Abstract
In order to maximize the benefit of prompt antimicrobial therapy and avoid the risk associated with inappropriate use of antimicrobial agents, patients with suspected sepsis must be rapidly differentiated from patients with systemic inflammatory response syndrome (SIRS). In combination with standard microbiological testing, a number of biomarkers have been recently evaluated for this purpose, and the performance characteristics of the most promising of these are reviewed.
INTRODUCTION
The title of this minireview is somewhat misleading, because sepsis is not a laboratory diagnosis and cannot be defined (at least not yet) using any number of discrete diagnostic assays in the absence of clinical criteria. A positive blood culture, for example, regardless of the organism's identity, is useful supplemental information but does not by itself indicate a septic state. In fact, the recovery of a potential pathogen from any site (1), including blood (2), occurs in less than 50% of all cases of sepsis, and contamination of blood cultures can cloud the interpretation. From the opposite direction, it is difficult to distinguish sepsis from systemic inflammatory response syndrome (SIRS) using clinical criteria alone (3).
A few definitions might help clarify the ambiguity here (4, 5). Some of these definitions have been refined over the years, but the general gist remains the same. “SIRS” was first defined in 1991 (4) and refers to “the systemic inflammatory response to a variety of severe clinical insults” (infectious or otherwise). It usually constitutes two or more of the following criteria: (i) temperature of >38°C or <36°C; (ii) heart rate of >90 beats/min; (iii) respiratory rate of >20 breaths/min or partial CO2 pressure (pCO2) of <32 mm Hg; (iv) white blood cell (WBC) count of >12,000 (12K)/μl or <4K/μl; or (v) >10% immature forms (i.e., bands). “Sepsis” has been defined as “the presence (probable or documented) of infection together with systemic manifestations of infection” (5). Having positive cultures supporting an infectious process adds credibility to the diagnosis but is not mandatory. Therefore, a patient can be classified as septic with ≥2 SIRS criteria and a clinical suspicion of infection without microbiological documentation. Bear this in mind when reading the remainder of this minireview. Most septic patients have SIRS, but not all SIRS patients are septic (Fig. 1). Severe sepsis adds an element of organ dysfunction or tissue hypoperfusion that is new and not explained by other causes of organ dysfunction (4). Finally, “septic shock” is “sepsis-induced hypotension persisting despite adequate fluid resuscitation” (4). Definitions specific for adult and pediatric populations are available (4–6).
FIG 1.
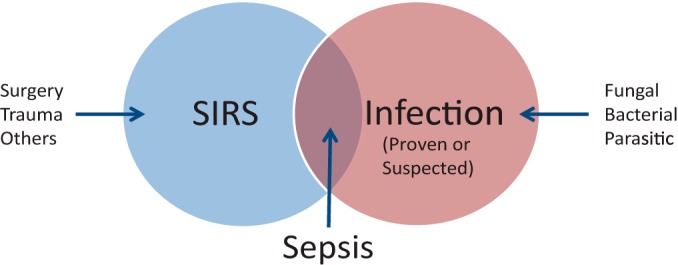
Venn diagram illustrating that sepsis lies at the intersection of infection and the systemic infection response syndrome (SIRS). Culture-proven infection is not a requirement, but a high clinical suspicion of infection suffices to define sepsis. The source of infection need not be blood/bacteremia but could be respiratory or abdominal or involve other sites. Many other noninfectious clinical conditions can lead to a clinical picture of SIRS, including major trauma or surgery, extensive burns, and pancreatitis, to name a few.
Sepsis is a major cause of morbidity and mortality among critically ill patients, and the management of septic shock profits from administration of early and appropriate antimicrobial therapy (7). Given that sepsis is only a subset of SIRS and that administration of antimicrobial therapy is not beneficial and indeed could generate risks in the absence of sepsis (3), the ultimate goal of biomarkers in this setting is to distinguish sepsis from SIRS. Is this possible with current or future laboratory assays excluding blood culture? The balance of this minireview will be devoted to biomarkers that have been or will be evaluated for this task. A word of caution: as one begins to review the current literature regarding the use of biomarkers to distinguish sepsis from SIRS, it is easy to be overcome by the dizzying collection of studies, variability in study design, numbers, and combinations of individual biomarkers evaluated, and the cutoff values applied toward individual evaluations. With that in mind, I will address the most prominent biomarkers of sepsis. You will notice that C-reactive protein (CRP) is not specifically addressed in this minireview but has been included where comparisons to other more contemporary biomarkers have been made.
