Abstract
Testing for E6/E7 mRNA in cells infected with high-risk (HR) human papillomavirus (HPV) might improve the specificity of HPV testing for the identification of cervical precancerous lesions. Here we compared the RNA-based Aptima HPV (AHPV) assay (Hologic) and the DNA-based Hybrid Capture 2 (HC2) HPV test (Qiagen) to liquid-based cytology (LBC) for women undergoing routine cervical screening. A total of 10,040 women, 30 to 60 years of age, were invited to participate in the study, 9,451 of whom were included in the analysis. Specimens were tested centrally by LBC, the AHPV test, and the HC2 test, and women who tested positive on any test were referred for colposcopy. Genotyping was performed on all HR-HPV-positive samples. Test characteristics were calculated based on histological review. As a result, we identified 90 women with cervical intraepithelial neoplasia grade 2+ (CIN2+), including 43 women with CIN3+. Sensitivity differences between the AHPV test and the HC2 test in detecting CIN2+ (P = 0.180) or CIN3+ (P = 0.0625) lesions were statistically nonsignificant. Of three CIN3 cases that were missed with the AHPV test, two cases presented lesion-free cones and one had a non-HR HPV67 infection. The specificity (<CIN2) and positive predictive value (CIN2+) of the AHPV test were significantly higher (both P < 0.001) than those of the HC2 test. The overall agreement between the tests was substantial (κ = 0.77). Finally, we present results for several possible triage strategies, based on the primary screening test being either the AHPV test or the HC2 test. In summary, the AHPV assay is both specific and sensitive for the detection of high-grade precancerous lesions and may be used in primary cervical cancer screening for women ≥30 years of age.
INTRODUCTION
The cervical cancer mortality rate in Germany has decreased dramatically since the introduction of gynecological screening for cervical cancer in 1971 (1). Annual opportunistic screening is performed by conventional cytology (Pap smear) and is covered by health insurance for women ≥20 years of age. Despite this extensive effort, 4,600 new cases of cervical cancer (and approximately 1,500 deaths attributable to cervical cancer) (2) and 150,000 cases of cervical cancer precursors (cervical intraepithelial neoplasia grade 3 [CIN3]) are diagnosed each year (3). Persistent infections with high-risk (HR) human papillomaviruses (HPVs) have been shown to be causal for the development of cervical precancerous lesions and cancer. This has led to the development and investigation of various HPV detection methods, and HR HPV testing in addition to cytology is now widely applied in cervical cancer screening programs (4–7). Three DNA-based and one RNA-based assay for HR HPV group detection and two HPV16/18 genotyping assays have been approved by the U.S. Food and Drug Administration (FDA) for cervical cancer screening (http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm330711.htm). These assays include the Digene Hybrid Capture 2 (HC2) high-risk HPV DNA test (Qiagen, Hilden, Germany), the Cervista HPV HR test (Hologic, San Diego, CA), the cobas HPV test (Roche, Pleasanton, CA), and the Aptima HPV (AHPV) assay (Hologic, San Diego, CA), as well as the Aptima HPV 16 18/45 genotype assay (Hologic, San Diego, CA) and the Cervista HPV 16/18 assay (Hologic, San Diego, CA) for HPV16/18 genotyping. The cobas HPV test with concurrent HPV16/18 genotyping was recently approved by the FDA for primary screening (http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm).
The HC2 test for the collective detection of at least 13 carcinogenic HPV types (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) (8) is a nucleic acid hybridization assay with signal amplification using microplate chemiluminescence for semiquantitative detection of HPV DNA in cervical specimens. The AHPV assay detects the HPV E6 and E7 mRNA of the 13 HR HPV types targeted by the HC2 assay as well as the class 2B type HPV66 (9). The AHPV test has been compared previously to the HC2 test (10–18); however, only three studies were conducted in a routine screening population (12, 14, 18) and only two of those studies performed a split-sample comparison with liquid-based cytology (LBC) and the HC2 test (12, 14).
Therefore, the objective of this study was to evaluate the AHPV assay in comparison to LBC and the HC2 test, with respect to clinical sensitivity and specificity for the detection of high-grade CIN, in women 30 to 60 years of age in a German routine screening population, using split cervical samples collected in ThinPrep collection medium. Cervical samples from 9,451 women were analyzed by LBC, the AHPV assay, and the HC2 assay and subsequently were evaluated based on reviewed histological findings.
MATERIALS AND METHODS
Participants.
Women 30 to 60 years of age who were undergoing routine cervical screening at three German centers, in Tübingen, Saarbrücken, and Freiburg, were invited to participate in the study (n = 10,040). Exclusion criteria for this study included hysterectomy or destructive therapy of the cervix, pregnancy, an abnormal cytological result within the past 6 months, HIV infection, and organ transplantation. Written informed consent was obtained from each participant, and the study protocol was approved by all relevant ethics committees (Ethik-Kommission Universitätsklinikum Tübingen, reference no. 475/2008MPG1; Ethik-Kommission Alfred-Ludwigs-Universität Freiburg, reference no. EK Freiburg 63/09; Ethik-Kommission Landesärztekammer Baden-Württemberg, reference no. B-2009-030f; Ethik-Kommission Ärztekammer des Saarlandes, reference no. 02/10).
Study design.
