Abstract
Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) has gained popularity in recent years for rapid bacterial identification, mostly at the genus or species level. In this study, a rapid method to identify the Escherichia coli flagellar antigen (H antigen) at the subspecies level was developed using a MALDI-TOF MS platform with high specificity and sensitivity. Flagella were trapped on a filter membrane, and on-filter trypsin digestion was performed. The tryptic digests of each flagellin then were collected and analyzed by MALDI-TOF MS through peptide mass fingerprinting. Sixty-one reference strains containing all 53 H types and 85 clinical strains were tested and compared to serotyping designations. Whole-genome sequencing was used to resolve conflicting results between the two methods. It was found that DHB (2,5-dihydroxybenzoic acid) worked better than CHCA (α-cyano-4-hydroxycinnamic acid) as the matrix for MALDI-TOF MS, with higher confidence during protein identification. After method optimization, reference strains representing all 53 E. coli H types were identified correctly by MALDI-TOF MS. A custom E. coli flagellar/H antigen database was crucial for clearly identifying the E. coli H antigens. Of 85 clinical isolates tested by MALDI-TOF MS-H, 75 identified MS-H types (88.2%) matched results obtained from traditional serotyping. Among 10 isolates where the results of MALDI-TOF MS-H and serotyping did not agree, 60% of H types characterized by whole-genome sequencing agreed with those identified by MALDI-TOF MS-H, compared to only 20% by serotyping. This MALDI-TOF MS-H platform can be used for rapid and cost-effective E. coli H antigen identification, especially during E. coli outbreaks.
INTRODUCTION
Escherichia coli food contamination can have serious consequences, such as hemolytic-uremic syndrome (HUS) (1, 2), with the 2011 Germany E. coli outbreak presenting a clear example (3). Therefore, the fast identification and typing of E. coli is important for tracking sources of contamination and reducing health risks. Traditional typing of E. coli is based mainly on two surface antigens of the bacteria for antiserum-based agglutination reactions: lipopolysaccharide (LPS) or O antigens for O typing and flagellar proteins or H antigens for H typing (4). The procedure for performing serotyping is time-consuming (2 to 12 days) and barely meets the need for fast diagnosis in outbreak situations. This is mainly due to the need to induce flagellar growth for optimizing agglutination reactions for H antigens. In addition, since there are many serotypes of H antigens, multiple agglutination steps have to be performed. There are 53 H types of E. coli flagella in total (designated H1 to H56; H13, H22, and H50 no longer exist) (4, 5). They are composed of polymerized flagellin proteins, each with an average molecular mass of approximately 50 kDa (36 to 60 kDa).
Since H antigens take longer to be typed with antisera, flagellar gene-based molecular methods, such as PCR-based flagellar gene sequencing and PCR-based restriction fragment length polymorphism (RFLP) analysis (4, 6, 7), have been developed over the past several years to type E. coli H antigens. These methods, although faster than serotyping, still take a few days to finish, and the results do not reveal whether the isolates are motile or not (6), an important feature of E. coli. In addition, multiple primers have to be designed for PCR-based H typing, a difficult task when examining unknown H types during an E. coli outbreak (4). Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS)-based methods have gained popularity in recent years in clinical microbiology laboratories and have been reported to successfully identify and classify bacteria (8–10). Currently, this MALDI-TOF MS platform can reach approximately 90% accuracy at the genus level and 80% accuracy at the species level for bacterial identification (11, 12). Moreover, the method is fast (12, 13) and cost-effective (14, 15), and it should prove useful in high-throughput bacterial screening and identification (16).
Recently, we reported an MS-based E. coli H antigen identification and classification method at the flagellar protein sequence level using a liquid chromatography-tandem MS (LC-MS/MS) platform (17, 18). The method applied a unique flagellar extraction and on-filter trypsin digestion method together with a curated E. coli flagellar protein sequence database for targeted and specific identification (19). The approach was termed MS-H and was able to identify and differentiate an E. coli H type in only a few hours after cell culture, with higher sensitivity and specificity than traditional serotyping (18). Since the MALDI-TOF platform can be used with higher throughput and less cost than with LC-MS/MS and is gaining popularity in clinical microbiology laboratories due to its ease of operation, in this study we have challenged the method to obtain subspecies-level identification and typing of E. coli flagella for the purpose of applying this technique for rapid H typing during an E. coli outbreak. We named this platform “MALDI-TOF MS-H.” Sample preparation was refined to optimize sensitivity, specificity, and reproducibility. Results were compared with those obtained by traditional serotyping, with conflicting results resolved by verifying flagellar gene sequences through whole-genome sequencing (WGS).
