hospitals had a higher prevalence of PTB and smear-positive disease than those recruited from the community. Individuals with PTB who are identified via presentation to health services tend to be more symptomatic, have more advanced disease, and have a higher rate of smear positivity than those identified through active case finding (14). Conversely, individuals with chronic cough in the community may be more likely to have other chronic respiratory problems.
Predictably, individual Xpert testing confirmed PTB in 44 smear-negative samples. The strong positive correlation between smear grade and Xpert semiquantitative M. tuberculosis level is in keeping with the findings described in previous reports (15). An Xpert finding of a very low/low or a high M. tuberculosis level was reasonably predictive of smear-negative or -positive disease, respectively, and could be used for monitoring the quality of smear microscopy. This information may also be useful for infection control purposes. Some discrepant results were observed, such as a sample with a 3+ smear but a very low Xpert M. tuberculosis level. This could be due to sampling during sputum smear preparation, as the bacilli are not evenly distributed in the specimen. These findings are similar to those presented in previous reports showing that Xpert is predictive of smear status only at the extremes of cycle threshold values (16).
The agreement between a pooled and an individual Xpert testing strategy was 99%, with pooled testing detecting 96% of individual Xpert M. tuberculosis-positive cases overall and 94% of cases from smear-negative pools. The latter is important, as Xpert is often used as a follow-on test in smear-negative individuals. Pooling of the samples did not appear to result in PCR inhibition, as no difference in the rate of failed tests was found. The maintenance of intrinsic assay performance and diagnostic accuracy suggests that pooling of sputum for Xpert testing is a technically feasible option.
There were five false-negative pools, each containing a single sample with a very low individual M. tuberculosis level. False-negative results likely occurred because the small amount of M. tuberculosis bacilli in these positive samples was diluted below the detection threshold. A similar loss of detection of low-level-positive samples has been reported with pooled testing of blood for HIV (6). We also observed a dilution effect in other pools containing one M. tuberculosis-positive sample, whereby the M. tuberculosis level in the pooled sample was lower than that in the individual sample. The effects of dilution could mean that the accuracy of pooled testing may vary between populations with different sputum bacillary loads. Although the dilution effect is important, Xpert cartridges with a much higher sensitivity are expected to be released in 2016 (17), and these cartridges may be able to detect the few specimens missed by the current assay in this study.
A further discrepant result was a positive pool containing all M. tuberculosis-negative samples on individual testing. This was an unexpected finding, as the assay is highly specific (4). It may have occurred because of an uneven distribution of bacilli in the processed sample, with the portion used for individual testing not containing bacilli (sampling variability), or because of cross contamination of the pooled samples. Practically, clinical decisions would be guided by the individual test result. In these instances, repeat individual testing may be beneficial.
One M. tuberculosis-positive pool provided a false RIF-sensitive result. This pool contained a mixture of RIF-resistant and RIF-sensitive isolates, which likely explains the discrepancy, as the Xpert MTB/RIF assay resistance requires 65% to 100% of the DNA present to be from the resistant isolate to produce a reliable RIF susceptibility result (18). A pooled testing strategy would still have identified the RIF-resistant isolate when samples were tested individually.
The pooled testing of sputum samples has the potential to save time compared to the time required for the testing of individual samples, and the time required for pooled testing is comparable to that required for batched testing. However, the calculation presented assumes that samples from positive pools for individual testing are available at the time of testing, that these are tested in batches of 4, and that there are no indeterminate or failed tests. The time savings would be particularly useful in busy laboratories that receive large numbers of sputum samples or in large screening programs where large numbers of patients are tested.
We were able to demonstrate that in an area with a high prevalence of TB, such as Nigeria, a pooled sputum testing strategy can reduce Xpert cartridge costs by up to 31%. The savings were substantially higher when pools consisted of samples collected in the community (41%) as opposed to samples collected in district hospitals (11%). This is a function of the lower disease prevalence in the community population and suggests that pooled Xpert testing may be best used to lower the costs of community-based active case finding programs. Furthermore, the higher specificity of Xpert than smear microscopy (99% versus 98%, respectively) would result in a lower number of false-positive results in community-based interventions. In these locations, the proportion of screened patients who have TB is lower than that in hospital settings, resulting in a lower predictive value of the test and the danger of a higher number of false-positive test results. This approach therefore would decrease the cost of active case finding approaches, and the higher specificity of Xpert would reduce the risk of false-positive results.
