Abstract
Taenia martis is a tapeworm affecting mustelids, with rodents serving as intermediate hosts. The larval stage (cysticercus) has been found before only rarely in humans or primates. We hereby describe a case of cerebral T. martis cysticercosis in a French immunocompetent patient, confirmed by DNA analyses of biopsy material.
CASE REPORT
In January 2012, a 44-year-old woman with no significant medical history except Verneuil's disease was admitted to the Strasbourg University Hospital for right hemiparesis and aphasia. Cerebral magnetic resonance imaging (MRI) revealed fluid attenuated inversion recovery (FLAIR) and diffusion signal abnormalities in a small area of the left temporal lobe cortex, associated with leptomeningeal gadolinium-based enhancement in the depth of the adjacent sulcus (Fig. 1 and 2). On examination, the patient was found to have a temperature of 38.5°C. She reported no travel history outside Europe and presented no immunosuppression. Meningoencephalitis was suspected, and the first examination of the cerebrospinal fluid (CSF) revealed lymphocytic pleocytosis, increased protein levels, and normal glucose concentration. Bacterial antigen detection in CSF was negative, as were CSF bacterial and fungal cultures. Acyclovir, cefotaxime, and amoxicillin were initiated. Five days later, a second lumbar puncture revealed 790 leukocytes/mm3 (70% lymphocytes, 30% eosinophils) and oligoclonal immunoglobulin bands.
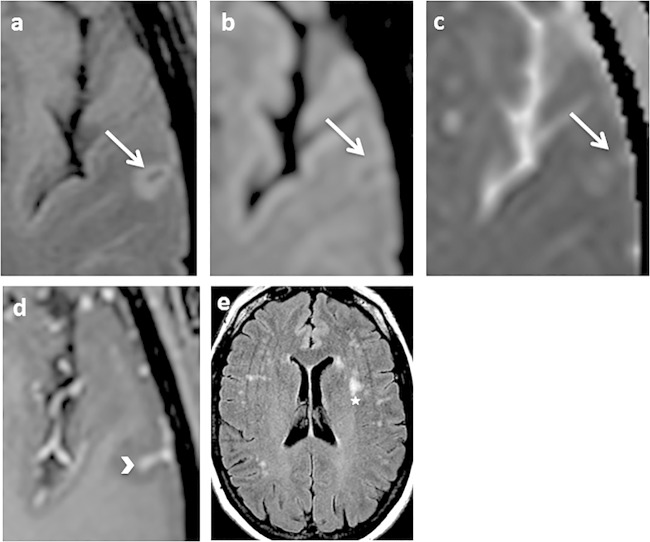
FIG 1.
Axial fluid attenuated inversion recovery (FLAIR) (a and e), axial diffusion (b), apparent diffusion coefficient map (c), and axial T1 spin echo (SE) after gadolinium injection (d). Shown are mild cortical FLAIR and diffusion hypersignal, with mild restriction of the diffusion coefficient in the left temporal lobe (arrow). An arrowhead shows leptomeningeal enhancement after gadolinium administration in the depth of the adjacent sulcus arrowhead. A star indicates white matter subcortical nonspecific hyperintensities.
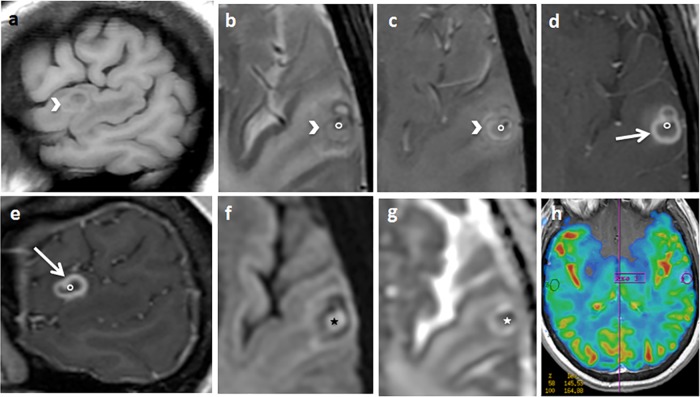
FIG 2.