MATERIALS AND METHODS
Bacterial strains and isolates.
Sixty-one E. coli reference strains representing all 53 H types were obtained from the ISO-certified national enteric reference center at the National Microbiology Laboratory, Public Health Agency of Canada in Winnipeg, Manitoba. Eighty-five clinical isolates were obtained from five Canadian provincial laboratories for public health: Alberta, Manitoba, Quebec, Newfoundland and Labrador, and Nova Scotia.
Flagellar purification and on-filter digestion.
Similar to LC-MS/MS-based H typing flagellar preparation (17), E. coli bacteria were grown at 37°C overnight on tryptic soy agar (TSA) plates with 5% sheep blood. A 10-μl loopful of culture was diluted in 1 ml of water and gently suspended using a pipette tip. Based on trial tests, the addition of lysozyme was omitted to avoid contamination of the flagellar digest to be used for peptide mass fingerprinting. The bacterial suspension was vortexed at maximum speed (Vortex-Genie 2; VWR) for 20 s and a 1-min break for a total of three cycles. After centrifugation for 20 min at 16,000 × g (Eppendorf 5417C), the supernatant was gently collected using a 1-ml syringe and passed through a 13-mm-diameter filter with a 0.20-μm pore size (Acrodisc; Pall). The filter was washed with 3 ml of water and then flushed with air using a 1-ml syringe. One hundred microliters of trypsin (MS grade; 100 μg per ml in 100 mM ammonium bicarbonate; Promega) was applied to the filter for digestion at 37°C for 2 h. The filter was flushed with 600 μl of water followed by air to collect the digest. For some isolates that required motility induction, cells were grown for 1 week on brain heart infusion (BHI) broth with 0.55% agar at room temperature to enable enough motility before reculturing the cells for flagellar extraction as performed above.
MALDI-TOF MS-H sample preparation.
MS detection with the AutoFlex MALDI-TOF system (Bruker Dalton, Germany) was explored first with two types of matrices: α-cyano-4-hydroxycinnamic acid (CHCA; Sigma) and 2, 5-dihydroxybenzoic acid (DHB; Sigma). For CHCA, 30 mg of the matrix dissolved in 200 μl acetonitrile and 100 μl 0.3% TFA (trifluoroacetic acid) was vortexed for 5 min and then centrifuged for 2 min at maximum speed (16,000 × g). One hundred microliters of supernatant was added to a clean 1.5-ml tube containing 300 μl of isopropanol and briefly vortexed. Thirty microliters of this mixture then was transferred to the center of a 9-by-9-grid MALDI target plate and quickly smeared throughout the grid using the pipette tip. This “precoat” then was allowed to air dry at room temperature for roughly 3 min. A second saturated CHCA matrix solution was prepared subsequently by adding 30 mg CHCA to 200 μl 0.1% TFA and 100 μl acetonitrile and was vigorously vortexed for 5 s to ensure a homogeneous solution. The mixture then was rotated on the vortex platform for 5 min and centrifuged for 2 min at maximum speed (16,000 × g). Five microliters of the matrix supernatant was added to 5 μl of each sample in a 0.2-ml tube and pipetted up and down to mix. Two microliters of each sample mixture was loaded onto each of the four precoated grid spots on the MALDI plate and left to air dry for roughly 3 min in a fume hood. For DHB, 48 mg of the chemical was mixed with 200 μl 0.1% TFA and 200 μl acetonitrile. The solution was vortexed for 5 s to ensure homogeneity, rotated on the vortex platform for 5 min, and centrifuged for 2 min at maximum speed (16,000 × g). Five microliters of sample was mixed with 5 μl of the DHB matrix solution in a 0.2-ml tube by pipetting up and down. Two microliters of each sample mixture was loaded onto each of the four grid spots of a MALDI plate and left to crystallize for roughly 3 min in a fume hood.
MALDI-TOF MS-H analysis and database search.