Eligible consenting women (n = 10,040) had single liquid-based cytology samples taken, using a Rovers Cervex-Brush, during the annual routine speculum examination. Samples were placed in ThinPrep transport medium according to the manufacturer's guidelines. Liquid-based cytology (LBC), the Digene Hybrid Capture 2 (HC2) high-risk HPV DNA test (Qiagen, Hilden, Germany), and the Aptima HPV (AHPV) assay (Hologic, San Diego, CA) were performed on all samples. All HR-HPV-positive samples were also genotyped by the INNO-LiPA HPV Genotyping Extra line probe assay (LiPA). All women with a positive result in any of the three screening tests (n = 699) were invited for colposcopy within 8 weeks after receiving their test results, along with a random 5% sample of women with triple negative results (n = 438). Colposcopy was carried out in specialized colposcopy clinics, and histological results were reported according to the CIN nomenclature. Biopsy specimens were taken from areas with a colposcopic impression of CIN. The colposcopist was not blinded to the screening test results.
Liquid-based cytology.
All samples were first analyzed by LBC. LBC results were evaluated according to the Munich nomenclature II and were translated into the Bethesda System (TBS) as described previously (19, 20). LBC results were considered negative when the result was Pap I/II (equivalent to negative for intraepithelial lesion or malignancy [NILM]) or Pap IIw (equivalent to inadequate or atypical cells of undetermined significance [ASCUS]); all other results were considered positive and resulted in referral for colposcopy.
HPV testing and genotyping.
Residual LBC samples were sent to Tübingen for HPV testing. In Tübingen, the samples were aliquoted and processed as follows. One 4-ml aliquot was subjected to the HC2 assay, one 1-ml aliquot was subjected to the AHPV assay, and another 1-ml aliquot was used for HPV genotyping. Sample processing was performed according to the manufacturer's specifications. Remaining samples were stored for quality assurance purposes.
Digene Hybrid Capture 2 high-risk HPV DNA (Qiagen, Hilden, Germany) testing was performed as described previously (20), using the Rapid Capture System 1 (RCS-1) according to the instructions. A cutoff value of relative light units/cutoff (RLU/CO) ratio of 1, equivalent to 1 pg of HPV DNA per 1 ml of sampling buffer, for positive test results was used in this study. PreservCyt specimens were retested when RLU/CO ratios between ≥1.0 and <2.5 were obtained. If the initial retest result was positive (RLU/CO ratio of ≥1.0), then the specimen was reported as positive. If the retest was negative (RLU/CO ratio of <1.0), then a second repeat test (third result) was performed to generate a final result.
The Aptima HPV assay (Hologic, San Diego, CA) was performed following the manufacturer's instructions. The recommended cutoff value of a signal/cutoff (S/CO) ratio of 1.0 at the time of study initiation was used in this study.
HPV genotyping of ThinPrep samples as well as paraffin-embedded cone biopsy specimens was carried out using the INNO-LiPA HPV Genotyping Extra test, as described previously (21, 22). One cone biopsy specimen that returned a LiPA result of HPV X was genotyped by nested PCR and subsequent sequence analysis. For the nested PCR, MY09/MY11 primers were used as the outer pair and GP5+/GP6+ primers were used as the inner pair, amplifying the L1 conserved region, as described previously (23).
Cytological and histological reviews.
All LBC-positive samples and samples with abnormal histological findings were collected by the respective clinical departments, and a blinded review was performed by independent external experts, for quality control. In cases of discrepant review readings, a second review was performed. The results were considered final when two of three diagnoses were identical.
Statistical analyses.
Women with adequate results from all three screening tests were included in the analysis (n = 9,451). Analyses are based on the original screening results and on the reviewed histological results.
As 93 women with abnormal screening results did not undergo colposcopy and 27 had inadequate colposcopies (17% of all women referred), the verification bias stemming from incomplete colposcopies needed to be adjusted by estimating what colposcopy results would have been observed if all women had participated. Conditional on the LBC results (cytology negative [NILM or ASCUS; Pap I/II or Pap IIw], low-grade positive [low-grade squamous intraepithelial lesion [LSIL]; Pap III or Pap IIID], or high-grade positive [high-grade squamous intraepithelial lesion [HSIL]; Pap IVa or Pap IVb]) and HPV detection (negative by the AHPV and HC2 tests, positive by the AHPV test or the HC2 test, positive by the AHPV and HC2 tests, or positive for HPV16 or HPV16/18), we used inverse probability of colposcopy weights in order to estimate what would have been observed if all women with at least one positive test result had an adequate colposcopy as well as adequate histological findings in the case of abnormal colposcopy results.
Because only 3.6% of the women with triple negative results had a colposcopy performed, we did not use inverse probability of colposcopy weights for this group, as this would have resulted in unstable estimates of sensitivity with wide confidence intervals (CIs). Instead, we assumed that LBC and HPV testing were conditionally independent for women with disease (CIN2+ or CIN3+, depending on the analysis), in order to estimate the number of pathological cases of CIN2 and CIN3+ among women with triple negative results without an adequate colposcopic examination (24). Hence, the assumption was that the sensitivity of LBC to detect disease identified by HPV testing would be the same as the sensitivity of LBC to detect disease missed by HPV testing. With missing histological results imputed, we were able to estimate the sensitivity, specificity, negative predictive value, and positive predictive value (PPV) of all three tests. When the sensitivity and/or the specificity of a test was 100%, the confidence intervals were based on the exact binomial for the observed number; otherwise, the confidence intervals were calculated using a normal approximation to the binomial distribution. We tested whether there were significant differences in the sensitivity and specificity of the HC2 test versus the AHPV test by using the discordant pairs and assuming a binomial distribution, and the confidence interval for the difference in specificity was calculated using the Wald test with the Bonett-Price Laplace adjustment (25). Kappa statistics were used to assess agreement between HPV tests, and these statistics were not adjusted for verification bias.
RESULTS
Screening results.