PCT
Procalcitonin (PCT) is the glycoprotein precursor of calcitonin and has no apparent physiologic activity (8). It is produced by C cells in the thymus but can be synthesized by leukocytes, liver, kidney, adipocytes, and muscle during periods of elevated inflammation (8–11). It has a serum half-life of 25 to 30 h, and in healthy individuals, serum levels remain <0.1 ng/ml. During periods of severe bacterial, fungal, or parasitic infection, serum levels can reach 100 ng/ml, but only modest increases are seen with viral infection or inflammation of a noninfectious nature. Increased serum PCT levels are also seen in cases of trauma and following major surgery (8, 9,) which decreases the specificity of this marker for the prediction of systemic infection.
The suggestion that PCT might be a useful biomarker of inflammation caused by a systemic infectious process or sepsis was first proposed in the early 1990s (8, 12). The kinetics of the PCT response are ideal for this purpose in that elevated serum concentrations occur within 2 to 4 h of the inflammatory insult, reach peak concentrations within 24 to 48 h, and fall rapidly when the disease process resolves (9, 10, 11). The magnitude and duration of elevated serum PCT levels seem to parallel disease severity and outcome (8, 10, 13). In practice, however, it appears that using PCT to distinguish sepsis from noninfectious SIRS is only modestly better than CRP, CRP plus WBC count, or the infection probability score (IPS), which considers CRP, WBCs, and four other parameters (3, 10, 14). However, the ability to discriminate sepsis from noninfectious SIRS using PCT seems possible for certain patient cohorts, e.g., neurological intensive care unit (ICU) patients (14). Further, PCT was also found to be significantly elevated in SIRS patients from standard medical wards with documented bacteremia versus SIRS patients without documented bacteremia and carried an impressive 92.9% negative predictive value (3). In a comprehensive review, Kibe et al. (10) concluded that PCT was better than CRP in the diagnosis or prognosis of sepsis but lacked the necessary accuracy to be used in the absence of clinical judgment. Moreover, PCT is considered more useful for ruling out than for confirming systemic bacterial infection, and serial low PCT values in the absence of positive cultures was sufficient reason to discontinue antimicrobial therapy (3, 10, 11, 13). Karzai et al. (8) noted that the sensitivity and specificity of PCT to predict a systemic infectious process depended on the cutoff values selected and the disease process considered (e.g., pancreatitis with or without infection, viral versus bacterial meningitis, etc.). If this were so, a single, uniform critical value for PCT might be impossible to establish (11). In an extensive meta-analysis of 13 case-control studies and 17 cohort studies, Ciriello et al. (15) could only show support for using PCT to predict the development of sepsis earlier among trauma patients from a wide ensemble of biomarkers (interleukin 1 [IL-1], IL-8, IL-10, neopterin, tumor necrosis factor alpha [TNF-α], selectins, intracellular adhesion molecule 1 [ICAM-1], and Toll-like receptor 9 [TLR-9], among others). They found no value for CRP in this setting. Persistently high PCT levels or secondary increases in PCT levels were found to be adequate predictors of sepsis and multiple organ failure and correlated well with increased mortality and severity scores, e.g., acute physiology and chronic health evaluation II (APACHE II) score, sequential organ failure assessment (SOFA) score, and simplified acute physiology score (SAPS) (9). In another meta-analysis of six randomized controlled trials, Agarwal and Schwartz (13) concluded that serial PCT measurements in the ICU significantly decreased the duration of, but not the initiation of, antimicrobial therapy. The length of stay in the ICU was reduced in two of six studies but showed no difference in the other four studies. There were no differences in mortality rate, relapse of infection, or mechanical ventilation between the PCT-guided or control patient groups, but as a biomarker of infection, PCT was more sensitive and specific than CRP was. They concluded that serial PCT measurements could substantially reduce antimicrobial use in the ICU without increased morbidity or mortality (9).