MALDI-TOF MS-H analysis was performed using an Autoflex III Smartbeam (Bruker Daltonics, Germany). Five thousand shots were accumulated for each sample spot, with no more than 1,000 shots per raster spot, using FlexControl v3.4. The mass range was set from 700 to 4,000 Da. Laser energy was set from 85 to 95% of the laser attenuator setting (75%). Analysis was performed using BioTools v.3.2 (Bruker Daltonics, Germany). Peptide standard mix II (Bruker Daltonics, Germany) was used for calibration prior to MS analysis with DHB as the matrix. A custom FASTA-formatted database for E. coli H types (see text S1 in the supplemental material) was created and updated using the sequences and serotype information found in the NCBI protein database (17–19). Peptide mass fingerprinting/profiling was used for identification and differentiation of flagellar proteins from the tested isolates. Search parameters of 300 ppm mass error tolerance for parent ions and one missed cleavage of trypsin digestion were used. Oxidation on methionine was chosen as a possible modification. The top Mascot scoring hit for each MALDI spot, together with 3 out of 4 repeated spots having the same identification, was used to determine the H type of each strain.
E. coli H serotyping.
E. coli H antigens were serotyped on the basis of methods of several publications (20–22), as summarized for our standard operation procedure. For motility induction, bacteria were streaked on MacConkey agar plates to check for purity and a single colony was selected. This colony was subcultured to a 0.25% Craigie tube and incubated overnight at 35°C ± 2°C. Motile E. coli bacteria should travel through the Craigie tube and up through the media using their flagella while developing their H antigen. E. coli then was selected from the top of the media and transferred to a 0.3% Craigie tube to further develop motility after incubation overnight at 35°C ± 2°C. To prepare the H antigen, Ewing's broth was added to the top of the 0.3% Craigie tube and gently drawn up and down so that the most motile bacteria, originally at the surface of the Craigie tube, became suspended fully in Ewing's broth. The suspension was incubated at 35°C ± 2°C for approximately 4 h and treated with formalin to kill the live bacteria and preserve the H antigen. The H antigen was diluted and screened first in antiserum pools prepared with 5 to 8 individual monovalent antisera. For any pool with a positive reaction, individual monovalent antisera were tested. Absorbed antisera were used for final confirmation of the H serotype for any occasional strains that cross-reacted with more than one monovalent antisera. The titers of all antisera had been determined previously with reference E. coli strains. A positive H serotype was obtained when the H antigen had an agglutination equivalent to or better than the reference titer for that antiserum.
Whole-genome sequencing.
Genomic DNA was extracted using the Epicentre Metagenomic DNA isolation kit for water (Mandel Canada) with overnight growth of bacteria, and the samples were prepared using a Nextera XT DNA sample preparation kit. Whole-genomic data of the isolates were acquired by 300-bp paired-end sequencing on the Illumina MiSeq using MiSeq reagent kit V (600 cycles; Illumina). An in silico analysis was performed on the sequences of the isolates, whereby data were assembled into contigs using SPAdes Assembler (v3.0). Contig output then was searched against a custom database containing E. coli flagellar gene sequences extracted from the NCBInr database.
RESULTS
Proof-of-principle testing of MALDI-TOF MS-H.
To verify the ability of MALDI-TOF to produce correct MS-H types for a variety of E. coli strains, three motile strains representing the most common H type (H7), the most common non-H7 type (H11), and the H type typically misidentified as H11 by serotyping (H21), together with a nonmotile strain control, were tested repeatedly. Two MALDI matrices, CHCA and DHB, were tested and compared first. The common H types were identified through mass profiling a few minutes after the targets were loaded, with database search results obtained shortly thereafter. The CHCA matrix gave the same specificity as DHB; however, its use produced lower confidence scores and sequence coverage (see Table S1 in the supplemental material). It also was found that although the signal intensity of tests performed using CHCA could be increased by adding a precoat of the matrix before sample loading (data for samples where precoating was not performed is not shown), H11 and H21 could be identified and differentiated only by MALDI-TOF using DHB; therefore, it was decided that DHB would be the matrix of choice for MALDI-TOF MS-H. This decision was not impeded by the fact that DHB matrix crystals are clumpy and known to produce hot spots during analysis (23, 24), as these manifestations should not affect total signal intensity. Repeated tests summarized in Table 1 indicated that the DHB matrix worked well to identify examined reference strains. While only a fraction (1/600) of the flagellin digest from a loopful culture (18) was used for each MALDI plate spot, all repeated tests produced identical and accurate H type results within minutes of sample loading.
TABLE 1.