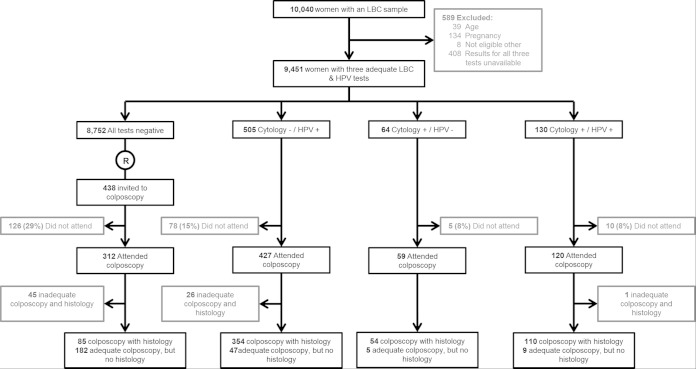
A screening sample was obtained from 10,040 women, of whom 9,451 were included in the analysis. The 589 excluded subjects included 181 who were ineligible and 408 for whom at least one of the three screening test results was not available, mostly due to insufficient residual material (Fig. 1). Of the 9,451 eligible women, 130 (1.4%) tested positive on at least one HPV test and had abnormal cytological findings, 64 (0.7%) had abnormal cytological findings but tested negative on both HPV tests, 505 tested positive on at least one of the two HPV tests but had normal cytological findings, and 8,752 (92.6%) had negative results for all three screening tests. A total of 699 samples tested positive in at least one test, and the numbers testing positive for each screening test were 194 (2.15%) for LBC, 464 (4.9%) for the AHPV test, and 580 (6.1%) for the HC2 test (Table 1). All 635 HPV-positive samples were genotyped using the INNO-LiPA HPV Genotyping Extra test, which indicated that 400 women (63%) presented with single HPV infections and 167 (26.3%) with multiple infections; 63 samples (9.9%) were LiPA negative and five samples failed. Further details about the genotype distribution are summarized in Table 2.
FIG 1.
Study flow chart. R, random selection of a 5% sample of women with triple negative results as a reference group.
TABLE 1.
Cytological results according to HPV test results for women included in the analysis
| HC2 test result | No. |
||||
|---|---|---|---|---|---|
| LBC positive |
LBC negative |
Total | |||
| AHPV test positive | AHPV test negative | AHPV test positive | AHPV test negative | ||
| Positive | 110 | 19 | 299 | 152 | 580 |
| Negative | 1 | 64 | 54 | 8,752 | 8,871 |
| Total | 111 | 83 | 353 | 8,904 | 9,451 |
TABLE 2.
Summary of genotype distributions, as determined by INNO-LiPA Genotyping Extra test
| HPV genotype | No. |
||
|---|---|---|---|
| Single infection | Multiple infection | Total | |
| 16 | 72 | 55 | 127 |
| 31 | 64 | 32 | 96 |
| 52 | 33 | 29 | 62 |
| 53 | 30 | 29 | 59 |
| 51 | 21 | 25 | 46 |
| 66 | 21 | 25 | 46 |
| 18 | 20 | 15 | 35 |
| 56 | 16 | 17 | 33 |
| 68 | 15 | 16 | 31 |
| 70 | 13 | 17 | 30 |
| 39 | 14 | 15 | 29 |
| 33 | 20 | 7 | 27 |
| 74 | 3 | 23 | 26 |
| 45 | 9 | 12 | 21 |
| 58 | 7 | 13 | 20 |
| 54 | 3 | 12 | 15 |
| 35 | 8 | 5 | 13 |
| 6 | 1 | 11 | 12 |
| 69 | 3 | 9 | 12 |
| 71 | 3 | 9 | 12 |
| 44 | 1 | 10 | 11 |
| 59 | 4 | 2 | 6 |
| 82 | 2 | 4 | 6 |
| 73 | 1 | 2 | 3 |
| Xa | 16 | 16 | |
| Total | 400 | 399 | 799 |
| Sample failed | 5 | ||
| HPV negative | 63 | ||
| Total | 867 | ||
X, HPV DNA was detected by INNO-LiPA genotyping but could not be correlated with a specific type.
Of the 699 women with abnormal screening results, 606 (86%) underwent colposcopy and 579 (83%) either had adequate negative colposcopy findings or an adequate biopsy specimen (Fig. 1). Among the 438 women with negative screening results who were invited for colposcopy, 312 (71%) attended and 267 (61%) either had adequate negative colposcopy findings or an adequate biopsy specimen.
A total of 90 women with CIN2 or worse (1% of the cohort) were identified, of whom 47 had confirmed cases of CIN2 and 43 had CIN3 or adenocarcinoma in situ (AIS); no cases of cancer were detected (Table 3). The HC2 test detected four more high-grade precancerous lesions than did the AHPV test, including three CIN3 cases, of which two presented with a lesion-free cone and one had an infection with HPV67 (which is not targeted by either the HC2 test or the AHPV test), as determined by PCR and subsequent sequencing analysis using the original ThinPrep sample and material from the paraffin-embedded cone biopsy specimen.
TABLE 3.