Sagittal T1 (a), axial T2 spin echo (SE) (b), T2 star-weighted angiography (SWAN) (c), axial (d) and sagittal (e) T1 SE after gadolinium injection, axial diffusion (f), an apparent diffusion coefficient map (g), and a cerebral blood volume map (h). An arrow indicates a left temporal cortical annular enhancing lesion. The border of the lesion is thick and partially spontaneously hyperintense on T1 and hypointense on T2, with T2 SWAN suggesting the presence of hemosiderin or calcium deposits (arrowhead). The contrast enhancement is predominant in the inner part of the border (arrow). The center of the lesion appears hyperintense on diffusion-weighted MRI, with restriction of the diffusion coefficient suggesting thick content (star). In the center of the lesion, there is a small enhancing nodule attached to the border, hypointense on T2 SWAN (circle). The cerebral blood volume is diminished (h). Significant perilesional edema is revealed.
Due to the eosinophilia, a complementary serological investigation was performed. Serology was weakly positive (1:60) for Taenia solium using T. solium antigens and immunofluorescence and negative for Echinococcus granulosus using Western blotting with Echinococcus sp. antigens (LDBIO Diagnostics, Lyon, France), Echinococcus multilocularis using enzyme-linked immunosorbent assay (ELISA) with Em2+ antigens, (Bordier Affinity Products SA, Crissier, Switzerland), Taenia saginata using T. saginata antigens and immunofluorescence, Schistosoma mansoni using ELISA with S. mansoni antigens (Bordier Affinity Products SA), Trichinella spiralis using ELISA with T. spiralis antigens (LDBIO Diagnostics), and Strongyloides stercoralis using ELISA with Strongyloides ratti antigens (Bordier Affinity Products SA). Stool examinations were negative for parasites. The patient fully recovered the following week. She was readmitted in May 2012 after another episode of aphasia. Cerebral MRI was then performed again, displaying a ring-enhancing lesion along with a small enhancing nodule attached to its border, associated with significant perilesional edema, in the same cortical area as that recorded on the first MRI.
Despite MRI revealing no evidence of neoangiogenesis, due to the cerebral blood volume being diminished, there were no clinical features that enabled the distinction from an inflammatory process due to either unknown infection or tumor, such as a glioblastoma or metastatic process. Surgical exeresis of the parasitic mass was performed. The pathological examination demonstrated no signs of a tumoral process, yet revealed a thick-walled parasitic cyst with dense fibrosis and intense mononuclear inflammation. The cyst contained dense fluid consisting of thick bright eosinophilic ribbons of membranous tissue and calcareous corpuscles, characteristic of cestodes. Several punch biopsies of the suspected lesion were carried out by means of laser capture microdissection.
DNA was extracted from the dissected tissues. PCR testing was negative for Echinococcus granulosus and E. multilocularis, though positive for Taenia spp. (1). Fragments of the mitochondrial cytochrome c oxidase subunit 1 (cox1), NADH dehydrogenase subunit 1 (nad1), and 12S rRNA (rRNAS) genes were amplified by PCR using primer pairs JB3/JB4.5 (2), JB11/JB12 (3), and Cest3/Cest5 (4), respectively. Both partial sequences of the cox1 (396-bp) and nad1 (488-bp) genes demonstrated 100% homology with published T. martis sequences (EU544557 and EU544607). For the rRNA gene, the sequence (263/265 bp) demonstrated 99.2% homology with a published T. martis sequence (JX415820). Lower sequence identities were also revealed between our specimen and T. crassiceps (90 and 82% for cox1 and nad1, respectively) or T. twitchelli (94 and 86% for cox1 and nad1, respectively). Cerebral cysticercosis diagnosis was thus established based on the presence of T. martis. A phylogenetic tree was created using the sequences obtained in this study as well as those of representative Taenia species accessed from GenBank (Fig. 3).
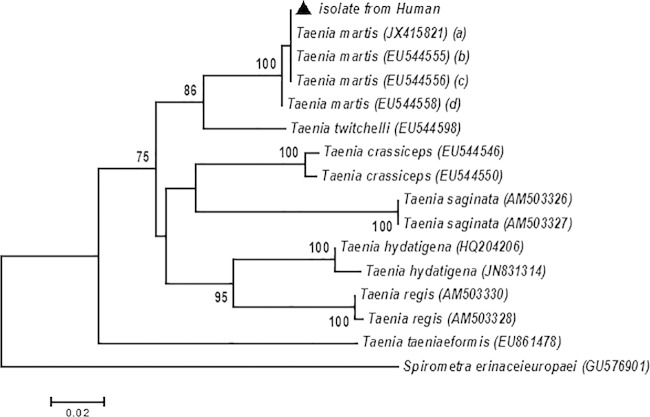
FIG 3.
