Abstract
In November 2011, The Rockefeller University Center for Clinical and Translational Science (CCTS), the Laboratory of Microbiology and Infectious Diseases, and Clinical Directors Network (CDN) launched a research and learning collaborative project with six community health centers in the New York City metropolitan area to determine the nature (clonal type) of community-acquired Staphylococcus aureus strains causing skin and soft tissue infections (SSTIs). Between November 2011 and March 2013, wound and nasal samples from 129 patients with active SSTIs suspicious for S. aureus were collected and characterized by molecular typing techniques. In 63 of 129 patients, the skin wounds were infected by S. aureus: methicillin-resistant S. aureus (MRSA) was recovered from 39 wounds and methicillin-sensitive S. aureus (MSSA) was recovered from 24. Most—46 of the 63–wound isolates belonged to the CC8/Panton-Valentine leukocidin-positive (PVL+) group of S. aureus clone USA300: 34 of these strains were MRSA and 12 were MSSA. Of the 63 patients with S. aureus infections, 30 were also colonized by S. aureus in the nares: 16 of the colonizing isolates were MRSA, and 14 were MSSA, and the majority of the colonizing isolates belonged to the USA300 clonal group. In most cases (70%), the colonizing isolate belonged to the same clonal type as the strain involved with the infection. In three of the patients, the identity of invasive and colonizing MRSA isolates was further documented by whole-genome sequencing.
INTRODUCTION
Staphylococcus aureus is the most common cause of bacterial infections in humans worldwide (1), and methicillin-resistant Staphylococcus aureus (MRSA) is the main cause of skin and soft tissue infections (SSTIs) in North America, with a single clone, USA300, accounting for 98% of these infections (2, 3).
The first human case of MRSA infection in the United States was reported in Boston, MA, in 1968 (4). MRSA was first detected in hospitals, and over the following decades, it became the main nosocomial pathogen around the world (5). In 1998, the prevalence of MRSA in 12 hospitals throughout the city of New York was assessed (6), and a single MRSA clone was found to be responsible for an overwhelming majority of MRSA infections. The same MRSA clone was subsequently identified as dominant in MRSA infections in 29 hospitals in the tristate area (7), and it was also identified in MRSA infections in Japan (8). This MRSA clone (multilocus sequence typing [MLST] clonal complex CC5, sequence type ST5, SCCmecII, and unique pulsed-field gel electrophoresis [PFGE] profile)—also known as the “New York/Japan clone” or “MRSA clone USA100”—became the most prevalent MRSA clone involved in MRSA infections in hospitals in the United States in the 1990s (9).
In 1993, a new MRSA clone emerged in Kimberley, Western Australia (10), in a community of patients without previous health care contact (community-acquired MRSA [CA-MRSA]). In the late 1990s, CA-MRSA also appeared in the United States and was responsible for the death of four otherwise healthy pediatric patients in Minnesota and North Dakota (11). These new CA-MRSA strains belonged to a clone (USA400/CC1/SCCmecIV) unrelated to the major MRSA clones that were frequent in hospitals in the United States during the same period.
In 2002 to 2003, another new clone of MRSA emerged in the United States among football players in Pennsylvania, followed soon after by prison inmates in Mississippi and athletes in Colorado, Indiana, and California (for a review, see reference 12). This clone, named USA300 (t008/ST8/SCCmecIVa/Panton-Valentine leukocidin positive [PVL+]/ACME+), is currently the most common MRSA clone in the community as well as in hospitals in the United States; it is frequently involved with infections of younger patients and causes high rates of sepsis and high rates of spread and mortality when infecting hospitalized patients (3).
In November 2011, The Rockefeller University Center for Clinical and Translational Science (CCTS), the Laboratory of Microbiology and Infectious Diseases, and Clinical Directors Network (CDN) established a multidisciplinary collaboration, the Community-Acquired MRSA (CA-MRSA) Project (CAMP) with six Community Health Centers (CHCs) strategically located in different parts of New York City and surrounding areas with the objective of tracking the presence of CA-MRSA among SSTIs and with the aim of improving diagnosis, therapy, and community awareness of this bacterium (40).
MATERIALS AND METHODS
The study was reviewed and approved by the Institutional Review Boards (IRBs) at Clinical Directors Network and The Rockefeller University.
Biological specimen collection and processing.
Following the Infectious Diseases Society of America guidelines for managing SSTIs (13), wounds were incised and drained, where possible, and in addition to wound specimens, additional surveillance specimens were acquired via nasal swabs. Wound and nasal specimens were sent in liquid Amies transport medium (Puritan Medical, Guilford, Maine, USA) to BioReference Laboratories (Elmwood Park, NJ, USA) for identification and antibiogram by the MicroScan system (Siemens, Munich, Germany). Cultures were tested against 12 antimicrobial agents, including penicillin, amoxicillin-clavulanic acid, cefazolin, oxacillin, clindamycin, erythromycin, gentamicin, levofloxacin, tetracycline, trimethoprim-sulfamethoxazole, vancomycin, and linezolid, according to the Clinical and Laboratory Standards Institute recommendations (14), and antibiograms were provided to the CHC clinician who was responsible for the patient's care.
S. aureus isolates and information on antibiotype were next sent to the Laboratory of Microbiology and Infectious Diseases at The Rockefeller University, New York, NY, for molecular characterization. S. aureus species confirmation was performed by growth on mannitol-salt agar (MSA; Difco, BBL, Becton Dickinson, Franklin Lakes, NJ, USA) and by testing coagulase production by the Staphaurex assay (Thermo Fisher Scientific, Lenexa, KS, USA).
Molecular identification: spa typing, MLST, PFGE, and staphylococcal cassette chromosome mec (SCCmec) typing.
Characterization by spa typing was performed as previously described (15), and spa types were determined using the RIDOM web server (http://spaserver.ridom.de/). The spa server was also used to predict sequence types (STs). When STs could not be determined from the spa server, the genetic background of the isolates was determined by MLST (16). Assignment of STs was done by submission of the DNA sequences of seven housekeeping genes (arcC, aroE, glpF, gmk, pta, tpi, and yqiL) to the online MLST database (http://www.mlst.net/). Clonal complexes were determined for the STs (17).
PFGE was then performed to identify isolates belonging to the same clonal complex. Bacterial DNA was restricted with SmaI enzyme, and the resulting fragments were separated by electrophoresis (18). Band patterns were compared manually according to guidelines to confirm classification (9, 19).