MALDI-TOF identification of E. coli flagella representing the most common serotypesa
| Strain no. (H type) | MS-H typeb | Sequence coveragec (%) for test: |
||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| EDL-933 (H7) | H7 | 71 | 66 | 68 |
| 09-1767 (H11) | H11 | 57 | 64 | 56 |
| 85-489 (H21) | H21 | 78 | 87 | 86 |
| E32511 (nonmotile) | No flagellin detected | NA | NA | NA |
The three most common E. coli strains and one nonmotile strain were independently cultured and analyzed by MS-H with DHB as the MALDI matrix on three separate occasions. Results were obtained by a Mascot database search.
The top hit resulting from mass profiling of at least three of four sample-loaded grid spots.
The average sequence coverage of MALDI grid spots (representing sample repeats) that gave correct identification. NA, not applicable.
During these exploratory tests, it was found that routine peptide mass fingerprinting assays were sufficient to type the flagella of examined E. coli reference strains. It also was found that MALDI-TOF-based tandem mass spectrometry by itself could not consistently identify E. coli H types with good reproducibility (data not shown). Lastly, even though prepared flagella were very pure (17), searches against public sequence databases did not result in unique identification of H types, as had been previously observed (19), through mass fingerprinting. Consequently, the updated curated database for E. coli H antigens with clear annotations of H types (see text S1 in the supplemental material), which had proven useful for LC-MS/MS (17–19), also was incorporated into the MALDI-TOF MS-H platform.
Diagnostic sensitivity and specificity tests with MALDI-TOF MS-H on all E. coli H types.
To demonstrate that MALDI-TOF MS-H should be able to correctly identify the H type of every E. coli H antigen, reference strains representing all 53 H types, together with five nonmotile strains, were isolated from overnight cultures of frozen stocks without motility induction and analyzed. As summarized in Table 2, the H types of 40 of 53 reference strains were detected directly and accurately after the first overnight culture. After motility induction for a week, 12 of the 13 remaining reference strains were correctly identified, indicating that MALDI-TOF MS-H performs with high diagnostic sensitivity (98%). For these 13 strains, samples taken from the first overnight culture also were tested after their tryptic digests were concentrated 10 times, and 11 out of 13 samples were identified correctly with a sensitivity of 96%. The combined sensitivity of these two approaches reached 100%. In addition, five nonmotile isolates could not be identified based on Mascot search data output, indicating that MALDI-TOF MS-H antigen identification also had high specificity. From these tests, we decided to apply the sample concentration approach for MALDI-TOF MS-H due to its faster data output (roughly 5 h after bacterial culture, including sample preparation and MS detection time) compared to that of motility induction, which took days for sample preparation.
TABLE 2.
Test summary of MALDI-TOF analysis performed on E. coli reference strains representing all 53 H antigensa
| Sample preparationb | MALDI-TOF resulte (% [no. identified/total no.]) |
|
|---|---|---|
| Correct | Incorrect | |
| Digests prepared from the first overnight culture of 53 strains (including 13 induced strains)c | 98.00 (52/53) | 2.00 (1/53) |
| Digests prepared from the first overnight culture of 53 strains (including concentrated samples from 13 strains)d | 96.00 (51/53) | 4.00 (2/53) |
| Two approaches combined | 100.00 (53/53) | 0.00 (0/53) |
Only 53 strains with clear H types were summarized (nonmotile strains were not included).
Unidentified reference strains from the first overnight culture were further motility induced and concentrated.
All motility-induced strains were identified except H45.
Tryptic digests were concentrated 10 times; all strains were identified except H35 and H41.
Correct or incorrect signifies the percentage of correctly identified or incorrectly identified E. coli H antigens.
Two H types (H35 and H41) that could be identified only by MALDI-TOF MS-H after motility induction (Table 2) were examined further by culturing and analyzing additional isolates of the same two H types. As can be seen from Table S2 in the supplemental material, some strains were identified while others were not, although all were characterized as reference strains. SDS-PAGE was used to observe the flagellin band intensity for flagella expressed by each isolate (17). Interestingly, isolates of the same H type showed different levels of flagellin band intensities, whereas the three strains not identified by MALDI-TOF MS-H displayed no flagellin band whatsoever (see Fig. S1 in the supplemental material). This finding confirmed that some reference strains, although motile when first isolated, could lose motility after long-term storage (17). Moreover, it was apparent that MALDI-TOF MS-H could detect flagellin over a wide range of expression levels.
Validation of MALDI-TOF MS-H by checking clinical isolates.