Detailed data on screening results, comparing LBC results and histological results according to high-risk-HPV test results
| Histological findings | Observed resultsa |
Verification bias-adjusted results |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal (Pap I or II) | ASCUS or AGC (Pap IIw) | ASC-H or AGUS (Pap III) | LSIL or HSIL (Pap IIID) | HSIL, CIS, or micro (Pap IVa or IVb) | Total | Normal (Pap I, II, or IIw) | Low grade (Pap III or IIID) | High grade, CIS, or micro (Pap IVa or IVb) | Total | |
| Histological data not available (no.) | ||||||||||
| Total | 8,582 | 236 | 6 | 21 | 3 | 8,848 | ||||
| AHPV test positive | 80 | 10 | 0 | 15 | 3 | 108 | ||||
| HC2 test positive | 119 | 10 | 0 | 17 | 3 | 149 | ||||
| <CIN2 (no.) | ||||||||||
| Total | 343 | 44 | 23 | 94 | 9 | 513 | 9,189.4 | 140.2 | 9.7 | 9,339.3 |
| AHPV test positive | 192 | 24 | 7 | 45 | 6 | 274 | 294.8 | 64.4 | 6.7 | 365.9 |
| HC2 test positive | 245 | 28 | 7 | 59 | 6 | 345 | 389.9 | 79.4 | 6.7 | 476.0 |
| CIN2 (no.) | ||||||||||
| Total | 26 | 5 | 2 | 8 | 6 | 47 | 41.5 | 11.6 | 6.6 | 59.7 |
| AHPV test positive | 23 | 5 | 2 | 6 | 6 | 42 | 34.8 | 9.4 | 6.6 | 50.8 |
| HC2 test positive | 23 | 5 | 2 | 7 | 6 | 43 | 35.0 | 10.5 | 6.6 | 52.1 |
| CIN3+ (no.) | ||||||||||
| Total | 20 | 1 | 2 | 5 | 15 | 43 | 26.1 | 9.1 | 16.7 | 52.0 |
| AHPV test positive | 18 | 1 | 1 | 5 | 15 | 40 | 23.4 | 7.1 | 16.7 | 47.2 |
| HC2 test positive | 20 | 1 | 2 | 5 | 15 | 43 | 26.1 | 9.1 | 16.7 | 52.0 |
| Total | ||||||||||
| Total no. | 8,971 | 286 | 33 | 128 | 33 | 9,451 | 9,257 | 161 | 33 | 9,451.0 |
| % AHPV test positive | 3.5 | 13.9 | 30.3 | 55.5 | 90.9 | 4.9 | 3.8 | 5.0 | 90.9 | 4.9 |
| % HC2 test positive | 4.5 | 15.4 | 33.3 | 68.8 | 90.9 | 6.1 | 4.9 | 6.1 | 90.9 | 6.1 |
AGC, atypical glandular cells; AGUS, atypical glandular cells of undetermined significance; CIS, carcinoma in situ; micro, microinvasive carcinoma.
Of the 9,257 women with negative cytological findings, 286 (3%) had Pap IIw results (inadequate or ASCUS). The proportion of women with Pap IIw results and a positive HPV result who were found to have CIN2+ was 2.1% (6/286 women), compared to 0.5% of women (46/8,971 women) with Pap I or II (NILM) findings. By comparison, 10.6% of women (17/161 women) with Pap III (atypical squamous cells with possible HSIL [ASC-H]) or IIID (LSIL to HSIL) findings and 63.6% of women (21/33 women) with Pap IVa or IVb (HSIL) findings had CIN2+ identified by histological examination (Table 3). The corresponding unadjusted positive predictive values for the AHPV and HC2 tests were 17.7% (82/464 cases) and 14.8% (86/580 cases), respectively. Among those testing negative with the AHPV and HC2 tests, the percentages found to have CIN2+ were 0.09% (8/8,987 women) and 0.05% (4/8,871 women), respectively. The extremely low rates of CIN2+ detected in women with negative AHPV and HC2 results may be partly due to the lack of colposcopy among those women. The results adjusted for verification bias are presented in Table 3. We estimate that, in addition to the 90 confirmed cases of CIN2+, there were 21.7 undetected cases of CIN2+ in the study cohort (12.7 extra CIN2 and 9.0 CIN3+ cases).
Cytological and histological reviews were performed by independent experts for quality assurance. In summary, 2% of the cytology-negative samples were upgraded, while 6% of the cytology-positive samples were negative at rescreening. Histological review of negative samples resulted in the upgrading of 1.6% of initially histology-negative results, and 15% of histology-positive samples were normal upon review.
Comparison of test characteristics.
After adjusting for verification bias, we estimated the sensitivity, specificity, positive predictive value, and negative predictive value of each screening test (Table 4). The sensitivities of the tests were slightly lower after adjustment. For the detection of CIN2+, the sensitivity values were 39.5% for LBC, 87.8% for the AHPV test, and 93.2% for the HC2 test. For the detection of CIN3+, the sensitivity values were 49.8% for LBC, 90.9% for the AHPV test, and 100.0% for the HC2 test. The corresponding specificities were 98.4% for LBC, 96.1% for the AHPV test, and 94.9% for the HC2 test. Consequently, the positive predictive value (PPV) for the AHPV test (21.1%) was somewhat better than that for the HC2 test (17.9%). Indeed, it was estimated that only 10.0 of 171 women who tested positive in the HC2 test but negative in the AHPV test had CIN2+, yielding a PPV for this combination of 5.8%. Women who tested positive in at least one HPV test had their samples typed for 24 HPV genotypes, including HPV16/18. HPV16 typing had sensitivities of 44.8% for CIN2+ and 59.0% for CIN3+ but had a high PPV of 38.8% (Table 4). The addition of HPV18-positive samples added 5.5 additional CIN2+ cases, of 29 that tested positive for HPV18 but negative for HPV16, giving a PPV of 19%.
TABLE 4.