The structure of the staphylococcal cassette chromosome mec (SCCmec) was determined by multiplex PCR (20), and SCCmec type IV subtyping was done by multiplex PCR (21). Ambiguous results were further tested by amplification of the ccrB gene (22) and comparing the sequences obtained with the ccrB typing tool available at the Instituto de Tecnologia Quimica e Biologica (ITQB) in Portugal. SCCmec was considered nontypeable (NT) when it was not possible to ascertain a class of mec complex and/or a type of ccrB.
Molecular characterization: detection of mecA, Panton-Valentine leukocidin (PVL), and ACME genes.
Appropriate DNA probes were used to test for the mecA gene, responsible for resistance to oxacillin and all beta-lactam antibiotics, and for the lukS and lukF genes (which encode PVL [Panton-Valentine leukocidin]) (23, 24).
The two main loci that make up ACME-I in USA300 strain FPR3757 (arcA and opp3) were amplified by PCR (25). This element was typed according to its structure: type I (arc and opp3 operons), type II (arc operon only), and type III (opp3 operon only) (26).
Antibiotic resistance phenotype of wound and nasal isolates.
Invasive and colonizing isolates from three patients that were characterized by whole-genome sequencing were also characterized for their oxacillin resistance phenotypes by population analysis profiles (PAPs) (27, 28).
Whole-genome sequencing.
Bacterial isolates from three patients were selected for whole-genome sequencing. Two patients (UHP/CAMP-016 and UHP/CAMP-022) carried USA300 isolates in both the wounds and the nares, while the third patient (UHP/CAMP-028) was infected and colonized by USA1100 strains. From each isolate, genomic DNA was extracted using a Wizard genomic DNA purification kit per the manufacturer's instructions (Promega, Madison, WI, USA).
Sequencing was carried out at the Genomics Core Facility of the Huck Institutes of the Life Sciences at Penn State University. DNA libraries were constructed with an Illumina TruSeq DNA-seq library preparation kit and had an average fragment size of 400 bp. The sequencing involved a 500-cycle run on an Illumina MiSeq to yield 2 by 250-bp paired-end reads.
Complete (i.e., ungapped) genomic sequences for MRSA strain USA300-FPR3757 and MRSA252 are in RefSeq and can be downloaded from the NCBI's website (http://www.ncbi.nlm.nih.gov/).
Read sequences for the CAMP isolates generated by this study.
The sequences for the CAMP isolates are in the Sequence Read Archive (SRA) and can be downloaded from the NCBI's website (http://www.ncbi.nlm.nih.gov/).
Selection of USA300-FPR3757 and MRSA252 as reference strains.
The DNAStar software SeqMan NGen version 12 was used to generate de novo assemblies of the read sequences. For each CAMP isolate, the number of contigs and the N50 statistic were in the ranges 7 to 11 and 481,266 to 1,121,951 bp, respectively. Due to the small read size of 250 bp and the lack of mate pair sequencing, it is likely that the contigs contain numerous assembly errors. Nevertheless, the contigs were useful because they could be blasted (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to determine the most closely related MRSA strain with a complete genomic sequence available in the NCBI nucleotide database. For CAMP-18, -19, -28, and -29 (wound and nasal isolates from patients UHP/CAMP-016 and UHP/CAMP-022, respectively), the most closely related strain was USA300-FPR3757, and for CAMP-36 and -37 (nasal and wound isolates from patient UHP/CAMP-028), it was strain MRSA252.
Reference-guided assemblies of the reads.
The DNAStar software NGen version 12 was used to generate reference-guided assemblies of the read sequences. For the isolates CAMP-18, -19, -28, and -29, a whole-genome multialignment was generated by mapping the read sequences of all four isolates to the complete genomic sequence for USA300-FPR3757. For CAMP-36 and -37, another whole-genome multialignment was generated by mapping the read sequences of both isolates to the complete genomic sequence for MRSA252. In each of the two multialignments, the mean read coverage on the reference was ≥50× for every isolate.
DNA molecules in the reference strains and in the related CAMP isolates.
From a whole-genome multialignment of the reads, it was clear which DNA molecules, besides the chromosome, were in both the reference and the CAMP isolates. Of the three plasmids present in USA300-FPR3757, pUSA01 was identified in each of the isolates CAMP-18, -19, -28, and -29, and pUSA02 and pUSA03 were in none of the four isolates. There are no plasmids in MRSA252.
Polymorphism discovery.
In each of the two whole-genome multialignments of the reads, the DNAStar software SeqMan Pro version 12 was used to call polymorphisms in the DNA molecules of the reference strains and the CAMP isolates. In each multialignment, polymorphisms of all types and sizes could be called relative to the reference with expected false-positive and false-negative error rates of effectively zero in 98% of the columns. Polymorphisms could not be called in the remaining 2% of the columns because they consist of highly repetitive sequences for which the read sequences mapped to multiple positions. Error rates were estimated by computer simulation as follows. For each of the reference strains—USA300-FPR3757 and MRSA252—a PERL script was used to introduce polymorphisms of all types and sizes into the complete genomic sequence—in order to generate 100 new synthetic mutated complete genomic sequences each with 1,000 polymorphisms. Next, the program WgSim (https://github.com/lh3/wgsim) was applied to the new mutated sequences in order to generate synthetic MiSeq read sequences with realistic sequencing error rates; then, SeqMan NGen was used to generate reference-guided assemblies of the reads as before; and then, SeqMan Pro was used to call polymorphisms as before. In the case of USA300-FPR3757 and MRSA252, this meant that a reference-guided assembly always consisted of the complete genomic sequence of the reference and also the reads from the clinical isolates (four for FPR3757 and two for MRSA252).
Phylogenetic tree construction.
The phylogenetic trees were manually constructed based on the discovered numbers of polymorphisms between the isolates. The more complex tree was constructed with the software REALPHY using its default settings (http://realphy.unibas.ch/fcgi/realphy) (29). Using the vernacular in the documentation for REALPHY, the reference genome was USA300-FPR3757, the query genomes were the reads for the CAMP isolates (this study) and the northern Manhattan isolates (30), the two reference genome alignments were merged in order to generate a single tree, and the tree was inferred by PhyML.
Accession numbers.