de Azevedo et al. (16) took a novel approach and measured the clearance of PCT (PCT-c) during the initial 24 and 48 h of treatment for severe sepsis and septic shock in the ICU. The area under the receiver-operator curve (AUROC) for 24- and 48-h PCT-c was 0.76 for both, and PCT-c was significantly higher in survivors (i.e., greater PCT clearance). Jekarl et al. (17) found that there were significant differences in PCT values between SIRS and sepsis groups, between the sepsis and severe sepsis/septic shock groups, and between patients with SIRS and those with severe sepsis/septic shock. In other words, PCT could not only differentiate sepsis from SIRS, but it could also classify sepsis severity. Of the various biomarkers this group evaluated, PCT showed the highest sensitivity, while IL-6 had the highest specificity for the prediction of sepsis and severe sepsis/septic shock. In this study, either PCT, IL-6, or CRP could differentiate SIRS group from sepsis group and severe sepsis/septic shock groups, which is not surprising, since CRP is upregulated by IL-6 (9).
MARKERS OF ENDOTHELIAL ACTIVATION/DYSFUNCTION
Evidence of endothelial activation or dysfunction generally precedes clinical signs of sepsis (18). Angiopoietins 1 and 2 (Ang-1 and Ang-2) are yin and yang ligands of the Tie-2 receptor expressed by endothelial cells (18). Tie-2 is thought to play a role in vascular integrity and angiogenesis. In a state of good health, Ang-1 levels are greater than Ang-2 levels, so the Ang-1–Tie-2 complex predominates. During inflammation, however, Ang-2 is released from endothelial cells, which shifts the equilibrium to Ang-2–Tie-2. The latter is prothrombotic and causes leaks in the microvasculature (18). Because of this interplay, Ang-2 levels and the Ang-1/Ang-2 ratio have been proposed as possible biomarkers of sepsis. The circulating levels of Ang-2 appear to correlate with severity scoring systems like APACHE and SOFA and with 28-day mortality (19). However, many issues remain before these markers are brought into mainstream diagnostics, including standardization of the analytical methods, cutoff values, and timing relative to sample collection during the disease process (19). Additional markers of endothelial damage or activation include soluble versions of P-selectin, E-selectin, vascular endothelial cadherin (VE-cadherin), ICAM-1, vascular endothelial growth factor (VEGF), and von Willebrand factor (vWF).
In a case-control study of patients admitted to the ICU over a 24-month period, Vassiliou et al. (18) found that within 2 h of admission, soluble E-selectin (sE-selectin) and soluble P-selectin (sP-selectin), PCT, and SOFA scores were significantly different between the septic and nonseptic groups. Multiple logistic and Cox regression analyses identified increased sE- and sP-selectins and SOFA scores with increased risk for sepsis and sepsis development. Only the soluble selectins (s-selectins) remained significantly associated using multiple Cox regression analysis. The calculated AUROCs were 0.789, 0.761, 0.659, and 0.539 for sP- and sE-selectin, PCT, and CRP, respectively (18). When trauma patients were considered separately, the s-selectins, PCT, CRP, and APACHE II score were significantly associated with the development of sepsis, but only the s-selectins remained after multiple regression analysis, while Cox regression and multiple Cox regression retained sE-selectin and CRP or sE-selectin alone as significant markers, respectively. No association was identified between sE- and sP-selectin and the Gram reaction of the pathogen involved (18). This study also found no association among the remaining endothelial function factors, including Ang-1 and -2, VEGF, vWF, and soluble ICAM-1 (sICAM-1).
In a similar study of ICU patients, de Pablo et al. (20) collected blood samples upon admission and at days 2, 7, 14, and 28 and looked for differences in sE-selectin, soluble vascular cell adhesion molecule 1 (sVCAM-1), sICAM-1, and sICAM-2. Ninety-two patients (52 with sepsis and 40 with noninfectious SIRS) were included. Compared to healthy controls, patients with sepsis had significantly increased levels of sVCAM-1, sICAM-1, and -2 which continued throughout the period monitored. sE-selectin was increased at admission and after 48 h in the ICU but decreased to normal levels thereafter. In the noninfectious SIRS group, sICAM-2 was increased upon admission but returned to normal, whereas sE-selectin was increased compared to healthy controls and remained elevated at 48 h. sVCAM-1 and sICAM-1 levels remained comparable to those of healthy controls. They concluded that sICAM-1 was better than sVCAM-1 and sE-selectin at discriminating between infectious and noninfectious SIRS using a cutoff of 904 ng/ml (sensitivity of 74.3% and specificity of 94.1%), that sE-selectin showed prognostic strength to define SIRS upon admission to the ICU, and that sE-selectin remained significantly higher among the nonsurvivor group. There was no difference between the SIRS survivor and nonsurvivor groups for sVCAM-1, sICAM-1, and sICAM-2 at admission or over the duration of the study period.
