LETTER
Bloodstream infections represent a major burden on the United States health care system, with the number of cases of severe sepsis exceeding 750,000 per year (1) and representing 10% of all intensive care unit (ICU) admissions (2). It has also been shown that patient survival rates directly correlate with the ability to identify these infections early, with a 7.6% increase in the mortality rate occurring for every hour treatment is delayed (3). To improve upon the reported time to identification of bloodstream infections in our laboratory, we implemented the use of the automated Verigene system (Nanosphere Inc., Northbrook, IL). The Verigene system is a multiplex, automated microarray-based nucleic acid diagnostic test for the identification of genus, species, and genetic resistance determinants from a broad panel of the most common Gram-negative and Gram-positive organisms isolated from blood cultures. Here we describe two bloodstream isolates that were reported as “not detected” when using the Verigene system but were subsequently identified as Klebsiella pneumoniae by both the Vitek 2XL identification/antimicrobial susceptibility testing and Vitek MS matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) systems (both from bioMérieux, Marcy l'Etoile, France). K. pneumoniae is included in the Verigene system Gram-negative blood culture panel, prompting us to investigate a potential false-negative result by the Verigene system. Our inquiry revealed that these isolates were actually another Klebsiella species, Klebsiella variicola.
A 27-year-old female with a history of systematic lupus erythematosus complicated by lupus nephritis presented to the medical ICU for acute hypoxic respiratory failure requiring intubation, attributed to a drug interaction. This patient's condition was further complicated by hematuria, kidney failure, pancytopenia, hemolytic anemia, oral thrush, and gastrointestinal bleeding from cytomegalovirus colitis. Multiple sets of blood cultures were drawn throughout the patient's hospital stay. Bottles from two blood culture sets drawn 3 weeks apart were flagged positive on the Bactec FX (Becton Dickinson, Sparks, MD) blood culture instrument and were found to be growing Gram-negative rods. First, the aerobic (BD Bactec Plus Aerobic/F culture vial) and anaerobic (BD Bactec Anaerobic/F culture vial) bottles from a peripheral blood draw were flagged positive, followed by the anaerobic bottle only from an arterial line blood draw.
The Verigene system was utilized as our first-line assay for rapid organism identification in positive blood cultures, but no organism was detected. Subsequently, the isolates grew on blood and MacConkey agar, followed by identification through utilization of Vitek 2XL and Vitek MS. Both instruments identified each of the isolates as K. pneumoniae with a 97% probability score from Vitek 2XL. Since K. pneumoniae is an organism included in the Verigene system Gram-negative blood culture panel, these isolates should have been identified if present in the positive blood cultures. Therefore, we began our investigation of what appeared to be two false-negative results.
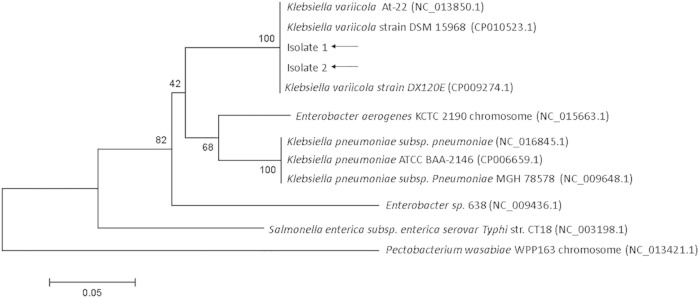
Bioinformatic and biochemical analyses were utilized to further characterize these two isolates. Our goal was to determine whether these isolates were indeed K. pneumoniae, as reported by the two Vitek instruments, or another closely related member of the genus Klebsiella that would not be identified by the Verigene system. In 2012, a Japanese group reported the human pathogenicity of K. variicola, a species with close genetic similarity to K. pneumoniae (4), prompting us to examine whether our isolates could be this species. Since >95% of K. variicola isolates have been shown not to ferment the carbohydrate adonitol (5) (whereas K. pneumoniae ferments this carbohydrate), we performed a biochemical analysis using Vitek 2XL to analyze adonitol fermentation. Neither isolate fermented adonitol, which supported our notion that these isolates were K. variicola. Next, a genome comparison study of K. pneumoniae and K. variicola identified KVAR_0717 (yggE) as a de novo target on the basis of sequence differences and a bioinformatic analysis was performed by amplification of this gene using Klebsiella species-specific primers (performed at ACGT Inc., Wheeling, IL) (Table 1), followed by nucleotide sequencing on an ABI 3730XL genetic analyzer to definitively identify both Klebsiella isolates to the species level (performed at Nanosphere Inc., Northbrook, IL). Bioinformatic analysis identified the two isolates as K. variicola with >99.5% confidence. On the basis of sequencing, a phylogenetic tree was generated that demonstrates the relatedness of these two isolates to other known bacterial sequences (Fig. 1) and clearly shows that these two isolates cluster with K. variicola.
TABLE 1.