Nucleotide sequence accession numbers are CP000255 and BX571856, respectively (25, 31). The BioProject numbers for microarray data are as follows: USA300 strain FPR3757, PRJNA58555; strain MRSA252, PRJNA57839; CAMP isolates, PRJNA277213.
RESULTS
One hundred twenty-nine patients with active skin and soft tissue infections (SSTIs) were enrolled from five Community Health Centers, and samples were obtained from each of the active SSTI lesions as well as the nares, for strain identification and characterization. The morphology of the wounds was documented by digital camera (Fig. 1), and a measuring tape was applied adjacent to the wound.
FIG 1.
Photographs of skin lesions in six patients enrolled in the study. Skin wounds of all patients were photographed with a digital camera to document the morphology/appearance of the skin and soft tissue infections. Samples taken from the wounds were subsequently used for the identification of the causative agents.
The study yielded a total of 104 S. aureus isolates: 63 recovered from wounds and 41 from nasal carriage. Of the 63 (49%) patients infected by S. aureus, 39 patients (35%) were infected by MRSA strains and 24 patients (19%) were infected by MSSA strains.
Characterization of the S. aureus isolates by molecular typing techniques.
Most of the S. aureus isolates—34 MRSA (54%) and 12 MSSA (19%) isolates—belonged to the USA300 clone (t008/ST8/SCCmecIVa/PVL+/ACME+) or to closely related clones (USA300 PFGE profile and ST8 but presenting differences in spa type, SCCmecIV subtype, and absence of PVL or ACME). Forty-one patients (65%) carried S. aureus in their nares: 19 (46%) carried MRSA and 22 (53%) carried MSSA; 23 of the nasal isolates belonged to the clonal group of USA300 (56% of S. aureus nasal isolates) (Fig. 2A and B).
FIG 2.
(A) Molecular types of S. aureus isolates recovered from wounds. (B) Molecular types of S. aureus isolates recovered from nares.
MRSA was involved in 61% of the abscesses (27/44), and 92% (24/27) of these belonged to the USA300 clone. Three patients presented with MRSA in the wounds and with MSSA in the nares; two cases had MSSA infections but MRSA carriage. In all these cases, the MRSA isolates belonged to the clonal group of USA300 while the MSSA strains belonged to very different clones (CC15, CC5, CC1, and CC30, respectively). Tables 1 and 2 show the clonal distribution of nasal and wound isolates and whether they were MRSA or MSSA.
TABLE 1.
Distribution of S. aureus isolates grouped by clonal complex
| MLST | No. of isolates |
||||
|---|---|---|---|---|---|
| Total | Wound |
Nasal |
|||
| MRSA | MSSA | MRSA | MSSA | ||
| CC8 | 69 | 34 | 12 | 17 | 6 |
| CC30 | 12 | 2 | 4 | 1 | 5 |
| CC5 | 6 | 1 | 2 | 1 | 2 |
| CC15 | 6 | 0 | 2 | 0 | 4 |
| CC121 | 3 | 0 | 1 | 0 | 2 |
| ST72 | 2 | 1 | 0 | 0 | 1 |
| CC1 | 1 | 0 | 0 | 0 | 1 |
| CC45 | 1 | 0 | 1 | 0 | 0 |
| CC88 | 1 | 1 | 0 | 0 | 0 |
| CC97 | 1 | 0 | 0 | 0 | 1 |
| CC152 | 1 | 0 | 1 | 0 | 0 |
| CC398 | 1 | 0 | 1 | 0 | 0 |
| Total | 104 | 39 | 24 | 19 | 22 |
TABLE 2.
Distribution of wound isolates belonging to clonal complex CC8
| S. aureus type and clone type | No. of wound isolates |
|---|---|
| MRSA | |
| USA300 (t008/ST8/SCCmecIVa/PVL+/ACME+) | 21 |
| Other spa types | 7 |
| PVL− | 0 |
| ACME− | 2 |
| Other | 4 |
| Total | 34 |
| MSSA | |
| USA300-like (t008/ST8/PVL+/ACME+) | 3 |
| Other spa types | 1 |
| PVL− | 0 |
| ACME− | 4 |
| Other | 4 |
| Total | 12 |
Of the 19 patients with MRSA in the nares, 14 were colonized by the same strain that caused the SSTI, and all except one of these strains belonged to the USA300 clone or closely related clones. Three patients carried MRSA in the nares, but no S. aureus was isolated in the wounds.
ACME was found only among isolates belonging to the CC8/USA300 clonal complex and was most prevalent among MRSA strains (29/39 MRSA wound infections and 10/19 MRSA nasal isolates). Four MSSA wound isolates that also carried the ACME virulence factor belonged to the same CC8/USA300 clonal complex. None of the 22 nasal MSSA isolates carried the ACME determinant.
The Panton-Valentine leukocidin (pvl) genes lukSF were detected in half (52.8%) of the wound isolates: in 94% of all MRSA strains and 75% of MSSA strains. Among all nasal isolates (84% MRSA and 31% MSSA) 46% carried the lukSF gene. All PVL+ strains belonged to the CC8/USA300 complex except for three patients whose isolates belonged to CC30 and CC88 (all of them also sharing with USA300 the SCCmecIVa cassette). Also, all strains belonging to the USA300 clonal group carried these genes except two nasal MRSA isolates that belonged to the “New York clone V” (ST8, SCCmecIVg/ACME−/PVL−) (24).
Twenty-two of the 63 patients (35%) who were identified with an SSTI carrying S. aureus reported previous episodes with similar lesions and described the present lesion as a recurrence. Of those 22 cases, 17 (77%) were identified as infections with MRSA, and all of them were infections by the USA300 clone. One patient had a previous diagnosis of MRSA and had a USA300 wound infection in the present study but did not carry the strain in the nares.
Antibiotic resistance of MRSA and MSSA isolates.
One hundred four S. aureus isolates, representing all nasal isolates and all but five wound cultures, were analyzed with antibiograms. None of the isolates were resistant to gentamicin, vancomycin, or linezolid. Forty-one isolates (28 MRSA and 13 MSSA strains) showed multiresistant profiles, i.e., were resistant to three or more families of antibiotics (Table 3). Four MRSA isolates were resistant to four families of antibiotics: β-lactams (penicillin, amoxicillin-clavulanic acid, cefazolin, and oxacillin), lincosamides (clindamycin), macrolides (erythromycin), and quinolones (levofloxacin), all belonging to USA300 or USA100 (“New York/Japan”) clones. The remaining 24 MRSA isolates that were resistant to three families of antibiotics showed various patterns of resistance: 21 isolates were resistant to β-lactams, macrolides, and quinolones (USA300 and USA100); one USA300 MRSA nasal isolate was resistant to β-lactams, lincosamides, and macrolides; one CC88 MRSA wound isolate was resistant to β-lactams, macrolides, and tetracyclines; and one CC8 MRSA nasal isolate was resistant to β-lactams, quinolones, and trimethoprim-sulfamethoxazole.