Neighbor-joining tree based on Cox1 sequences, showing the phylogenetic relationships between the Taenia martis isolate from humans and the other tapeworm species. The black triangle refers to the sequence generated by this study, and the GenBank accession numbers of each strain are given in parentheses. The letters a to d in parentheses refer to Taenia martis isolated from humans (a), Myodes glareolus (b), Apodemus sylvaticus (c), and Myodes rufocanus (d), respectively. Bootstrap values of >70% are included to indicate branch support. Neighbor-joining analysis was processed using MEGA V.5.2. software, and the branch support was evaluated by means of a bootstrap test with 1,000 replicates (14).
Surgical exeresis of the parasitic mass was performed, and the patient was treated with praziquantel (50 mg/kg body weight/day) for 15 days and albendazole (15 mg/kg/day) for 1 month, combined with corticosteroids (1 mg/kg/day). Three years thereafter, no signs of recurrence were observed.
The patient lives in the forested part of the French Vosges region, consisting of a woody mountainous area at medium altitude with temperate climate in eastern France. She has three cats and a dog, with a garden where she grows vegetables, with free access to the nearby forest. Fecal samples collected from her cats and dog were investigated, and taeniid eggs were detected in one cat and the dog. The eggs were identified as Taenia taeniaeformis (4). T. martis was not identified. Fecal samples collected from the house's surroundings, suspected to originate from martens, were also negative for T. martis.
Several cestode species are currently known to be human parasites. Some are capable of invading the central nervous system (CNS), namely, Taenia solium and Echinococcus granulosus. Neurocysticercosis caused by T. crassiceps or T. multiceps is less common yet should be considered in the differential diagnosis of CNS infections. T. martis cysticercosis was recently reported for the very first time in a human eye (5).
T. martis is usually found in adult stages in the small intestines of stone martens. Other carnivores, including mustelids of the Mustela, Meles, Lutra, and Gulo genera, and more rarely, canids of the Canis and Vulpes genera, as well as wild cats, may also act as definitive hosts (6). Rodents and other small mammals serve as intermediate hosts and harbor the parasite in its larval stage (cysticerci). In rodents, cysticerci are usually found in the pleural and peritoneal cavities. The distribution of T. martis ranges from Europe (T. m. martis subspecies) to North America and Russia (T. m. americana) (6). In Europe, T. martis has been observed in martens and rodents in Italy, Germany, The Netherlands, Belgium, Spain, Poland, Belarus, and Switzerland (7–9). Recently, fatal T. martis cysticercosis was identified in a Tonkean macaque (Macaca tonkeana) in eastern France near Strasbourg (Alsace region) (10) and in a ring-tailed lemur (Lemur catta) in a zoological garden in Rome, Italy (11). These recent cases are evidence of potential lethal outcomes in monkeys and offer particular epidemiological value as indicators of existing environmental contamination with T. martis eggs. So far, the only human case that has been described involved the eye of an immunocompetent woman in Germany. The patient exhibited a subretinal tumor-like structure resembling a cestode larva, which was treated with albendazole and dexamethasone, and the cyst was removed. The patient lived in southwestern Germany on the east side of the Rhine River, just across from the Alsace area where our case occurred, and the consumption of contaminated homegrown vegetables was suspected as causative risk factor (5). In our patient, the T. martis cysticerci developed in the brain, and once treated, her clinical evolution turned out favorable. Both cases displayed one unique lesion, potentially due to the nonmultiplying nature of T. martis larvae, thus limiting the risk of generalization and recurrence (6). Transmission to intermediate hosts occurs via the oral route by way of food or water contaminated by fecal matter. In our case, the potential risk factors could have been the consumption of wild berries or garden-grown vegetables contaminated by infected marten or fox feces, as the patient lives near the forest and the intrusion of wild animals into the garden is possible. The involvement of her pet animals could not be excluded either, even though the corresponding fecal examinations were negative at the time of sample collection. It is highly probable that a natural T. martis life cycle existed in the environment of the house, given that small rodents (voles, mice, rats, and squirrels) and carnivores (martens and foxes) were regularly observed in close vicinity. In France, the prevalence of T. martis in wildlife remains largely unknown, with only a few reports available (12, 13). However, the recent observation of a peritoneal T. martis cysticercosis in a Tonkean macaque in the Alsace region indicates that local contaminations of this particular environment with eggs of this tapeworm species may occur.