sTREM-1
Triggering receptor expression on myeloid cells 1 (TREM-1) is a part of the immunoglobulin superfamily and is expressed on the surfaces of phagocytic cells, with enhanced expression occurring after exposure of cells to fungi and bacteria (9, 21). TREM-1 amplifies the inflammatory response initiated by Toll-like receptors (TLRs) and is upregulated in the presence of bacterial antigens but not in the presence of noninfectious mediators of inflammation. A soluble form of TREM-1 (sTREM-1) can be detected in plasma with enzyme-linked immunoassays. Based on these properties, soluble and surface-associated TREM-1 have been evaluated as a potential means of separating noninfectious SIRS from sepsis.
Oku et al. (21) noted that among patients admitted to the ICU, septic patients had significantly decreased expression of TREM-1 on phagocytic cell surfaces by flow cytometry (neutrophils more pronounced than monocytes), while noninfectious SIRS patients had enhanced cellular expression compared to healthy controls. The decreased expression of TREM-1 on the surfaces of neutrophils from septic patients was inversely correlated with IL-6 and CRP levels. Conversely, sTREM-1 plasma levels were significantly higher in septic patients compared to noninfectious SIRS patients and in survivors versus nonsurvivors from both groups. sTREM-1 levels also correlated positively with SOFA scores. A second study involving ICU patients (22) found that septic patients had significantly elevated levels of sTREM-1 (and PCT and CRP) compared to SIRS patients, but there were no differences in sTREM-1 and PCT for blood culture-positive or -negative septic patients. When a cutoff value of 108.9 pg/ml was applied, the sensitivity and specificity for differentiating sepsis from SIRS were 83% and 81%, respectively, with an AUROC of 0.868 (95% confidence interval [95% CI], 0.798 to 0.938). A meta-analysis of 11 studies provided a combined sensitivity and specificity of 79% and 80%, respectively, and an AUROC of 0.87 (95% CI, 0.84 to 0.89) for sTREM-1 to identify patients with sepsis (9).
DcR3
Decoy receptor 3 (DcR3) belongs to the tumor necrosis factor (TNF) receptor family along with DcR1 and -2 and osteoprotegerin (23, 24). It binds three TNF family cytokines (FasL, LIGHT [homologous to lymphotoxin, exhibits inducible expression and competes with herpes simplex virus glycoprotein D for binding to herpesvirus entry mediator, a receptor expressed on T lymphocytes], and TL1A [TNF-like cytokine 1A]) and can protect cancer cells in vitro from Fas/FasL-mediated apoptosis. In healthy individuals, DcR3 serum levels are extremely low, but expression is elevated in a variety of chronic inflammatory diseases and in patients with bacterial infection. Expression is also upregulated in response to lipopolysaccharide (LPS) exposure.
Two studies examined the use of serum DcR3 to distinguish between sepsis and SIRS (23, 24). The first study included 118 healthy, age-matched controls, 24 patients with a diagnosis of sepsis (variable infectious sources), and 43 patients classified as having SIRS (negative for any pathogen on culture). Serum was collected within 24 h of ICU admission, and APACHE II scores were determined for each individual. Mean serum DcR3 concentrations were calculated for each group with the following results: 0.91 ± 0.56 ng/ml for the healthy control group, 2.62 ± 1.46 ng/ml for the SIRS group, and 6.11 ± 2.58 ng/ml for the septic group. These values were statistically significantly different between groups, and there was good correlation between DcR3 levels and APACHE II scores. The AUROC for the healthy control group versus SIRS group was 0.910 (95% CI, 0.870 to 0.950), the AUROC for the healthy control group versus the sepsis group was 0.992 (95% CI, 0.984 to 1.000), and the AUROC for the septic group versus the SIRS group was 0.896 (95% CI, 0.820 to 0.973). To distinguish the septic and noninfected SIRS groups, the nominal cutoff value of DcR3 was 2.85 ng/ml, which provided a sensitivity and specificity of 95.8% and 67.4%, respectively.