Sensitivity, specificity, and positive and negative predictive values
| Test | Sensitivity for CIN3+ (% [95% CI]) | Sensitivity for CIN2+ (% [95% CI]) | Specificity for <CIN2 (% [95% CI]) | PPV for CIN2+ (% [95% CI]) | NPV for <CIN2 (% [95% CI]) |
|---|---|---|---|---|---|
| LBC | 49.8 (34.7–64.9) | 39.5 (29.4–49.5) | 98.4 (98.1–98.7) | 22.7 (16.3–29.1) | 99.3 (99.1–99.5) |
| HR HC2 test | 100 (91.8–100) | 93.2 (87.1–99.2) | 94.9 (94.1–95.7) | 17.9 (14.5–21.4) | 99.9 (99.8–100) |
| AHPV test | 90.9 (81.1–100) | 87.8 (80.2–95.5) | 96.1 (95.5–96.7) | 21.1 (17.0–25.2) | 99.8 (99.7–100) |
| HPV 16 test | 59.0 (44.2–73.7) | 44.8 (34.5–55.1) | 99.2 (98.9–99.4) | 38.8 (29.4–48.1) | 99.3 (99.1–99.5) |
| HPV 16/18 test | 63.1 (48.6–77.6) | 49.7 (39.3–60.1) | 98.9 (98.6–99.2) | 35.2 (27.0–43.4) | 99.4 (99.2–99.6) |
The differences in the sensitivities of the HC2 and AHPV tests for CIN2+ and CIN3+ were not statistically significant (P values of 0.180 and 0.0625, respectively); however, the difference in specificity of 96.1% for the AHPV test versus 94.9% for the HC2 test (difference, 1.2% [95% CI, 0.87% to 1.48%]) was significantly greater (P < 0.001). Of special interest is the comparison of the AHPV and HC2 test results for individual samples. Overall, there was 97.6% agreement for the 9,451 samples, with a Cohen's kappa value of 0.77. Although the agreement was 88.9% among the 90 known cases of CIN2+, the kappa value was only 0.11. It is also of interest to note the lack of agreement (only 78.6%) among samples shown to be positive for either HPV16 or HPV18 DNA; 21 samples were positive in the HC2 test but negative in the AHPV test, and 13 were AHPV assay positive but HC2 assay negative.
Triage strategies.
In Table 5, we present the results of a number of possible triage strategies, based on the primary screening test being either the AHPV test or the HC2 test. Triage using LBC or HPV16/18 typing would yield higher PPVs for immediate colposcopy, but a smaller proportion of cases would be diagnosed immediately. For instance, using HPV16/18 results among AHPV-assay-positive women would yield a PPV for immediate colposcopy of 36.9%, with an immediate sensitivity (for CIN2+) of 45.8%. Triaging AHPV-assay-positive women using LBC and HPV16/18 typing and referring women who tested positive in either triage test would increase the sensitivity of immediate colposcopy to 60.0%, with a PPV of 32.6%. The sensitivity of such a strategy for CIN3+ would be increased to 72.0%.
TABLE 5.
Strategies for triage to colposcopy, adjusted for verification bias, for women who tested HPV positive and sensitivity and PPV for CIN2+ histology
| Testing and referral | No. referred to colposcopy | No. of cases found immediatelya | Referral/case ratio | Immediate sensitivity for CIN2+ (% [95% CI]) | PPV of referral for CIN2+ (% [95% CI]) |
|---|---|---|---|---|---|
| Cytology only | 194 | 44 | 4.4 | 39.5 (29.4–49.5) | 22.7 (16.3–29.1) |
| Triage of HC2-test-positive cases | |||||
| Refer all to colposcopy | 580 | 104.0 | 5.6 | 93.2 (87.1–99.2) | 17.9 (14.5–21.4) |
| Refer if AHPV test positive | 409 | 94.1 | 4.3 | 84.2 (75.9–92.6) | 23.0 (18.5–27.5) |
| Refer if HPV16/18 positive | 146 | 54.4 | 2.6 | 48.7 (38.3–59.1) | 37.3 (28.6–46.0) |
| Refer if cytology positive | 129 | 43.0 | 3.0 | 38.5 (28.4–48.5) | 33.3 (24.5–42.1) |
| Refer if HPV16/18 positive and/or cytology positive | 227 | 71.2 | 3.2 | 63.8 (53.6–74.0) | 31.4 (24.8–38.1) |
| Triage of AHPV-test-positive cases | |||||
| Refer all to colposcopy | 464 | 98.1 | 4.7 | 87.8 (80.2–95.5) | 21.1 (17.0–25.2) |
| Refer if HC2 test positive | 409 | 94.1 | 4.3 | 84.2 (75.9–92.6) | 23.0 (18.5–27.5) |
| Refer if HPV16/18 positive | 139 | 51.2 | 2.7 | 45.8 (35.5–56.2) | 36.9 (28.0–45.7) |
| Refer if cytology positive | 111 | 39.9 | 2.8 | 35.7 (25.9–45.5) | 35.9 (26.3–45.6) |
| Refer if HPV16/18 positive and/or cytology positive | 206 | 67.0 | 3.1 | 60.0 (49.6–70.3) | 32.6 (25.6–39.6) |
Verification bias-adjusted results for an estimated total of 111.7 CIN2+ cases.
DISCUSSION
This study evaluated and compared the performances of the Digene Hybrid Capture 2 (HC2) high-risk HPV DNA test (Qiagen, Hilden, Germany) and the Aptima HPV (AHPV) assay (Hologic, San Diego, CA) to liquid-based cytology (LBC) and histology in a routine screening cohort of 10,040 women in Germany. We found that both HPV tests were highly sensitive in detecting high-grade cervical lesions (CIN2+ and CIN3+).