TABLE 3.
Antibiotic resistance profiles of S. aureus isolates
| Antibiotic family(-ies)a | No. of isolates |
Clonal complex(es)b | Origin | No. of patients with both wound and nasal USA300 isolates | |
|---|---|---|---|---|---|
| MRSA | MSSA | ||||
| BLAs + CLI + ERY + LEV + TET | 0 | 1 | CC15 | Nasal | |
| BLAs + CLI + ERY + LEV | 4 | 2 | CC5, CC8, CC15 | Wound, nasal | 1 patient with USA300 |
| BLAs + ERY + TET | 1 | 2 | CC8, CC88 | Wound, nasal | 1 patient with USA300 |
| BLAs + ERY + LEV | 21 | 0 | CC5, CC8 | Wound, nasal | 2 patients with USA300, 1 patient with USA300-like |
| BLAs + CLI + ERY | 1 | 7 | CC5, CC8, CC15, CC30 | Wound, nasal | 1 patient with USA300 |
| BLAs + LEV + TMP-SMX | 1 | 0 | CC8 | Nasal | |
| BLAs + ERY | 7 | 5 | CC8, CC30, CC121 | Wound, nasal | 1 patient with USA300 |
| BLAs + TET | 3 | 1 | CC5, CC8 | Wound, nasal | 1 patient with USA300 |
| BLAs + TMP-SMX | 0 | 1 | CC8 | Nasal | |
BLAs, β-lactam antibiotics; penicillin, amoxicillin-clavulanic acid, cefazolin, and oxacillin in the case of MRSA isolates and penicillin alone for MSSA isolates. CLI, clindamycin; ERY, erythromycin; LEV, levofloxacin; TET, tetracycline; TMP-SMX, trimethoprim-sulfamethoxazole.
Clonal complex as defined by MLST. CC5, clonal complex of the HA-MRSA strain USA100 (“New York/Japan” clone); CC8, clonal complex of the CA-MRSA strain USA300 clone.
One MSSA nasal isolate belonging to CC15 was resistant to antibiotics of five different families: β-lactams (penicillin, amoxicillin-clavulanic acid, and cefazolin, but not oxacillin), lincosamides, macrolides, quinolones, and tetracyclines. Two wound MSSA isolates were resistant to four families of antibiotics: some β-lactams, lincosamides, macrolides, and quinolones (CC15 and CC8). Nine isolates were resistant to three families of antibiotics: two isolates resistant to β-lactams, macrolides, and tetracyclines (nasal and wound isolates of a single patient with a CC8/PVL+ profile) and seven isolates resistant to β-lactams, lincosamides, and macrolides (CC5, CC8, CC15, CC30, and CC121).
As mentioned above, 14 patients infected with MRSA also carried the same strain in the nares, and in 13 of these cases, the MRSA strains belonged to the USA300 group; in one patient the CA-MRSA recovered from the nares and the wound belonged to the “South-West Pacific clone” (USA1100/t665/CC30/SCCmecIVa/PVL+/ACME−).
Antibiotic resistance phenotypes of invasive and colonizing isolates.
Invasive and colonizing isolates from three patients (UHP-CAMP-016, -CAMP-022, and -CAMP-028) whose isolates were also analyzed by whole-genome sequencing showed identical oxacillin-resistant phenotypes as indicated by the superimposable population analysis profiles (Fig. 3).
FIG 3.
Population analysis profiles of the nasal and wound isolates from three selected patients. UHP/CAMP-016 is a patient who carries in both the wound and the nares the same USA300 strain (t008/ST8/SCCmecIVa/PVL+/ACME+); UHP/CAMP-022 is another patient who carries in wound and nares a variant of USA300 (t052/ST8/SCCmecIVa/PVL+/ACME+); UHP/CAMP-028 is a patient who carries a USA1100 strain (t665/ST1472/SCCmecIVa/PVL+/ACME−) in both the wound and the nares.
Comparison of wound and nares isolates in three patients by whole-genome sequencing.
Two of the three patients (UHP/CAMP-016 and UHP/CAMP-022) were infected and colonized by MRSA isolates belonging to the USA300 group (t008 and t052/ST8/SCCmecIVa/PVL+/ACME+). The third patient (UHP/CAMP-028) was infected and colonized by CA-MRSA USA1100.
For each of the three pairs of nares and wound isolates CAMP-18 and -19, CAMP-28 and -29, and CAMP-36 and -37, we did whole-genome shotgun sequencing to elucidate phylogenetic relationships and identify polymorphisms of all types and sizes. For each of these six isolates, we identified the most closely related MRSA strain available in the NCBI nucleotide database (http://www.ncbi.nlm.nih.gov/) with a complete (i.e., ungapped) genomic sequence. For CAMP-18, -19, -28, and -29, that strain is USA300 FPR3757, and for CAMP-36 and -37, it is MRSA252.
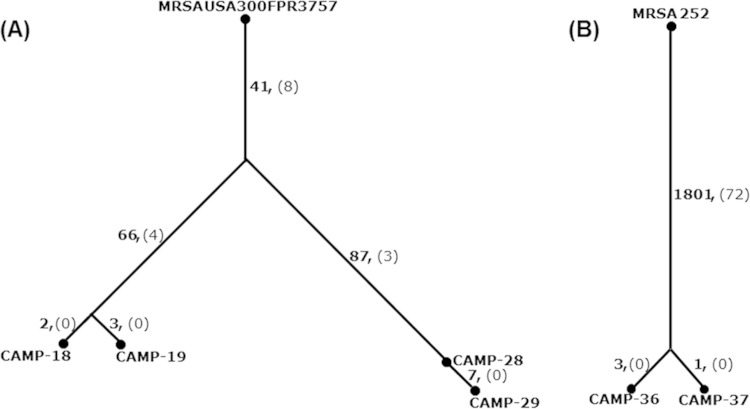
The tree in Fig. 4 depicts the phylogenetic relationships and numbers of polymorphisms. It can be seen that for each pair of nares and wound isolates, they differ by only a few point mutations. Isolates CAMP-18 and -19 differed from one another by 5 point mutations, isolates CAMP-28 and -29 differed by 7 point mutations, and CAMP-36 and -37 differed by 4 point mutations. These mutations are described in Table 4 and—in more detail—in Table S1 in the supplemental material. It is unclear if any of the listed mutations are important for colonization of the nares or infection of the wound. Curiously, one of the mutations in the wound isolate CAMP-19 is in a gene, SAUSA300_1059, with homology to exotoxins.