In summary, we hereby describe the first case of a human cerebral infection with T. martis. Treatment comprising praziquantel and albendazole as well as surgical exeresis led to a complete cure. Our study further documents the susceptibility of humans to larval T. martis infections. Even though the number of diagnosed cases is low, we cannot rule out the possibility that cases may have been missed in the past due to the absence of molecular analysis. We therefore believe that cysticercosis of both T. martis and T. crassiceps should be added to the differential diagnosis of unusual tumor-like CNS lesions.
REFERENCES
- 1.Georges S, Villard O, Filisetti D, Mathis A, Marcellin L, Hansmann Y, Candolfi E. 2004. Usefulness of PCR analysis for diagnosis of alveolar echinococcosis with unusual localizations: two case studies. J Clin Microbiol 42:5954–5956. doi: 10.1128/JCM.42.12.5954-5956.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowles J, Blair D, McManus DP. 1992. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasitol 54:165–173. doi: 10.1016/0166-6851(92)90109-W. [DOI] [PubMed] [Google Scholar]
- 3.Bowles J, McManus DP. 1993. NADH dehydrogenase 1 gene sequences compared for species and strains of the genus Echinococcus. Int J Parasitol 23:969–972. doi: 10.1016/0020-7519(93)90065-7. [DOI] [PubMed] [Google Scholar]
- 4.Trachsel D, Deplazes P, Mathis A. 2007. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology 134:911–920. doi: 10.1017/S0031182007002235. [DOI] [PubMed] [Google Scholar]
- 5.Eberwein P, Haeupler A, Kuepper F, Wagner D, Kern WV, Muntau B, Racz P, Agostini H, Poppert S. 2013. Human infection with marten tapeworm. Emerg Infect Dis 19:1152–1154. doi: 10.3201/eid1907.121114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loos-Frank B. 2000. An up-date of Verster's (1969) 'Taxonomic revision of the genus Taenia Linnaeus' (Cestoda) in table format. Syst Parasitol 45:155–183. doi: 10.1023/A:1006219625792. [DOI] [PubMed] [Google Scholar]
- 7.Ribas A, Torre I, Feliu C, Arrizabalaga A, Casanova JC. 2009. Helminth communities of the bank vole Myodes glareolus (Rodentia, Arvicolinae) in two populations: Montsenyu Natural Park (north-eastern-Spain) and Pi Natural Reserve (French Pyrenees). Rev Ibero-Latinoam Parasitol Int 1:73–81. [Google Scholar]
- 8.Mathy A, Hanosset R, Adant S, Losson B. 2009. The carriage of larval Echinococcus multilocularis and other cestodes by the musk rat (Ondatra zibethicus) along the Ourthe River and its tributaries (Belgium). J Wildl Dis 45:279–287. doi: 10.7589/0090-3558-45.2.279. [DOI] [PubMed] [Google Scholar]
- 9.Reperant LA, Hegglin D, Tanner I, Fischer C, Deplazes P. 2009. Rodents as shared indicators for zoonotic parasites of carnivores in urban environments. Parasitology 136:329–337. doi: 10.1017/S0031182008005428. [DOI] [PubMed] [Google Scholar]
- 10.Brunet J, Pesson B, Chermette R, Regnard P, Grimm F, Deplazes P, Ferreira X, Sabou M, Pfaff AW, Abou-Bacar A, Candolfi E. 2014. First case of peritoneal cysticercosis in a non-human primate host (Macaca tonkeana) due to Taenia martis. Parasit Vectors 7:422. doi: 10.1186/1756-3305-7-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Liberato C, Berrilli F, Meoli R, Friedrich KG, Di Cerbo P, Cocumelli C, Eleni C. 2014. Fatal infection with Taenia martis metacestodes in a ring-tailed lemur (Lemur catta) living in an Italian zoological garden. Parasitol Int 63:695–697. doi: 10.1016/j.parint.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Joyeux C, Bear JG. 1934. Sur quelques cestodes de France. Arch Mus Natl Hist Nat (Paris) 6:157–171. [Google Scholar]
- 13.Ribas A, Milazzo C, Foronda P, Casanova JC. 2004. New data on helminths of stone marten, Martes foina (Carnivora, Mustelidae), in Italy. Helminthologia 41:59–61. [Google Scholar]
- 14.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]