The second study consisted of 25 cases of sepsis and 23 patients with noninfectious SIRS. A collection of 46 healthy blood donors comprised the control group. In this study, there was a statistically significant difference in age between the septic group (older) and the SIRS group, and there was also a significant correlation between DcR3 serum concentrations and age. However, similar to the previous report, significant differences were seen between the DcR3 concentrations of all three groups. To distinguish sepsis from SIRS, the AUROC was 0.958 (95% CI, 0.857 to 0.995) using a cutoff value of 3.24 ng/ml, with an associated sensitivity and specificity of 96% and 82.6%, respectively.
suPAR
The soluble urokinase-type plasminogen activator receptor (suPAR) is a component of the urokinase-type plasminogen activator system whose main function includes cell migration and adhesion, tissue remodeling, and pericellular proteolytic processes (9, 23). Under conditions of inflammation and infection, including bacteremia, malaria, and certain viral infections, including HIV, suPAR serum levels (predominantly expressed by WBCs) are elevated.
Hoenigl et al. (25) compared the early diagnostic accuracies of suPAR, PCT, IL-6, CRP, and WBC count to differentiate SIRS patients with and without positive blood cultures in a prospective fashion. The study included 132 adult patients with SIRS, of whom 55 had positive blood cultures (15 with Gram-positive bacteremia and 40 with Gram-negative bacteremia). Seventy-seven patients with negative blood cultures served as controls. Only suPAR, PCT, and IL-6 could distinguish SIRS patients with or without positive blood cultures independent of Gram reaction (CRP could not differentiate patients with Gram-negative bacteremia from controls). AUROC determinations were similar for suPAR, PCT, and IL-6 (0.726 [95% CI, 0.638 to 0.814], 0.44 [95% CI, 0.650 to 0.838], and 0.735 [95% CI, 0.632 to 0.838], respectively) but superior to CRP and WBC count. The AUROC was maximized when suPAR, PCT, and IL-6 were combined (0.804 [95% CI, 718 to 0.890]). Further, initial suPAR serum levels were significantly associated with 28-day mortality. Loonen et al. (26) evaluated the use of four biomarkers, suPAR, PCT, CRP, and neutrophil/lymphocyte count ratio (NLCR), as a way to screen patients who might be candidates for more costly direct-from-blood nucleic acid amplification testing (NAAT) for microbial DNA compared to blood culture as the reference standard. They found that suPAR, PCT, and NLCR were all significantly elevated in patients with positive blood culture and that the AUROC was best for PCT (0.806) and worst for CRP (0.485). The AUROC for the NLCR (0.77), however, was nearly as good as PCT, but the comparative cost of determining the NLCR was much less. Incidentally, the performance of the two NAAT assays which amplify a variety of bacterial DNA targets by PCR was quite inadequate, generating sensitivity and specificity values of 11 and 96% and 37 and 77%, respectively. In a study of 539 adult patients admitted to the emergency department, Uusitalo-Seppälä et al. (27) found that elevated plasma suPAR, PCT, CRP, and IL-6 levels could be used to distinguish patients with severe sepsis from patients without SIRS (with and without bacterial infection), SIRS, and sepsis, while a high suPAR level alone was the optimum marker of case fatality. In contrast and using a meta-analysis of 10 studies of critically ill patients, Backes et al. (28) found that suPAR levels held no diagnostic value toward differentiating septic from nonseptic ICU patients but were significantly higher in nonsurvivors (septic or not) and correlated well with disease severity and organ failure scores.
TRANSCRIPTOME ANALYSIS
One of the more exciting approaches toward the diagnosis of sepsis is the evaluation of differential gene expression between SIRS patients with or without sepsis versus healthy controls. Sepsis was once thought to be a manifestation of a hyperinflammatory response to infection, but a new paradigm is emerging suggesting a shift in immune regulation during the course of sepsis from inflammation to immunosuppression/dysfunction (29). The latter has been linked to a state of endotoxin (LPS) tolerance, possibly indicative of immune exhaustion (30).