Primers used to amplify KVAR_0717 (yggE)a
| Amplification target | Amplicon size (bp) | Primer direction | Primer sequence |
|---|---|---|---|
| Klebsiella spp. | 330 | Forward | AGAATGCTTTTCCCGTTCTGA |
| Forward | AGAATGCTTTTGCCGTTCTGA | ||
| Forward | AGAATGCTTTTACCGTTCTGA | ||
| Forward | AGGATGCTTTTGCCATTCTGA | ||
| Reverse | TGGAGAAACGACAGTGAAGTT |
The primers shown were used to amplify the KVAR_0717 gene of Klebsiella spp. in order to use this amplified sequence for genetic sequencing.
FIG 1.
The phylogeny of clinical isolates 1 and 2 was determined by the neighbor-joining method from partial sequences of KVAR_017 (yggE) and was prepared with MEGA6 software. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches. All reference sequences were obtained from NCBI, and NCBI reference sequence numbers are included.
Our investigation revealed that both Vitek 2XL and Vitek MS misidentified the isolates in question as K. pneumoniae, when further analysis revealed that they were both actually K. variicola. Vitek 2XL uses sugar fermentation reactions to biochemically determine bacterial identity, and Vitek MS uses the profile of bacterial proteins to determine identity. In contrast, the Verigene system, which is a microarray-based nucleic acid diagnostic test and includes K. pneumoniae in its identification panel, did not misidentify the isolates and correctly reported them as not identified. Since K. variicola is closely related to K. pneumoniae, it is difficult to distinguish between the two species by commonly used methods such as 16S rRNA gene sequencing (6, 7). The Verigene system is able to distinguish between the two species because the K. pneumoniae probes used in the assay have a low enough sequence identity with K. variicola (88% versus 100% for K. pneumoniae) to select against K. variicola as a target. K. variicola was first isolated from plants (6) and has previously been identified as a cause of fatal sepsis (4). Isolates of K. variicola have previously been shown to have lower antibiotic resistance rates than other Klebsiella species (5). A recent study by Maatallah et al. also found lower antibiotic resistance rates but linked K. variicola bloodstream infections to a higher mortality rate than infections caused by K. pneumoniae. Also, no additional known virulence factors in K. variicola were identified that could explain the increased risk of death (8). These findings demonstrate that lower antibiotic resistance rates do not necessarily correlate with better treatment outcomes in K. variicola infections. With this in mind, proper identification of K. variicola will be critical in the further evaluation of its clinical significance as an underreported infectious agent and in the development of more tailored K. variicola treatment options. Since both Vitek 2XL and Vitek MS are widely used for isolate identification in clinical labs, our data reveal one way in which K. variicola infections may be currently underestimated.
ACKNOWLEDGMENTS
We thank the Bioinformatics team at Nanosphere Inc. for their assistance in the bioinformatic analysis. We also thank Donald Bouyer, Nicole Mendell, Lucas Blanton, and Patricia A. Valdes for their assistance in generation of the phylogenetic tree.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, van der Poll T. 2013. Severe sepsis and septic shock. N Engl J Med 369:2063. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Cheang M. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 4.Seki M, Gotoh K, Nakamura S, Akeda Y, Yoshii T, Miyaguchi S, Inohara H, Horii T, Oishi K, Iida T, Tomono K. 2013. Fatal sepsis caused by an unusual Klebsiella species that was misidentified by an automated identification system. J Med Microbiol 62:801–803. doi: 10.1099/jmm.0.051334-0. [DOI] [PubMed] [Google Scholar]
- 5.Brisse S, van Himbergen T, Kusters K, Verhoef J. 2004. Development of a rapid identification method for Klebsiella pneumoniae phylogenetic groups and analysis of 420 clinical isolates. Clin Microbiol Infect 10:942–945. doi: 10.1111/j.1469-0691.2004.00973.x. [DOI] [PubMed] [Google Scholar]
- 6.Rosenblueth M, Martínez L, Silva J, Martínez-Romero E. 2004. Klebsiella variicola, a novel species with clinical and plant-associated isolates. Syst Appl Microbiol 27:27–35. doi: 10.1078/0723-2020-00261. [DOI] [PubMed] [Google Scholar]
- 7.Alves MS, Dias RC, de Castro AC, Riley LW, Moreira BM. 2006. Identification of clinical isolates of indole-positive and indole-negative Klebsiella spp. J Clin Microbiol 44:3640–3646. doi: 10.1128/JCM.00940-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maatallah M, Vading M, Kabir MH, Bakhrouf A, Kalin M, Nauclér P, Brisse S, Giske CG. 2014. Klebsiella variicola is a frequent cause of bloodstream infection in the Stockholm area, and associated with higher mortality compared to K. pneumoniae. PLoS One 9:e113539. doi: 10.1371/journal.pone.0113539. [DOI] [PMC free article] [PubMed] [Google Scholar]