After preliminary testing of MALDI-TOF MS-H on reference strains was completed, a standard operating protocol was established for this platform and a second study was designed to compare the serotyping results and MALD-TOF-MS-H results of 85 clinical isolates. For serotyping, an ISO-certified operating standard (17) was always used. The workflow of the MALDI-TOF MS-H method for the validation of clinical isolates is shown in Fig. 1 and briefly described here. (i) A positive-control sample (reference strain O157:H7) was analyzed to confirm the performance of the mass spectrometer. (ii) Cell culture was performed for three consecutive days on TSA plates with 5% sheep blood; culture was analyzed daily to ensure sufficient signal would be achieved with DHB matrix (23, 25) and also to avoid using sluggish cells occasionally seen during the early phase of cell culture (18, 26). (iii) Tryptic digests were concentrated 10 times in a vacuum dryer before applying the sample to the MALDI plate. (iv) Four spots per sample were loaded to observe the reproducibility of results; if identical H types were obtained from at least three spots with confidence based on the Mascot peptide mass fingerprinting database search for a top hit, then that top hit would be characterized as the flagellum type. (v) If necessary, motility induction was performed for 1 week for any unidentified strains in BHI broth with 0.55% agar to enable enough flagellar production for successful flagellin identification.
FIG 1.
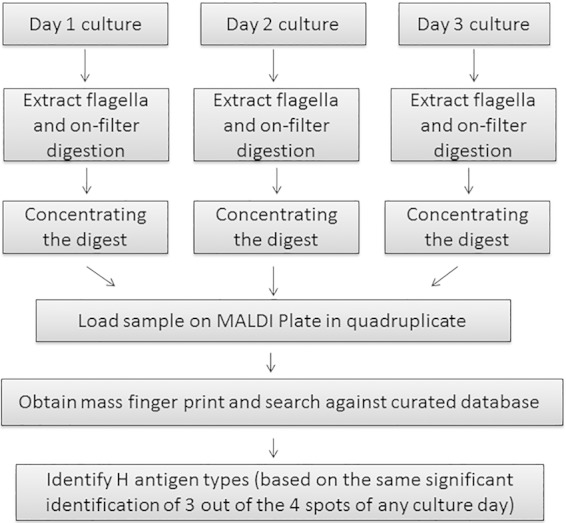
MALD-TOF MS-H workflow.
Table 3 demonstrates that the results of MALDI-TOF MS-H were in good agreement (88%; 82.35% + 5.88%) with those of serotyping. Consecutive cultures for 3 days, performing MALDI-TOF MS-H each day, did enhance the positive identification rate in total (see Table S3 in the supplemental material). For the 12% (10 isolates) in disagreement between serotyping and MALDI-TOF MS-H, flagellar genetic data were analyzed by whole-genome sequencing (WGS) and were found to translate more often to the results of mass spectrometry than to those of serotyping (for MALDI-TOF analysis, 6 out of 10 [60%] were identified correctly, whereas 4 out of 10 [40%] were incorrect; for serotyping, 2 out of 10 [20%] were identified correctly, whereas 8 out of 10 [80%] were incorrect). WGS also uncovered two flagellar sequences that did not agree with the types identified by either method. Overall, these results show that MALDI-TOF MS-based E. coli H typing possesses higher accuracy than traditional serotyping, which is currently the gold standard.
TABLE 3.
Summary of serotyping and MALDI-TOF-based H antigen identification on 85 clinical E. coli isolates
| Serotype identification | MALDI-TOF result (% [no. identified/total no.]) |
||||
|---|---|---|---|---|---|
| Correct |
Incorrecta |
Total | |||
| Non-MIb | MIc | Non-MIb | MIc | ||
| Correct | 82.35 (70/85) | 5.88 (5/85) | 0.00 (0/85) | 2.35 (2d/85) | 90.59 (77/85) |
| Incorrectc | 7.06 (6e/85) | 0.00 (0/85) | 0.00 (0/85) | 2.35 (2f/85) | 9.41 (8/85) |
| Total | 95.29 (81/85) | 4.71 (4/85) | 100.00 (85/85) | ||
Whole-genome sequencing (WGS) was performed.
Non-MI, non-motility induction.
MI, motility induction.
WGS produced the same results as serotyping but not MALDI-TOF.
WGS produced the same results as MALDI-TOF but not serotyping.
WGS produced different results than both serotyping and MALDI-TOF.