The predicted estimates of cost savings are comparable to, if slightly less than, what we obtained. The marginally lower values may be because the predictive model does not account for any potential clustering of positive samples that may have occurred. The estimates also support the use of a pool size of three or four in the study population. In locations with a different PTB epidemiology, the most appropriate pool size may differ, as smaller pools may be appropriate in areas of high TB prevalence. For example, in hospital patients it would be preferable to use a pool sample size of three, which would produce higher cost savings than a pool of four samples, while in the community, a pool of four or even five would result in higher savings.
From a safety and practical point of view, pooling of sputum samples already processed with the Xpert sample reagent (SR) is superior to pooling of unprocessed samples. Processing of sputum samples with the SR virtually eliminates biohazard risks (19) and liquefies the sample, facilitating easier transfer, and if a pool tests positive, the technician simply has to add the remaining portion of the samples into individual cartridges. The extra steps involved in pooled testing heighten the potential for laboratory errors, particularly if the laboratory is dealing with large numbers of samples. Therefore, strict adherence to good laboratory practices is required, including careful handling and labeling of samples and pools and clear record keeping.
Limitations of the study include incomplete demographic data, absent smear status, and an inability to retest failed results for some individuals. HIV coinfection status was also unavailable, although it was likely to be commonplace. Improved participant information would have aided interpretation of the findings, but its absence is not expected to affect the results. We were unable to use sputum culture, which would have helped resolve the results for pools with discrepant results. The simultaneous testing of pooled and individual samples was required to determine agreement, and that testing approach varies from how a pooled testing system would work in practice. Furthermore, as the same investigator performed both the pooled and individual Xpert MTB/RIF tests, the investigator was not blinded to the results of the other set of tests when performing a particular set of tests. However, as the test is fully automated and the results are objective, knowledge of the results of the other tests is not expected to bias the results. The participants consisted primarily of adults with suspected PTB; therefore, the results should not be generalized to other patient populations.
Conclusion.
An Xpert MTB/RIF pooled sputum testing strategy had a high level of agreement with individual Xpert testing at a reduced cost. The findings suggest that a pooled testing approach has the potential to increase the affordability of Xpert testing, as the cost of the test is not expected to change in the near future. This strategy would be especially suited for use in active case finding programs and in locations where the proportion of positive cases is expected to be low. Further studies with the high-sensitivity Xpert cartridges may increase the agreement between the single and pooled testing strategies and should be explored.
ACKNOWLEDGMENTS
We are grateful for the support of the staff of Zankli Tuberculosis Research Laboratory, the Federal Capital Territory, and the National Tuberculosis and Leprosy Control programs.
None of us has a conflict of interest to declare.
The study was conceived by L.E.C., L.L., S.T.A., J.O., and O.M.; data collection and sputum processing were conducted by O.M., S.T.A., O.O., and N.E.; data analysis and interpretation were conducted by L.E.C., O.M., M.B., E.R.A., and R.D.; and L.E.C., O.M., and M.B. wrote the first draft of the manuscript. We all contributed to the final manuscript.