Pena et al. (31) examined early-stage sepsis patients for evidence of LPS tolerance using gene expression profiling of peripheral blood mononuclear cells (PBMCs) by microarray analysis. Using high-throughput sequencing of cDNAs from PBMCs, they described an “endotoxin tolerance signature (ETS)” profile consisting of 99 differentially expressed genes uniquely found in LPS-tolerant PBMCs but not in inflammatory PBMCs or nonstimulated control cells. Conversely, they were also able to define an “inflammatory signature (IS)” based on gene expression of inflammatory PBMCs, but not LPS-tolerant cells. They then evaluated 11 individual patient cohorts. One in-house cohort consisted of 22 patients with confirmed sepsis plus 10 additional cohorts representing 571 early sepsis patients and 160 healthy controls from a meta-analysis. They found that septic patients from all cohorts produced an expression profile highly associated with the ETS compared to controls. The IS was significantly associated with septic patients in eight cohorts but at a consistently weaker association than the ETS. Further, the ETS was present in septic patients as early as day 1 after ICU admission and maintained through day 3. Next, they performed a prospective observational evaluation of 72 patients with early suspected sepsis. Of these, 37 had confirmed sepsis. Once again, the ETS was significantly enriched among patients with sepsis but not in the others and in the culture-positive versus the culture-negative sepsis group. The ETS was significantly associated with the development of organ failure and disease severity. Notably, ETS was not enriched in other critically ill patients (kidney transplant rejection or myocardial infarction). Finally, they were able to pare the gene set down from 99 to 31 without loss of performance. The 31-gene expression set actually improved the ability to discriminate septic patients with positive culture, those requiring admission to the ICU, and individuals with subsequent organ failure.
CONCLUSIONS
This minireview presents only a small fraction of the number of potential biomarkers that have been evaluated for the purpose of predicting sepsis or differentiating patients with sepsis or noninfectious SIRS. As of 2010, 178 individual biomarkers were examined in 3,370 studies (32), and those numbers have likely increased considerably since then. While the ultimate goal is a noble one, it is clear (at least to the author) that there will likely not be a single home run hit any time soon to satisfy this requirement. This is understandable, because SIRS and sepsis are heterogeneous collections of disease processes defined not only by individual host response but also by the numerous infectious, presumed infectious, or noninfectious triggers that generate SIRS. More likely, a combination of biomarkers more capable of supporting the clinical diagnosis or predicting the development of sepsis will emerge. However, it is unlikely that there will be a “one size fits all” approach to accommodate different patient populations (emergency department, ICU, neurology, trauma, adults, pediatrics, etc.), a single specimen type (whole blood, plasma, and serum), and a single cutoff value in the absence of a single reference standard. From the existing literature, this goal also seems unlikely until some form of standardization or procedural norm is established for conducting future studies (2). Further, no single marker carries the necessary sensitivity, specificity, or positive or negative predictive value to stand on its own. However, not all is lost. We are beginning to appreciate the hidden value buried in some of these biomarkers—e.g., using PCT to support antimicrobial stewardship, determining the duration of antimicrobial therapy, and ruling out rather than comfirming sepsis (2, 3, 10, 13). In that sense, the use of transcriptome analysis to differentiate sepsis from SIRS and to predict patient risk for developing sepsis and organ failure seems to be a promising approach that could be developed over time into a nonbiased database of differential gene expression using standardized sequence-based and hybridization technologies. Studies such as these could also lead to future biomarker discovery, including gene products that at present seem nonsequitur to the sepsis response profile. To reiterate the introduction, sepsis is not (yet) a laboratory-based diagnosis but a laboratory-supported diagnosis initiated by clinical impression and patient presentation.