The HC2 assay detected four more high-grade precancerous lesions than the AHPV assay, including three CIN3 cases. Of the three CIN3+ cases missed by the AHPV assay, two presented with a lesion-free cone and one had an infection with the non-HR-type HPV67. The detection of the two lesion-free cases by the HC2 assay may be explained by the fact that the HC2 test targets HPV DNA, which is more stable than mRNA. Some rare HPV67-related invasive cervical carcinoma (ICC) cases have previously been reported (26, 27), and HPV67 prevalence is higher in ICC than among women with normal cytological findings (28), which is why the IARC has classified HPV67 as possibly carcinogenic (class 2B carcinogens) (9). Despite HPV67 not being a target of the HC2 test, previous reports demonstrated cross-reactivity with HPV67 (29), which explains why this case was detected by the HC2 assay.
The nonsignificantly lower sensitivity of the AHPV test in detecting CIN2+ (P = 0.180) and CIN3+ (P = 0.0625) is in line with earlier studies (11, 12, 17). However, more-recent reports demonstrate equal (10, 14–16) or higher (13, 18) sensitivity of the AHPV test, compared to that of the HC2 test. All of those studies also reported higher specificities for the AHPV test, which is supported by our data showing a significantly (P < 0.001) increased specificity of 96.1% for the AHPV test, compared to 94.9% for the HC2 test (difference, 1.2% [95% CI, 0.87% to 1.48%]). This might be due to the fact that, by measuring mRNA levels, the AHPV assay detects actively infected cells, whereas DNA-based assays such as the HC2 assay do not distinguish between intracellular viral DNA and extracellular viral DNA, which may represent contamination (e.g., with virus particles), as shown previously in transmission studies in young couples (30).
The Cohen's kappa value confirmed substantial agreement between the overall test performances. The small value (κ = 0.11) for high-grade lesions is due to the different CIN2+ detection rates of the two assays.
Despite our finding that the AHPV assay has a lower positivity rate than the HC2 assay, the generally high positivity rates of both assays as standalone tests suggest that perhaps HPV-positive women should not all be referred for immediate colposcopy. It is noteworthy that we used the manufacturer-recommended cutoff value for the AHPV assay, which was valid at the beginning of this study (S/CO ratio of 1.0). When we applied the new FDA-approved cutoff value of a S/CO ratio of 0.5 to our data set, 46 additional HR-HPV-positive cases were detected, of which only four had histological results available (all CIN1 or less). The cutoff S/CO ratio of 0.5 did not have any effect on the sensitivity and specificity results of the AHPV assay.
Current practice in Germany involves an annual cytological screening. However, the poor sensitivity of cytology demands the development of more-accurate screening schemes. Strategies for the improvement of early diagnosis of CIN2+ cases have been assessed for the AHPV assay based on the primary screening test being cytology (10–18). Adjunctive testing to cytology, however, leads to reduced combined sensitivity. The AHPV test has also been suggested as the primary cervical cancer screening test in a few studies (10, 13, 14). HPV DNA screening, with certain requirements, has already been identified as an alternative option for primary cervical cancer screening (31–33). A triage scenario in which AHPV-assay-positive women underwent HPV16 or HPV18 testing and/or cytology showed immediate sensitivity for CIN2+ similar to that of triage of HC2-assay-positive women by the same methods but led to 9.3% fewer referrals for colposcopy (Table 5). Our results also imply that only slightly more women would be referred (206 women with the AHPV assay versus 194 with cytology only) but a larger number (>50%) of additional CIN2+ cases would be identified (71.2 cases for the AHPV assay versus 44 for cytology only), compared to the use of cytology as a primary standalone test. Current practice (screening with cytology) would have referred 194 cytology-positive women for colposcopy, immediately detecting 44 CIN2+ cases (39.4%) in our study cohort. With the use of cytology as triage for either the AHPV test or the HC2 test, 111 and 129 women, respectively, would have been referred, with 39.2 and 43 cases being detected immediately. This means that the number of referrals may be drastically reduced, by more than 30%, while the number of detected cases remains unchanged. A similar result was obtained with HPV16/18 screening as the triage test, but the referral/case ratios of 2.6 for the HC2 assay and 2.7 for the AHPV assay are more in favor of HPV16/18 genotyping. This result highlights the importance of HPV16/18 genotyping, as the estimated relative risk for prevalent high-grade disease is significantly higher for those who test HPV16/18 positive, compared to HR HPV positive (34–36).
It should generally be noted that, without increasing the number of referrals (from 194 cases for screening with cytology only), only a maximum of 48.7% of the total CIN2+ patients would have been identified in any tested triage scenario. Therefore, it seems plausible to refer double-positive patients for colposcopy, while the follow-up strategy for HPV-positive women who were negative in triage would be based on shorter repeat screening intervals, which means that cases that were missed in the first screening round would likely be identified in follow-up testing.
In summary, the AHPV assay was found to be more specific and to have a higher PPV than the HC2 assay. Although the AHPV assay appeared to be less sensitive, we estimate that only 5.8% of those testing positive by the HC2 assay but negative by the AHPV assay had CIN2 or worse. In fact, triaging AHPV-assay-positive women using LBC and/or HPV16/18 typing and referring women positive by either triage test would increase the sensitivity of immediate colposcopy to 60.0%, with a PPV of 32.6%, and very similar results would be obtained by triaging HC2-test-positive women in the same way. In conclusion, the AHPV test is both sensitive and specific for the detection of high-grade intraepithelial neoplasia of the cervix and, with an appropriate triage strategy, can be considered a primary cervical cancer screening option for women ≥30 years of age.