FIG 4.
Phylogeny of MRSA isolates recovered from wounds and nasal colonization sites of three patients: comparison by whole-genome sequencing. CAMP-18 and CAMP-19 are the wound and nasal isolates, respectively, from patient UHP/CAMP-016. CAMP-28 and CAMP-29 are the nasal and wound isolates, from patient UHP/CAMP-022. CAMP-36 and CAMP-37 are the nasal and wound isolates, respectively, of patient UHP/CAMP-028. CAMP-18 and CAMP-19 were both characterized as USA300 (t008/ST8/SCCmecIVa/PVL+/ACME+); CAMP-28 and CAMP-29 belonged to a variant of USA300 (t052/ST8/SCCmecIVa/PVL+/ACME+); CAMP-36 and CAMP-37 belonged to the clone USA1100 (t665/CC30/SCCmecIVa/PVL+/ACME−). Beside each tree branch are listed the numbers of point mutations (boldface) and larger structural variants (in parentheses) between the chromosomes of the ancestor and descendant.
TABLE 4.
Mutations in CAMP isolatesg
| Mutation presencea | Reference MRSA strain and position | Positionb | Changec | Type | Proximal gened |
|||
|---|---|---|---|---|---|---|---|---|
| Referencee | N315f | Name | Function | |||||
| CAMP-18, not CAMP-19 | FPR3757 | 357216 | C→T | Intergenic | SAUSA300_0307 | SA0295 | Acid phosphatase? | |
| 1181875 | T→C | Synonymous | SAUSA300_1080 | SA1029 | ftsZ | Cell division | ||
| CAMP-19, not CAMP-18 | FPR3757 | 1159271 | C→T | Nonsynonymous | SAUSA300_1059 | SA1009 | Exotoxin? | |
| 1777369 | T→— | Frameshift | SAUSA300_1622 | SA1499 | tig | Molecular chaperone | ||
| 1858349 | A→G | Synonymous | SAUSA300_1686 | SA1561 | murC | Cell wall synthesis | ||
| CAMP-28, not CAMP-29h | ||||||||
| CAMP-29, not CAMP-28 | FPR3757 | 157471 | C→A | Nonsynonymous | SAUSA300_0138 | SA0131 | deoD | Purine metabolism |
| 1043552 | —→A | Intergenic | SAUSA300_0954 | SA0904 | Antibiotic resistance? | |||
| 1387815 | G→A | Nonsynonymous | SAUSA300_1260 | SA1197 | tyrA | Shikimate pathway | ||
| 1768026 | T→C | Synonymous | SAUSA300_1614 | SA1491 | hemL | Porphyrin biosynthesis | ||
| 2031280 | T→A | Intergenic | SAUSA300_1871 | SA1706 | ||||
| 2335992 | G→A | Intergenic | SAUSA300_2158 | |||||
| 2348652 | A→— | Intergenic | SAUSA300_2167 | |||||
| CAMP-36, not CAMP-37 | 252 | 142269 | C→A | Intergenic | SAR0129 | SA0122 | butA | Acetoin formation |
| 1042026 | T→— | Frameshift | SAR0994 | SA0881 | ||||
| 2105113 | C→T | Nonsynonymous | SAR2012 | SA1735 | ||||
| CAMP-37, not CAMP-36 | 252 | 2707338 | G→A | Nonsynonymous | SAR2622 | SA2330 | Transcriptional regulator? | |
CAMP-18, CAMP-28, and CAMP-36 are nares isolates. CAMP-19, CAMP-29, and CAMP-37 are wound isolates.
Nucleotide position on the chromosome of the reference strain. No mutations were observed on plasmids.
Nucleotide change on the Watson strand where sense (Watson or Crick) is defined by the reference. The convention used is [original]→[new], where “a to —” means there is a deletion and “— to a” means there is an insertion.
If mutation is in a gene, that gene; if mutation is in intergenic sequence between divergently or tandemly transcribed genes, the nearest downstream gene; and if mutation is in intergenic sequence between convergently transcribed genes, the nearest gene.
Name of gene in reference.
Name of orthologous gene in strain N315.
For more detailed descriptions, see Table S1 in the supplemental material.
No mutations were found in CAMP-28 that were not found in CAMP-29.
We compared the CAMP isolates to isolates of the same MRSA clone characterized in a recent outbreak by MRSA clone USA300 in northern Manhattan (30). Uhlemann et al. shotgun sequenced the whole genomes of about 400 MRSA isolates recovered during the outbreak (30). The tree in Fig. 5 depicts the phylogenetic relationships between the CAMP and the northern Manhattan isolates. Figure 5 shows that the genotypes of the four CAMP USA300 isolates are within the genetic variation seen in the northern Manhattan isolates. Therefore, the CAMP and the northern Manhattan isolates of USA300 may all be part of the same outbreak.
FIG 5.
Relationship of the CAMP USA300 isolates to isolates of the same clone from the outbreak in northern Manhattan. Uhlemann et al. shotgun sequenced the whole genomes of about 400 MRSA isolates recovered during a USA300 outbreak in northern Manhattan (30). The tree illustrates the phylogenetic relationship between the CAMP and Uhlemann et al. isolates as inferred from whole-genome analysis. Due to the sheer number of isolates, the branches are densely packed. Of the northern Manhattan isolates, the vast majority are USA300 and so comprise the main body of the tree (highlighted in blue), and a very small minority are non-USA300 isolates and represent distant outgroups (highlighted in red). The USA300 isolates CAMP-18, -19, -28, and -29 fall well within the main body of the tree and not on the periphery (see arrows). Thus, the genotypes of the four CAMP USA300 isolates are encompassed by the genetic variation seen in the northern Manhattan USA300 isolates. Thus, the CAMP and northern Manhattan USA300 isolates may all be part of the same outbreak. Not surprisingly, the non-USA300 isolates CAMP-36 and -37 fall among the distant outgroups (see arrows).