Biography

W. Michael Dunne, Jr., Ph.D., is Vice President of Research and Development, North America, for bioMérieux, Inc. Prior to joining bioMerieux, he was the medical director of the diagnostic microbiology laboratory at Barnes-Jewish Hospital and Professor of Pathology and Immunology, Molecular Microbiology, Pediatrics and Medicine at Washington University School of Medicine in St. Louis from 2000 to 2011 and remains on the faculty there. During that time, he established a Committee on Postgraduate Educational Programs (CPEP) training program in Medical and Public Health Microbiology, which is still active under the direction of Dr. Carey-Ann Burnham. He has previously served as medical director of microbiology laboratories at Henry Ford Hospital (Detroit, MI), Texas Children's Hospital (Baylor College of Medicine, Houston, TX) and Children's Hospital of Wisconsin (Medical College of Wisconsin, Milwaukee, WI, where he had received his Ph.D. in 1982). Dr. Dunne is a Diplomate of the American Board of Medical Microbiology and a Fellow of the American Academy of Microbiology, Infectious Diseases Society of America, and Canadian College of Microbiology. He served as an editor of the Journal of Clinical Microbiology for 10 years and remains on the editorial board.
REFERENCES
- 1.Vincent J-L, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall J-R, Payen D. 2006. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34:344–353. doi: 10.1097/01.CCM.0000194725.48928.3A. [DOI] [PubMed] [Google Scholar]
- 2.Hall TC, Bilku DK, Al-Leswas D, Horst C, Dennison AR. 2011. Biomarkers for the differentiation of sepsis and SIRS: the need for the standardization of diagnostic studies. Ir J Med Sci 180:793–798. doi: 10.1007/s11845-011-0741-1. [DOI] [PubMed] [Google Scholar]
- 3.Ratzinger F, Schuardt M, Eichbichler K, Tsirkinidou I, Bauer M, Haslacher H, Mitteregger D, Binder M, Burgmann H. 2013. Utility of sepsis biomarkers and the infection probability score to discriminate sepsis and systemic inflammatory response syndrome in standard care patients. PLoS One 8:e82946. doi: 10.1371/journal.pone.0082946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams & Wilkins Co. 1992. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874. [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal S, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reihnart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent J-L, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. 2013. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Crit Care Med 41:580–624. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric Sepsis. 2005. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Sanotti S, Tailberg L, Gurka D, Kumar A, Cheang M. 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 8.Karzai W, Oberhoffer M, Meier-Hellmann A, Reinhart K. 1997. Procalcitonin—a new indicator of the systemic response to severe infection. Infection 6:329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henriquez-Camacho C, Losa J. 2014. Biomarkers for sepsis. Biomed Res Int 2014:547818. doi: 10.1155/2014/547818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kibe S, Adams K, Barlow G. 2011. Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob. Chemother 66(Suppl 2):ii33–ii40. doi: 10.1093/jac/dkq523. [DOI] [PubMed] [Google Scholar]
- 11.Riedel S. 2012. Procalcitonin and the role of biomarkers in the diagnosis and management of sepsis. Diagn Microbiol Infect Dis 73:221–227. doi: 10.1016/j.diagmicrobio.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. 1993. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 341:515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal R, Schwartz DN. 2011. Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin Infect Dis 53:379–387. doi: 10.1093/cid/cir408. [DOI] [PubMed] [Google Scholar]
- 14.Tian G, Pan S-Y, Ma G, Liao W, Su Q-G, Gu B-C, Qin K. 2014. Serum levels of procalcitonin as a biomarker for differentiating between sepsis and systemic inflammatory response syndrome in the neurological intensive care unit. J Clin Neurosci 21:1153–1158. doi: 10.1016/j.jocn.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Ciriello V, Gudipati S, Stavrou PZ, Kanakaris NK, Bellamy MC, Giannoudis PV. 2013. Biomarkers predicting sepsis in polytrauma patients: current evidence. Injury 44:1680–1692. doi: 10.1016/j.injury.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 16.de Azevedo JRA, Torres OJM, Beraldi RA, Ribas CAPM, Malafaia O. 2015. Prognostic evaluation of severe sepsis and septic shock: procalcitonin clearance vs Δ sequential organ failure assessment. J Crit Care 30:219.e9–219.e12. doi: 10.1016/j.jcrc.