DISCUSSION
In the past few years, we have attempted to use the LC-MS/MS platform for E. coli H typing (17, 18), and it has proven to be a robust method for H typing at the molecular level. To transfer this experience to the more popular and easier-to-use MALDI-TOF platform, we first tested the most common pathogenic serotypes of E. coli, such as O157:H7 and O26:H11, and found mass fingerprinting was sufficient to resolve the H types of these strains accurately. This was due to the high purity of flagellar preparation (17), a critical component of peptide mass fingerprinting. Peptide mass fingerprinting rendered sample testing extremely fast, as sample replicates were analyzed in just minutes. This approach certainly proved much faster than traditional serotyping, but it also was faster than the LC-MS/MS platform, for which a column cleanup procedure had to be performed for consecutive and high-throughput sample testing (18). A curated database still was crucial for accurate H type identification due to the clear and specific annotation of each flagellar antigen (19).
During the expanded test on all of the E. coli flagellar H types from reference strains, the majority of antigens (40 of 53) were detected on the first day of cell culture, while the rest were detected by concentrating the tryptic digests or motility induction. We suspected that some strains would lose motility or had limited motility due to long-term storage at low temperatures, as observed previously (17); hence, further sample enrichment was required to enhance the loading capacity during mass fingerprinting. After either motility induction or sample concentration through vacuum drying, these difficult-to-culture strains were identified successfully. We also found that CHCA, which often was used as a matrix for biotyping microorganisms (27–29), did not give as high a confidence (Mascot score) as DHB. This may be attributed to the clumps or hot spots (29) formed by DHB crystals which emit strong signals upon contact with laser energy. Thus, DHB was selected as the matrix for clinical sample testing, although the hot spots were reported to have reproducibility problems in other applications (23, 24). This reproducibility issue could be mitigated partially by repeating the experiment consecutively for 3 days, testing four replicates per sample, and requiring three of four replicates to display the same identification with confidence.
The result from the comparison of MALDI-TOF MS-H and serotyping results on 85 clinical isolates indicated that the MALDI-TOF MS-H method was an accurate and sensitive method for E. coli H antigen identification and typing. It had a much shorter turnaround time, higher throughput, and lower cost than serotyping. MALDI-TOF MS-H identified 54 isolates with no false positives, while serotyping identified 56 with six false positives (confirmed by WGS). MALDI-TOF MS-H also identified one isolate that was designated undetermined by serotyping. Due to the sluggish (26) growth of some clinical isolates, motility induction in BHI broth with 0.55% agar for 1 week enabled the isolation of the most highly motile bacteria for successful flagellin identification. It was noteworthy that MALDI-TOF MS-H generally did not require motility induction of bacterial isolates, unlike the serotyping procedure, with H types being easily identified with good accuracy. When confident results could not be obtained, this further induction of motility was performed for 1 week instead of a maximum of 2 weeks in the serotyping procedure. Although we tested and compared the results of each 3-day sample for the purposes of method development and validation, any daylong culture with a positive MS-H type was virtually sufficient to provide MS-H typing and further testing was not needed, because the identification was sequence specific with no false-positive result observed.
In conclusion, the MALDI-TOF-MS-H method described in this study, in conjunction with peptide mass fingerprinting, can be used to identify and type E. coli flagella with high speed, high specificity, and high sensitivity. It is simple, straightforward, and cost-effective. This platform should be particularly useful during E. coli outbreak situations for the provision of quick H type classifications.
Supplementary Material
ACKNOWLEDGMENTS
We thank Larissa Domish and Angela Sloan for their contributions at the later stage of this project and the Genomics Core and Bioinformatics Core at the National Microbiology Laboratory, Public Health Agency of Canada, for providing related services and support.
We also appreciate Genomics Research and Development Initiative (GRDI) funding from the Public Health Agency of Canada, which supported this project.
We have no competing financial interests to declare.
H. Chui, M. Chan, and D. Hernandez performed MALDI-TOF MS-H sample and whole-genome sequencing sample preparation; P. Chong and S. McCorrister performed MALDI-TOF MS-H testing; L. A. M. Peterson and A. Robinson performed serotyping; M. Walker performed whole-genome sequencing and data analysis; L. Chui, J. Wylie, S. Bekal, S. Ratnam, and D. J. M. Haldane provided clinical isolates and critical writing; M. Drebot, B. Xu, C. Nadon, and J. D. Knox contributed project ideas and critical writing; G. Westmacott contributed to database creation; and K. Cheng and G. Wang contributed project ideas, method design, data summary, and manuscript writing.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00593-15.