DISCUSSION
S. aureus was identified in 63 of the 129 cases of infections in this study, and 30% of wounds were infected by MRSA. Of the six skin lesions shown in Fig. 1, MRSA was recovered from only two of the cases. Thus, S. aureus, and more specifically MRSA-caused SSTI, cannot be diagnosed by observation alone—additional laboratory analysis is necessary for a correct diagnosis and treatment.
Both ACME and PVL seem to play important roles in the prevalence of USA300. Many of the isolates in our study (40/69 CC8 isolates) did not present with the complete characteristic pattern t008/ST8/SCCmecIVa/PVL+/ACME+. Both the cassette chromosome and the mobile element encoding enzymes involved with arginine catabolism are known to be susceptible to genetic modifications, including a total or partial loss from the bacteria (32). Over the years, evolution may have caused changes in the spa gene, generating variants that still belong to the same clonal complex (33). Many wound isolates characterized in this study were closely related but not identical to CA-MRSA USA300 (t008/ST8/SCCmecIVa/PVL+/ACME+). This may indicate that the isolates were derivatives of the original clone, and while lacking some of the virulence determinants, they still shared a genetic background that facilitates rapid clonal spread.
CA-MRSA strains may not be more virulent than many MSSA clones. The clinical importance of CA-MRSA may reside in a combination of factors: a better fitness related to the smaller SCCmec cassettes; higher growth rates; ACME-mediated resistance to host defenses; increased virulence due to the PVL toxin (34); and resistance to multiple antibiotics, which complicates treatment and increases the risk of recurrence (35).
We recovered 39 MRSA strains from the wounds: 38 of these were CA-MRSA and one strain was a representative of the health care-acquired MRSA (HA-MRSA) clone CC5/USA100 (Table 1). Most of the infections were due to a single MRSA clone, USA300, which is currently the most prevalent clone of CA-MRSA in the United States and specifically in New York City (30). ACME was restricted to strains belonging to the USA300 CA-MRSA clone or clones closely related to it. Interestingly, ACME was also found in four methicillin-sensitive S. aureus (MSSA) isolates recovered from wounds, grouping all CC8/USA300 strains in what Tenover and Goering described as a family of isolates showing >80% similarity by PFGE typing (12). In all patients carrying an ACME+ strain in the nares (all of them MRSA), the same strain was also recovered from the wounds. ACME was absent from all MSSA nasal cultures.
Panton-Valentine leukocidin is a virulence factor frequently associated with CA-MRSA (94% of all MRSA wound isolates in our study). However, in our study, the prevalence of the genes lukS-lukF was also very high among MSSA (75%) wound isolates. Most (66%) of these MSSA wound isolates belonged to the same CC8 clonal complex as the USA300 clone, which would suggest that these strains were derivatives of the original USA300 clone that have lost the SCCmec cassette while preserving the genetic background that would still provide for superior survival and virulence (36, 37). Most nasal MRSA isolates (84%) and some MSSA nasal isolates (30%) presented as PVL+, but in these cases, the same strain was also detected in the wound culture of the same patient.
Whole-genome sequencing and PAPs.
The identity of wound and carriage (nasal) isolates was documented by standard typing methods. In three patients, pairs of wound and carriage isolates (nasal isolates) were also compared by whole-genome sequencing and by detailed characterization of the resistance phenotypes using population analysis profiles (28) (Fig. 3). The three patients included UHP/CAMP-016 and UHP/CAMP-022, each infected and colonized by MRSA strains of the USA300 group (t008 and t052/ST8/SCCmecIVa/PVL+/ACME+), and a third patient, UHP/CAMP-028, who was infected and colonized by CA-MRSA USA1100 (t665/ST1472/SCCmecIVa/PVL+/ACME−).
Whole-genome sequencing showed that the pairs of isolates recovered from the infection and colonization sites of the same patient were very similar in terms of DNA sequence (Fig. 4). Population analysis profiles (PAPs) showed that the bacterial isolates from the wound and nares of the same patient also exhibited identical—heterogeneous—resistance to oxacillin. The PAPs of bacteria recovered from the two patients infected by USA300 were virtually identical and were different from the PAP of strains recovered from patient UHP/CAMP-028, who was infected by a MRSA strain belonging to the USA1100 clone (Fig. 3).
In conclusion, the CA-MRSA clone USA300 was identified as the most prevalent S. aureus clone causing SSTIs in metropolitan New York City community health centers. Methicillin-susceptible variants of the same clone that share most of the genetic background of USA300 were also prevalent among isolates causing SSTIs.
Clinical presentation alone is not sufficient to predict the nature of the pathogen associated with SSTIs; bacterial cultures and molecular identification are needed to determine the true prevalence and spread of MRSA clones.
Nasal carriage cannot be used as a predictor of S. aureus SSTI infection. Many patients who were found to have active infections by MRSA did not carry the pathogen in the nares (22 patients presenting with MRSA only in the wound versus 14 patients with MRSA both in the wound and in the nares). Nevertheless, MRSA nasal carriage often accompanied MRSA wound infections by the same strain. In the present study, we performed an extensive screening on some patients presenting with recurrent skin and soft tissue infections with MRSA in which carriage was checked both in the nares and at nine additional body sites (pharynx, axillae, cubital folds, inguinal folds, and patellar folds). In some patients, the nasal carriage was negative (data not shown), and in one USA300-infected patient, the infecting MRSA strain was found only in the inguinal and patellar folds (38).
A recent study described an outbreak and extensive spread of MRSA belonging to the clonal type USA300 in northern Manhattan (30) during the same time period in which our CAMP study was performed. Comparison of the sequencing data presented in this communication with the sequencing data available from the northern Manhattan outbreak clearly indicates the involvement of strains with an identical clonal type.
Given the capacity of the USA300 MRSA clone to adhere to and survive on skin and on a variety of inert surfaces, control steps should include screening for carriage by close contacts and by fomites in homes and public transportation (39), which may represent potential reservoirs of these dangerous MRSA clones.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH-NCATS grant 8 UL1 TR000043 to B. S. Coller (principal investigator [PI]) and by a 2011 CTSA Administrative Supplement Award to J. N. Tobin (PI). Additional support was provided by U.S. Public Health Service award 2 RO1 AI457838-15 to A. Tomasz (PI), AHRQ grant 1 P30-HS-021667 to J. N. Tobin (PI), and PCORI grant CER-1402-10800 to J. N. Tobin (PI).