ACKNOWLEDGMENTS
This study was supported by an unconditional grant from Hologic Inc. to the University Hospital Tübingen. Hologic had no influence on the study protocol and no access to the primary data and was not involved in the analysis and writing of the manuscript.
We thank H. Ikenberg for performing cytological reviews. Furthermore, we thank all collaborating gynecologists, as well as all women who participated in this study.
REFERENCES
- 1.Schenck U, von Karsa L. 2000. Cervical cancer screening in Germany. Eur J Cancer 36:2221–2226. doi: 10.1016/S0959-8049(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 2.Robert Koch-Institut. 2013. Krebs in Deutschland 2009/2010. Robert Koch-Institut, Berlin, Germany. [Google Scholar]
- 3.Siebert U, Sroczynski G, Hillemanns P, Engel J, Stabenow R, Stegmaier C, Voigt K, Gibis B, Holzel D, Goldie SJ. 2006. The German cervical cancer screening model: development and validation of a decision-analytic model for cervical cancer screening in Germany. Eur J Public Health 16:185–192. doi: 10.1093/eurpub/cki163. [DOI] [PubMed] [Google Scholar]
- 4.Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, Koliopoulos G, Naucler P, Sankaranarayanan R, Peto J. 2012. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 30(Suppl 5):F88–F99. doi: 10.1016/j.vaccine.2012.06.095. [DOI] [PubMed] [Google Scholar]
- 5.Dillner J. 2013. Primary human papillomavirus testing in organized cervical screening. Curr Opin Obstet Gynecol 25:11–16. doi: 10.1097/GCO.0b013e32835c5d10. [DOI] [PubMed] [Google Scholar]
- 6.Castle PE, de Sanjose S, Qiao YL, Belinson JL, Lazcano-Ponce E, Kinney W. 2012. Introduction of human papillomavirus DNA screening in the world: 15 years of experience. Vaccine 30(Suppl 5):F117–F122. doi: 10.1016/j.vaccine.2012.05.071. [DOI] [PubMed] [Google Scholar]
- 7.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC, Wentzensen N, Downs LS Jr, Spitzer M, Moscicki AB, Franco EL, Stoler MH, Schiffman M, Castle PE, Myers ER. 2012. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 137:516–542. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 8.Iftner T, Villa LL. 2003. Chapter 12: human papillomavirus technologies. J Natl Cancer Inst Monogr 2003:80–88. doi: 10.1093/oxfordjournals.jncimonographs.a003487. [DOI] [PubMed] [Google Scholar]
- 9.Arbyn M, Tommasino M, Depuydt C, Dillner J. 2014. Are 20 human papillomavirus types causing cervical cancer? J Pathol 234:431–435. doi: 10.1002/path.4424. [DOI] [PubMed] [Google Scholar]
- 10.Clad A, Reuschenbach M, Weinschenk J, Grote R, Rahmsdorf J, Freudenberg N. 2011. Performance of the Aptima high-risk human papillomavirus mRNA assay in a referral population in comparison with Hybrid Capture 2 and cytology. J Clin Microbiol 49:1071–1076. doi: 10.1128/JCM.01674-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. 2009. Clinical performance of the APTIMA HPV assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol 45(Suppl 1):S55–S61. doi: 10.1016/S1386-6532(09)70009-5. [DOI] [PubMed] [Google Scholar]
- 12.Monsonego J, Hudgens MG, Zerat L, Zerat JC, Syrjanen K, Halfon P, Ruiz F, Smith JS. 2011. Evaluation of oncogenic human papillomavirus RNA and DNA tests with liquid-based cytology in primary cervical cancer screening: the FASE study. Int J Cancer 129:691–701. doi: 10.1002/ijc.25726. [DOI] [PubMed] [Google Scholar]
- 13.Monsonego J, Hudgens MG, Zerat L, Zerat JC, Syrjanen K, Smith JS. 2012. Risk assessment and clinical impact of liquid-based cytology, oncogenic human papillomavirus (HPV) DNA and mRNA testing in primary cervical cancer screening (the FASE study). Gynecol Oncol 125:175–180. doi: 10.1016/j.ygyno.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Nieves L, Enerson CL, Belinson S, Brainard J, Chiesa-Vottero A, Nagore N, Booth C, Perez AG, Chavez-Aviles MN, Belinson J. 2013. Primary cervical cancer screening and triage using an mRNA human papillomavirus assay and visual inspection. Int J Gynecol Cancer 23:513–518. doi: 10.1097/IGC.0b013e318280f3bc. [DOI] [PubMed] [Google Scholar]
- 15.Ratnam S, Coutlee F, Fontaine D, Bentley J, Escott N, Ghatage P, Gadag V, Holloway G, Bartellas E, Kum N, Giede C, Lear A. 2011. Aptima HPV E6/E7 mRNA test is as sensitive as Hybrid Capture 2 assay but more specific at detecting cervical precancer and cancer. J Clin Microbiol 49:557–564. doi: 10.1128/JCM.02147-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reuschenbach M, Clad A, von Knebel Doeberitz C, Wentzensen N, Rahmsdorf J, Schaffrath F, Griesser H, Freudenberg N, von Knebel Doeberitz M. 2010. Performance of p16INK4a-cytology, HPV mRNA, and HPV DNA testing to identify high grade cervical dysplasia in women with abnormal screening results. Gynecol Oncol 119:98–105. doi: 10.1016/j.ygyno.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Szarewski A, Ambroisine L, Cadman L, Austin J, Ho L, Terry G, Liddle S, Dina R, McCarthy J, Buckley H, Bergeron C, Soutter P, Lyons D, Cuzick J. 2008. Comparison of predictors for high-grade cervical intraepithelial neoplasia in women with abnormal smears. Cancer Epidemiol Biomarkers Prev 17:3033–3042. doi: 10.1158/1055-9965.EPI-08-0508. [DOI] [PubMed] [Google Scholar]