REFERENCES
- 1.Gyles CL. 2007. Shiga toxin-producing Escherichia coli: an overview. J Anim Sci 85:E45–E62. doi: 10.2527/jas.2006-508. [DOI] [PubMed] [Google Scholar]
- 2.Mayer CL, Leibowitz CS, Kurosawa S, Stearns-Kurosawa DJ. 2012. Shiga toxins and the pathophysiology of hemolytic uremic syndrome in humans and animals. Toxins (Basel) 4:1261–1287. doi: 10.3390/toxins4111261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin J, Cui Y, Zhao X, Rohde H, Liang T, Wolters M, Li D, Belmar Campos C, Christner M, Song Y, Yang R. 2011. Identification of the Shiga toxin-producing Escherichia coli O104:H4 strain responsible for a food poisoning outbreak in Germany by PCR. J Clin Microbiol 49:3439–3440. doi: 10.1128/JCM.01312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray PR. 1999. Manual of clinical microbiology. ASM Press, Washington, DC. [Google Scholar]
- 5.Wang L, Rothemund D, Curd H, Reeves PR. 2003. Species-wide variation in the Escherichia coli flagellin (H-antigen) gene. J Bacteriol 185:2936–2943. doi: 10.1128/JB.185.9.2936-2943.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos Moreno AC, Cabilio Guth BE, Baquerizo Martinez M. 2006. Can the fliC PCR-restriction fragment length polymorphism technique replace classic serotyping methods for characterizing the H antigen of enterotoxigenic Escherichia coli strains? J Clin Microbiol 44:1453–1458. doi: 10.1128/JCM.44.4.1453-1458.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machado J, Grimont F, Grimont PA. 2000. Identification of Escherichia coli flagellar types by restriction of the amplified fliC gene. Res Microbiol 151:535–546. doi: 10.1016/S0923-2508(00)00223-0. [DOI] [PubMed] [Google Scholar]
- 8.Clark CG, Kruczkiewicz P, Guan C, McCorrister SJ, Chong P, Wylie J, van Caeseele P, Tabor HA, Snarr P, Gilmour MW, Taboada EN, Westmacott GR. 2013. Evaluation of MALDI-TOF mass spectroscopy methods for determination of Escherichia coli pathotypes. J Microbiol Methods 94:180–191. doi: 10.1016/j.mimet.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Neville SA, Lecordier A, Ziochos H, Chater MJ, Gosbell IB, Maley MW, van Hal SJ. 2011. Utility of matrix-assisted laser desorption ionization-time of flight mass spectrometry following introduction for routine laboratory bacterial identification. J Clin Microbiol 49:2980–2984. doi: 10.1128/JCM.00431-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel R. 2013. MALDI-TOF mass spectrometry: transformative proteomics for clinical microbiology. Clin Chem 59:340–342. doi: 10.1373/clinchem.2012.183558. [DOI] [PubMed] [Google Scholar]
- 11.Bessede E, Angla-Gre M, Delagarde Y, Sep Hieng S, Menard A, Megraud F. 2011. Matrix-assisted laser-desorption/ionization biotyper: experience in the routine of a university hospital. Clin Microbiol Infect 17:533–538. doi: 10.1111/j.1469-0691.2010.03274.x. [DOI] [PubMed] [Google Scholar]
- 12.Sogawa K, Watanabe M, Sato K, Segawa S, Ishii C, Miyabe A, Murata S, Saito T, Nomura F. 2011. Use of the MALDI BioTyper system with MALDI-TOF mass spectrometry for rapid identification of microorganisms. Anal Bioanal Chem 400:1905–1911. doi: 10.1007/s00216-011-4877-7. [DOI] [PubMed] [Google Scholar]
- 13.Schneiderhan W, Grundt A, Worner S, Findeisen P, Neumaier M. 2013. Work flow analysis of around-the-clock processing of blood culture samples and integrated MALDI-TOF mass spectrometry analysis for the diagnosis of bloodstream infections. Clin Chem 59:1649–1656. doi: 10.1373/clinchem.2012.198218. [DOI] [PubMed] [Google Scholar]
- 14.Jadhav S, Sevior D, Bhave M, Palombo EA. 2014. Detection of Listeria monocytogenes from selective enrichment broth using MALDI-TOF mass spectrometry. J Proteomics 97:100–106. doi: 10.1016/j.jprot.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Bailey D, Diamandis EP, Greub G, Poutanen SM, Christensen JJ, Kostrzew M. 2013. Use of MALDI-TOF for diagnosis of microbial infections. Clin Chem 59:1435–1441. doi: 10.1373/clinchem.2013.204644. [DOI] [PubMed] [Google Scholar]