We thank Vikas Koundal (Department of Biochemistry and Molecular Biology, Penn State University) for help in the additional purification and concentration of some DNA samples prior to genome sequencing. We greatly appreciate the efforts of the participating community health centers, including Brookdale Family Center (Brooklyn, NY), Hudson River Healthcare (Peekskill, NY), Manhattan Physicians Group (New York, NY), Open Door Family Health Center (Ossining, NY), and Urban Health Plan (Bronx, NY); the BioReference Laboratories, Inc.; and the collaboration of the clinicians, office staff, and patients.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00591-15.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thurlow LR, Joshi GS, Richardson AR. 2012. Virulence strategies of the dominant USA300 lineage of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). FEMS Immunol Med Microbiol 65:5–22. doi: 10.1111/j.1574-695X.2012.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett FF, McGehee RF Jr, Finland M. 1968. Methicillin-resistant Staphylococcus aureus at Boston City Hospital. Bacteriologic and epidemiologic observations. N Engl J Med 279:441–448. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira DC, Tomasz A, de Lencastre H. 2002. Secrets of success of a human pathogen: molecular evolution of pandemic clones of meticillin-resistant Staphylococcus aureus. Lancet Infect Dis 2:180–189. doi: 10.1016/S1473-3099(02)00227-X. [DOI] [PubMed] [Google Scholar]
- 6.Roberts RB, de Lencastre A, Eisner W, Severina EP, Shopsin B, Kreiswirth BN, Tomasz A. 1998. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in 12 New York hospitals. MRSA Collaborative Study Group. J Infect Dis 178:164–171. [DOI] [PubMed] [Google Scholar]
- 7.Roberts RB, Chung M, de Lencastre H, Hargrave J, Tomasz A, Nicolau DP, John JF Jr, Korzeniowski O. 2000. Distribution of methicillin-resistant Staphylococcus aureus clones among health care facilities in Connecticut, New Jersey, and Pennsylvania. Microb Drug Resist 6:245–251. doi: 10.1089/mdr.2000.6.245. [DOI] [PubMed] [Google Scholar]
- 8.Aires de Sousa M, de Lencastre H, Santos Sanches I, Kikuchi K, Totsuka K, Tomasz A. 2000. Similarity of antibiotic resistance patterns and molecular typing properties of methicillin-resistant Staphylococcus aureus isolates widely spread in hospitals in New York City and in a hospital in Tokyo, Japan. Microb Drug Resist 6:253–258. doi: 10.1089/mdr.2000.6.253. [DOI] [PubMed] [Google Scholar]
- 9.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol 41:5113–5120. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Udo EE, Pearman JW, Grubb WB. 1993. Genetic analysis of community isolates of methicillin-resistant Staphylococcus aureus in Western Australia. J Hosp Infect 25:97–108. doi: 10.1016/0195-6701(93)90100-E. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 1999. From the Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin resistant Staphylococcus aureus—Minnesota and North Dakota, 1979–1999. JAMA 282:1123–1125. doi: 10.1001/jama.282.12.1123-JWR0922-2-1. [DOI] [PubMed] [Google Scholar]
- 12.Tenover FC, Goering RV. 2009. Methicillin-resistant Staphylococcus aureus strain USA300: origin and epidemiology. J Antimicrob Chemother 64:441–446. doi: 10.1093/jac/dkp241. [DOI] [PubMed] [Google Scholar]
- 13.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 14.CLSI. 2013. Performance standards for antimicrobial susceptibility testing; 23rd informational supplement CLSI document M100-S23. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.Aires-de-Sousa M, Boye K, de Lencastre H, Deplano A, Enright MC, Etienne J, Friedrich A, Harmsen D, Holmes A, Huijsdens XW, Kearns AM, Mellmann A, Meugnier H, Rasheed JK, Spalburg E, Strommenger B, Struelens MJ, Tenover FC, Thomas J, Vogel U, Westh H, Xu J, Witte W. 2006. High interlaboratory reproducibility of DNA sequence-based typing of bacteria in a multicenter study. J Clin Microbiol 44:619–621. doi: 10.1128/JCM.44.2.619-621.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol 38:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feil EJ, Cooper JE, Grundmann H, Robinson DA, Enright MC, Berendt T, Peacock SJ, Smith JM, Murphy M, Spratt BG, Moore CE, Day NP. 2003. How clonal is Staphylococcus aureus? J Bacteriol 185:3307–3316. doi: 10.1128/JB.185.11.3307-3316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung M, de Lencastre H, Matthews P, Tomasz A, Adamsson I, Aires de Sousa M, Camou T, Cocuzza C, Corso A, Couto I, Dominguez A, Gniadkowski M, Goering R, Gomes A, Kikuchi K, Marchese A, Mato R, Melter O, Oliveira D, Palacio R, Sa-Leao R, Santos Sanches I, Song JH, Tassios PT, Villari P. 2000. Molecular typing of methicillin-resistant Staphylococcus aureus by pulsed-field gel electrophoresis: comparison of results obtained in a multilaboratory effort using identical protocols and MRSA strains. Microb Drug Resist 6:189–198. doi: 10.1089/mdr.2000.6.189. [DOI] [PubMed] [Google Scholar]
- 19.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milheirico C, Oliveira DC, de Lencastre H. 2007. Update to the multiplex PCR strategy for assignment of mec element types in Staphylococcus aureus. Antimicrob Agents Chemother 51:3374–3377. doi: 10.1128/AAC.00275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milheirico C, Oliveira DC, de Lencastre H. 2007. Multiplex PCR strategy for subtyping the staphylococcal cassette chromosome mec type IV in methicillin-resistant Staphylococcus aureus: ‘SCCmec IV multiplex’. J Antimicrob Chemother 60:42–48. doi: 10.1093/jac/dkm112. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira DC, Santos M, Milheirico C, Carrico JA, Vinga S, Oliveira AL, de Lencastre H. 2008. CcrB typing tool: an online resource for staphylococci ccrB sequence typing. J Antimicrob Chemother 61:959–960. doi: 10.1093/jac/dkn021. [DOI] [PubMed] [Google Scholar]