The project was funded by a Wave II TB REACH award (project number T9-370-114) and the EDCTP (SP.2011.41304.021) and its cofunders (the Medical Research Council [MRC] of the United Kingdom and the Instituto de Salud Carlos III [ISCIII] of Spain).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.WHO. 2014. Global tuberculosis report 2014. WHO, Geneva, Switzerland. [Google Scholar]
- 2.Parsons LM, Somoskovi A, Gutierrez C, Lee E, Paramasivan CN, Abimiku A, Spector S, Roscigno G, Nkengasong J. 2011. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev 24:314–350. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colebunders R, Bastian I. 2000. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. Int J Tuberc Lung Dis 4:97–107. [PubMed] [Google Scholar]
- 4.Lawn SD, Mwaba P, Bates M, Piatek A, Alexander H, Marais BJ, Cuevas LE, McHugh TD, Zijenah L, Kapata N, Abubakar I, McNerney R, Hoelscher M, Memish ZA, Migliori GB, Kim P, Maeurer M, Schito M, Zumla A. 2013. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis 13:349–361. doi: 10.1016/S1473-3099(13)70008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin ZZ, Pai M, Van Gemert W, Sahu S, Ghiasi M, Creswell J. 2015. How is Xpert MTB/RIF being implemented in 22 high tuberculosis burden countries? Eur Respir J 45:549–554. doi: 10.1183/09031936.00147714. [DOI] [PubMed] [Google Scholar]
- 6.Emmanuel JC, Bassett MT, Smith HJ, Jacobs JA. 1988. Pooling of sera for human immunodeficiency virus (HIV) testing: an economical method for use in developing countries. J Clin Pathol 41:582–585. doi: 10.1136/jcp.41.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindan C, Mathur M, Kumta S, Jerajani H, Gogate A, Schachter J, Moncada J. 2005. Utility of pooled urine specimens for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in men attending public sexually transmitted infection clinics in Mumbai, India, by PCR. J Clin Microbiol 43:1674–1677. doi: 10.1128/JCM.43.4.1674-1677.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morandi PA, Schockmel GA, Yerly S, Burgisser P, Erb P, Matter L, Sitavanc R, Perrin L. 1998. Detection of human immunodeficiency virus type 1 (HIV-1) RNA in pools of sera negative for antibodies to HIV-1 and HIV-2. J Clin Microbiol 36:1534–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mine H, Emura H, Miyamoto M, Tomono T, Minegishi K, Murokawa H, Yamanaka R, Yoshikawa A, Nishioka K, Japanese Red Cross NAT Research Group . 2003. High throughput screening of 16 million serologically negative blood donors for hepatitis B virus, hepatitis C virus and human immunodeficiency virus type-1 by nucleic acid amplification testing with specific and sensitive multiplex reagent in Japan. J Virol Methods 112:145–151. doi: 10.1016/S0166-0934(03)00215-5. [DOI] [PubMed] [Google Scholar]
- 10.Westreich DJ, Hudgens MG, Fiscus SA, Pilcher CD. 2008. Optimizing screening for acute human immunodeficiency virus infection with pooled nucleic acid amplification tests. J Clin Microbiol 46:1785–1792. doi: 10.1128/JCM.00787-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peeling RW, Toye B, Jessamine P, Gemmill I. 1998. Pooling of urine specimens for PCR testing: a cost saving strategy for Chlamydia trachomatis control programmes. Sex Transm Infect 74:66–70. doi: 10.1136/sti.74.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. 1998. Laboratory services in tuberculosis control. Part II. Microscopy. Report WHO/TB/98.258. WHO, Geneva, Switzerland. [Google Scholar]
- 13.Raboud JM, Sherlock C, Schechter MT, Lepine DG, O'Shaughnessy MV. 1993. Combining pooling and alternative algorithms in seroprevalence studies. J Clin Microbiol 31:2298–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward HA, Marciniuk DD, Pahwa P, Hoeppner VH. 2004. Extent of pulmonary tuberculosis in patients diagnosed by active compared to passive case finding. Int J Tuberc Lung Dis 8:593–597. [PubMed] [Google Scholar]
- 15.Theron G, Peter J, van Zyl-Smit R, Mishra H, Streicher E, Murray S, Dawson R, Whitelaw A, Hoelscher M, Sharma S, Pai M, Warren R, Dheda K. 2011. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med 184:132–140. doi: 10.1164/rccm.201101-0056OC. [DOI] [PubMed] [Google Scholar]
- 16.Theron G, Pinto L, Peter J, Mishra HK, Mishra HK, van Zyl-Smit R, Sharma SK, Dheda K. 2012. The use of an automated quantitative polymerase chain reaction (Xpert MTB/RIF) to predict the sputum smear status of tuberculosis patients. Clin Infect Dis 54:384–388. doi: 10.1093/cid/cir824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cepheid. 2014. Cepheid, FIND & Rutgers announce collaboration for next-generation innovations to game changing Xpert MTB/RIF test. Cepheid, Sunnyvale, CA. [Google Scholar]
- 18.Blakemore R, Story E, Helb D, Kop J, Banada P, Owens MR, Chakravorty S, Jones M, Alland D. 2010. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol 48:2495–2501. doi: 10.1128/JCM.00128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banada PP, Sivasubramani SK, Blakemore R, Boehme C, Perkins MD, Fennelly K, Alland D. 2010. Containment of bioaerosol infection risk by the Xpert MTB/RIF assay and its applicability to point-of-care settings. J Clin Microbiol 48:3551–3557. doi: 10.1128/JCM.01053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]