- 16.Christner M, Trusch M, Rohde H, Kwiatkowski M, Schluter H, Wolters M, Aepfelbacher M, Hentschke M. 2014. Rapid MALDI-TOF mass spectrometry strain typing during a large outbreak of Shiga-toxigenic Escherichia coli. PLoS One 9:e101924. doi: 10.1371/journal.pone.0101924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng K, Drebot M, McCrea J, Peterson L, Lee D, McCorrister S, Nickel R, Gerbasi A, Sloan A, Janella D, Van Domselaar G, Beniac D, Booth T, Chui L, Tabor H, Westmacott G, Gilmour M, Wang G. 2013. MS-H: a novel proteomic approach to isolate and type the E. coli H antigen using membrane filtration and liquid chromatography-tandem mass spectrometry (LC-MS/MS). PLoS One 8:e57339. doi: 10.1371/journal.pone.0057339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng K, Sloan A, Peterson L, McCorrister S, Robinson A, Walker M, Drew T, McCrea J, Chui L, Wylie J, Bekal S, Reimer A, Westmacott G, Drebot M, Nadon C, Knox JD, Wang G. 2014. Comparative study of traditional flagellum serotyping and liquid chromatography-tandem mass spectrometry-based flagellum typing with clinical Escherichia coli isolates. J Clin Microbiol 52:2275–2278. doi: 10.1128/JCM.00174-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng K, Sloan A, McCorrister S, Babiuk S, Bowden TR, Wang G, Knox JD. 2014. Fit-for-purpose curated database application in mass spectrometry-based targeted protein identification and validation. BMC Res Notes 7:444. doi: 10.1186/1756-0500-7-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross RJ, Rowe B. 1985. Serotyping of Escherichia coli, p 345–363. In Sussman M. (ed), The virulence of Escherichia coli: reviews and methods. Academic Press, London, United Kingdom. [Google Scholar]
- 21.Kauffmann F. 1947. The serology of the coli group. J Immunol 57:71–100. [PubMed] [Google Scholar]
- 22.Prager R, Strutz U, Fruth A, Tschape H. 2003. Subtyping of pathogenic Escherichia coli strains using flagellar (H)-antigens: serotyping versus fliC polymorphisms. Int J Med Microbiol 292(7-8):477–486. [DOI] [PubMed] [Google Scholar]
- 23.Albrethsen J. 2007. Reproducibility in protein profiling by MALDI-TOF mass spectrometry. Clin Chem 53:852–858. doi: 10.1373/clinchem.2006.082644. [DOI] [PubMed] [Google Scholar]
- 24.Szajli E, Feher T, Medzihradszky KF. 2008. Investigating the quantitative nature of MALDI-TOF MS. Mol Cell Proteomics 7:2410–2418. doi: 10.1074/mcp.M800108-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Williams TL, Andrzejewski D, Lay JO, Musser SM. 2003. Experimental factors affecting the quality and reproducibility of MALDI TOF mass spectra obtained from whole bacteria cells. J Am Soc Mass Spectrom 14:342–351. doi: 10.1016/S1044-0305(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 26.Orskov F, Orskov I. 1992. Escherichia coli serotyping and disease in man and animals. Can J Microbiol 38:699–704. doi: 10.1139/m92-115. [DOI] [PubMed] [Google Scholar]
- 27.Bizzini A, Durussel C, Bille J, Greub G, Prod'hom G. 2010. Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J Clin Microbiol 48:1549–1554. doi: 10.1128/JCM.01794-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carbonnelle E, Mesquita C, Bille E, Day N, Dauphin B, Beretti JL, Ferroni A, Gutmann L, Nassif X. 2011. MALDI-TOF mass spectrometry tools for bacterial identification in clinical microbiology laboratory. Clin Biochem 44:104–109. doi: 10.1016/j.clinbiochem.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 29.Richter SS, Sercia L, Branda JA, Burnham CA, Bythrow M, Ferraro MJ, Garner OB, Ginocchio CC, Jennemann R, Lewinski MA, Manji R, Mochon AB, Rychert JA, Westblade LF, Procop GW. 2013. Identification of Enterobacteriaceae by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry using the VITEK MS system. Eur J Clin Microbiol Infect Dis 32:1571–1578. doi: 10.1007/s10096-013-1912-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