- 23.Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, Vandenesch F, Etienne J. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis 29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira DC, de Lencastre H. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 46:2155–2161. doi: 10.1128/AAC.46.7.2155-2161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 26.Diep BA, Stone GG, Basuino L, Graber CJ, Miller A, des Etages SA, Jones A, Palazzolo-Ballance AM, Perdreau-Remington F, Sensabaugh GF, DeLeo FR, Chambers HF. 2008. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus. J Infect Dis 197:1523–1530. doi: 10.1086/587907. [DOI] [PubMed] [Google Scholar]
- 27.Dordel J, Kim C, Chung M, Pardos de la Gandara M, Holden MT, Parkhill J, de Lencastre H, Bentley SD, Tomasz A. 2014. Novel determinants of antibiotic resistance: identification of mutated loci in highly methicillin-resistant subpopulations of methicillin-resistant Staphylococcus aureus. mBio 5(2):e01000. doi: 10.1128/mBio.01000-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomasz A, Nachman S, Leaf H. 1991. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob Agents Chemother 35:124–129. doi: 10.1128/AAC.35.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertels F, Silander OK, Pachkov M, Rainey PB, van Nimwegen E. 2014. Automated reconstruction of whole-genome phylogenies from short-sequence reads. Mol Biol Evol 31:1077–1088. doi: 10.1093/molbev/msu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uhlemann AC, Dordel J, Knox JR, Raven KE, Parkhill J, Holden MT, Peacock SJ, Lowy FD. 2014. Molecular tracing of the emergence, diversification, and transmission of S. aureus sequence type 8 in a New York community. Proc Natl Acad Sci U S A 111:6738–6743. doi: 10.1073/pnas.1401006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holden MT, Feil EJ, Lindsay JA, Peacock SJ, Day NP, Enright MC, Foster TJ, Moore CE, Hurst L, Atkin R, Barron A, Bason N, Bentley SD, Chillingworth C, Chillingworth T, Churcher C, Clark L, Corton C, Cronin A, Doggett J, Dowd L, Feltwell T, Hance Z, Harris B, Hauser H, Holroyd S, Jagels K, James KD, Lennard N, Line A, Mayes R, Moule S, Mungall K, Ormond D, Quail MA, Rabbinowitsch E, Rutherford K, Sanders M, Sharp S, Simmonds M, Stevens K, Whitehead S, Barrell BG, Spratt BG, Parkhill J. 2004. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci U S A 101:9786–9791. doi: 10.1073/pnas.0402521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fritz SA, Hogan PG, Singh LN, Thompson RM, Wallace MA, Whitney K, Al-Zubeidi D, Burnham CA, Fraser VJ. 2014. Contamination of environmental surfaces with Staphylococcus aureus in households with children infected with methicillin-resistant S aureus. JAMA Pediatr 168:1030–1038. doi: 10.1001/jamapediatrics.2014.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golding GR, Campbell JL, Spreitzer DJ, Veyhl J, Surynicz K, Simor A, Mulvey MR. 2008. A preliminary guideline for the assignment of methicillin-resistant Staphylococcus aureus to a Canadian pulsed-field gel electrophoresis epidemic type using spa typing. Can J Infect Dis Med Microbiol 19:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hudson LO, Murphy CR, Spratt BG, Enright MC, Terpstra L, Gombosev A, Hannah P, Mikhail L, Alexander R, Moore DF, Huang SS. 2012. Differences in methicillin-resistant Staphylococcus aureus strains isolated from pediatric and adult patients from hospitals in a large county in California. J Clin Microbiol 50:573–579. doi: 10.1128/JCM.05336-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otto M. 2013. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med 64:175–188. doi: 10.1146/annurev-med-042711-140023. [DOI] [PubMed] [Google Scholar]
- 36.Highlander SK, Hulten KG, Qin X, Jiang H, Yerrapragada S, Mason EO Jr, Shang Y, Williams TM, Fortunov RM, Liu Y, Igboeli O, Petrosino J, Tirumalai M, Uzman A, Fox GE, Cardenas AM, Muzny DM, Hemphill L, Ding Y, Dugan S, Blyth PR, Buhay CJ, Dinh HH, Hawes AC, Holder M, Kovar CL, Lee SL, Liu W, Nazareth LV, Wang Q, Zhou J, Kaplan SL, Weinstock GM. 2007. Subtle genetic changes enhance virulence of methicillin resistant and sensitive Staphylococcus aureus. BMC Microbiol 7:99. doi: 10.1186/1471-2180-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller LG, Perdreau-Remington F, Bayer AS, Diep B, Tan N, Bharadwa K, Tsui J, Perlroth J, Shay A, Tagudar G, Ibebuogu U, Spellberg B. 2007. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis 44:471–482. doi: 10.1086/511033. [DOI] [PubMed] [Google Scholar]
- 38.Balachandra S, Pardos de la Gandara M, Salvato S, Urban T, Parola C, Khalida C, Kost RG, Evering TH, Pastagia M, D'Orazio BM, Tomasz A, de Lencastre H, Tobin JN. 2015. Recurrent furunculosis caused by a community-acquired Staphylococcus aureus strain belonging to the USA300 clone. Microb Drug Resist 21:237–243. doi: 10.1089/mdr.2014.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conceicao T, Diamantino F, Coelho C, de Lencastre H, Aires-de-Sousa M. 2013. Contamination of public buses with MRSA in Lisbon, Portugal: a possible transmission route of major MRSA clones within the community. PLoS One 8:e77812. doi: 10.1371/journal.pone.0077812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tobin JN, Kost RG, Tomasz A, Jenks N, Frei CR, Balachandra S, Khalida C, D'Orazio B, Urban T, Salvato S, Parola C, Burgess R, Chinea C, Coffran C, Dladla N, Budhrani R, Leinberger-Jabari A, Pastagia M, Evering T, Pardos de la Gandara M, de Lencastre H, Coller BS, Du LC, Treviño LB, Treviño SB. 2014. PBRN networks conduct the full spectrum of translational research studies of CA-MRSA treatment and recurrence in community health centers, poster P36. Abstr North Am Prim Care Res Group (NAPCRG) Pract Based Res Netw (PBRN) Conf, Bethesda, MD, 30 June to 1 July 2014 http://www.napcrg.org/Portals/51/Documents/PBRN%20Conf%20Handouts/Poster%20Session%20II%20Abstracts.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.