- 18.Wu R, Belinson SE, Du H, Na W, Qu X, Wu R, Liu Y, Wang C, Zhou Y, Zhang L, Belinson JL. 2010. Human papillomavirus messenger RNA assay for cervical cancer screening: the Shenzhen Cervical Cancer Screening Trial I. Int J Gynecol Cancer 20:1411–1414. doi: 10.1111/IGC.0b013e3181f29547. [DOI] [PubMed] [Google Scholar]
- 19.Petry KU, Menton S, Menton M, van Loenen-Frosch F, Gomes HD, Holz B, Schopp B, Garbrecht-Buettner S, Davies P, Boehmer G, van den Akker E, Iftner T. 2003. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 88:1570–1577. doi: 10.1038/sj.bjc.6600918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boehmer G, Wang L, Iftner A, Holz B, Haedicke J, von Wasielewski R, Martus P, Iftner T. 2014. A population-based observational study comparing Cervista and Hybrid Capture 2 methods: improved relative specificity of the Cervista assay by increasing its cut-off. BMC Infect Dis 14:674. doi: 10.1186/s12879-014-0674-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petry KU, Luyten A, Justus A, Iftner A, Strehlke S, Schulze-Rath R, Iftner T. 2012. Prevalence of low-risk HPV types and genital warts in women born 1988/89 or 1983/84: results of WOLVES, a population-based epidemiological study in Wolfsburg, Germany. BMC Infect Dis 12:367. doi: 10.1186/1471-2334-12-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimm M, Iftner T, Altaki H, Iftner A, Peters JP, Munz A, Reinert S. 2014. Detection of mutation-specific epidermal growth factor receptor (E746–A750del) and lack of detection of human papillomavirus in oral squamous cell carcinoma. Int J Oral Maxillofac Surg 43:1199–1205. doi: 10.1016/j.ijom.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Fuessel Haws AL, He Q, Rady PL, Zhang L, Grady J, Hughes TK, Stisser K, Konig R, Tyring SK. 2004. Nested PCR with the PGMY09/11 and GP5+/6+ primer sets improves detection of HPV DNA in cervical samples. J Virol Methods 122:87–93. doi: 10.1016/j.jviromet.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Sasieni P. 2001. Estimating prevalence when the true disease status is incompletely ascertained. Stat Med 20:935–949. doi: 10.1002/sim.907. [DOI] [PubMed] [Google Scholar]
- 25.Bonett DG, Price RM. 2012. Adjusted Wald confidence interval for a difference of binomial proportions based on paired data. J Educ Behav Stat 37:479–488. doi: 10.3102/1076998611411915. [DOI] [Google Scholar]
- 26.Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, Markowitz LE. 2007. Prevalence of HPV infection among females in the United States. JAMA 297:813–819. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 27.Halec G, Alemany L, Lloveras B, Schmitt M, Alejo M, Bosch FX, Tous S, Klaustermeier JE, Guimera N, Grabe N, Lahrmann B, Gissmann L, Quint W, Bosch FX, de Sanjose S, Pawlita M. 2014. Pathogenic role of the eight probably/possibly carcinogenic HPV types 26, 53, 66, 67, 68, 70, 73 and 82 in cervical cancer. J Pathol 234:441–451. doi: 10.1002/path.4405. [DOI] [PubMed] [Google Scholar]
- 28.Bzhalava D, Guan P, Franceschi S, Dillner J, Clifford G. 2013. A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology 445:224–231. doi: 10.1016/j.virol.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Castle PE, Solomon D, Wheeler CM, Gravitt PE, Wacholder S, Schiffman M. 2008. Human papillomavirus genotype specificity of Hybrid Capture 2. J Clin Microbiol 46:2595–2604. doi: 10.1128/JCM.00824-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Widdice L, Ma Y, Jonte J, Farhat S, Breland D, Shiboski S, Moscicki AB. 2013. Concordance and transmission of human papillomavirus within heterosexual couples observed over short intervals. J Infect Dis 207:1286–1294. doi: 10.1093/infdis/jit018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, Radberg T, Strander B, Johansson B, Forslund O, Hansson BG, Rylander E, Dillner J. 2007. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 357:1589–1597. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 32.Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, Voorhorst FJ, Verheijen RH, van Groningen K, Boon ME, Ruitinga W, van Ballegooijen M, Snijders PJ, Meijer CJ. 2007. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 370:1764–1772. doi: 10.1016/S0140-6736(07)61450-0. [DOI] [PubMed] [Google Scholar]
- 33.Meijer CJ, Berkhof J, Castle PE, Hesselink AT, Franco EL, Ronco G, Arbyn M, Bosch FX, Cuzick J, Dillner J, Heideman DA, Snijders PJ. 2009. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer 124:516–520. doi: 10.1002/ijc.24010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjaer SK, Frederiksen K, Munk C, Iftner T. 2010. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 102:1478–1488. doi: 10.1093/jnci/djq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright TC Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL. 2011. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 136:578–586. doi: 10.1309/AJCPTUS5EXAS6DKZ. [DOI] [PubMed] [Google Scholar]
- 36.Khan MJ, Castle PE, Lorincz AT, Wacholder S, Sherman M, Scott DR, Rush BB, Glass AG, Schiffman M. 2005. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 97:1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]