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Jekarl DW, Lee S-Y, Lee J, Park Y-J, Kim Y, Park JH, Wee JH, Choi SP. 2013. Procalcitonin as a diagnostic marker and IL-6 as a prognostic marker for sepsis. Diagn Microbiol Infect Dis 75:342–347. doi: 10.1016/j.diagmicrobio.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Vassiliou AG, Mastora Z, Orfanos SE, Jahaj E, Maniatis NA, Koutsoukou A, Armaganidis A, Kotanidou A. 2014. Elevated biomarkers of endothelial dysfunction/activation at ICU admission are associated with sepsis development. Cytokine 69:240–247. doi: 10.1016/j.cyto.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Mussap M, Cibecchini F, Noto A, Fanos V. 2013. In search of biomarkers for diagnosing and managing neonatal sepsis: the role of angiopoietins. J Matern Fetal Neonatal Med 26(Suppl 2):24–26. doi: 10.3109/14767058.2013.830411. [DOI] [PubMed] [Google Scholar]
- 20.de Pablo R, Monserrat J, Reyes E, Díaz D, Rodríguez-Zapata M, de la Hera A, Prieto A, Álvarez-Mon M. 2013. Circulating sICAM-1 and sE-selectin as biomarker of infection and prognosis in patients with systemic inflammatory response syndrome. Eur J Intern Med 24:132–138. doi: 10.1016/j.ejim.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Oku R, Oda S, Nakada T-A, Sadahiro T, Nakamura M, Hirayama Y, Abe R, Tateishi Y, Ito M, Iseki T, Hirasawa H. 2013. Differential pattern of cell-surface and soluble TREM-1 between sepsis and SIRS. Cytokine 61:112–117. doi: 10.1016/j.cyto.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Su L, Han B, Liu C, Liang L, Jiang Z, Deng J, Yan P, Jia Y, Feng D, Xie L. 2012. Value of soluble TREM-1, procalcitonin, and C-reactive protein serum levels as biomarkers for detecting bacteremia among sepsis patients with new fever in intensive care units: a prospective cohort study. BMC Infect Dis 12:157–167. doi: 10.1186/1471-2334-12-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou Y-Q, Xu P, Zhang M, Han D, Peng L, Liang D-Y, Yang S, Zhang Z, Hong J, Lou X-L, Zhang L, Kim S. 2012. Serum decoy receptor 3, a potential new biomarker for sepsis. Clin Chim Acta 413:744–748. doi: 10.1016/j.cca.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Kim S, Mi L, Zhang L. 2012. Specific elevation of DcR3 in sera of sepsis patients and its potential role as a clinically important biomarker of sepsis. Diagn Microbiol Infect Dis 73:312–317. doi: 10.1016/j.diagmicrobio.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoenigl M, Raggam RB, Wagner J, Valentin T, Leitner E, Seeber K, Zollner-Schwetz I, Krammer W, Prüller Grisold AJ, Krause R. 2013. Diagnostic accuracy of soluble urokinase plasminogen activator receptor (suPAR) for prediction of bacteremia in patients with systemic inflammation syndrome. Clin Biochem 46:225–229. doi: 10.1016/j.clinbiochem.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Loonen AJM, de Jager CPC, Tosserams J, Kusters R, Hilbink M, Wever PC, van den Brule AJC. 2014. Biomarkers and molecular analysis to improve bloodstream infection diagnostics in an emergency care unit. PLoS One 9:e87315. doi: 10.1371/journal.pone.0087315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uusitalo-Seppälä R, Tarkka M, Aittoniemi J, Koskinen P, Leino A, Vahlberg T, Rintala EM. 2012. Soluble urokinase-type plasminogen activator receptor in patients with suspected infection in the emergency room: a prospective cohort study. J Intern Med 272:247–256. doi: 10.1111/j.1365-2796.2012.02569.x. [DOI] [PubMed] [Google Scholar]
- 28.Backes Y, van der Sluijs KF, Mackie DP, Tacke F, Koch A, Tenhunen JJ, Schultz MJ. 2012. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med 38:1418–1428. doi: 10.1007/s00134-012-2613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotchkiss RS, Monneret G, Payen D. 2013. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavaillon J-M, Adib-Conquy M. 2006. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care 10:233. doi: 10.1186/cc5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pena OM, Hancock DG, Lyle NH, Linder A, Russell JA, Xia J, Fjell CD, Boyd JH, Hancock REW. 2014. An endotoxin tolerance signature predicts sepsis and organ dysfunction at initial clinical presentation. EBioMedicine 1:64–71. doi: 10.1016/j.ebiom.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierrakos C, Vincent J-L. 2010. Sepsis biomarkers: a review. Crit Care 14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]


