Abstract
The routes of transmission of a newly emerged Tembusu virus (TMUV, Flavivirus) in ducks in China remain unclear. Our epidemiological data show that TMUV is spread in winter, when mosquitos are inactive, which suggests that nonvector transmission routes are involved in the spread of TMUV. Furthermore, in vivo studies indicate that TMUV can be transmitted efficiently among ducks by both direct contact and aerosol transmission. This finding has important implications for the control of infection with this novel TMUV in the field.
TEXT
In April 2010, a novel Tembusu virus (TMUV)-associated disease, characterized by retarded growth, high fever, loss of appetite, decline in egg production, and death, emerged in ducks in China (1–5). Since then, TMUV has continuously infected ducks and caused significant economic loss in China. TMUV is a member of the Ntaya virus group in the genus Flavivirus of the family Flaviviridae (6, 7). TMUV was first isolated from mosquitoes in Kuala Lumpur, Malaysia, in 1955; since then, TMUV has been isolated from Culex species mosquitos in Malaysia and Thailand (6, 7). However, the natural reservoir, transmission route, and epidemic situation of TMUV in China remain unclear. To better understand the prevalence and potential transmission route of TMUV in China, we conducted active surveillance of TMUV in ducks at different time points in China.
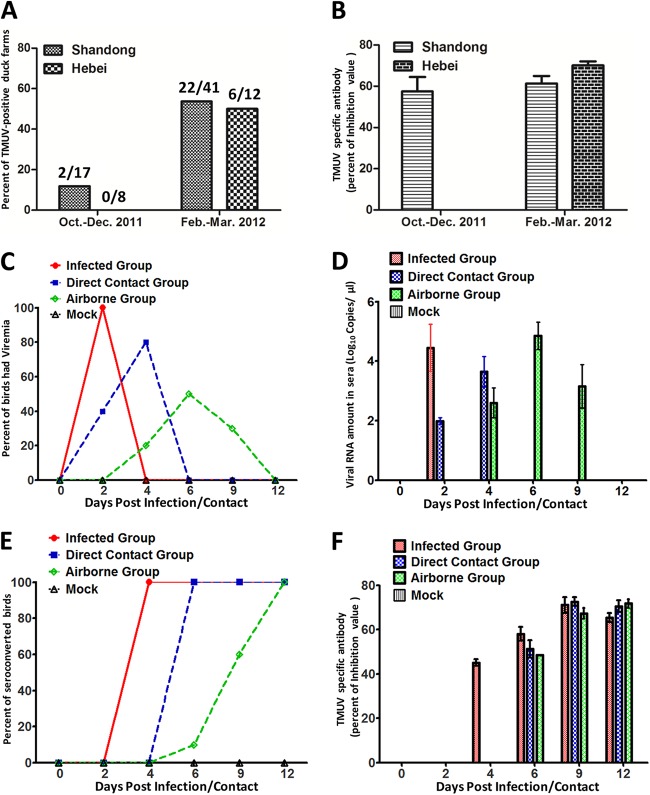
A total of 1,200 serum samples were collected randomly from 53 duck farms in Shandong and Hebei Provinces. Samples were collected from 27- to 380-day-old ducks from 25 farms in the fall (October to December 2011) and 53 farms in the late winter (February and March 2012), respectively. The farms investigated in winter included all of the fall sites (except one farm in Laiwu) (see Fig. S1 in the supplemental material). The presence of TMUV-specific antibody was determined by a blocking enzyme-linked immunosorbent assay (ELISA) based on monoclonal antibody (MAb) 1F5, which is a neutralizing MAb that binds specifically to the E protein of TMUV. A blocking ELISA based on this MAb was developed for the detection of neutralization-related antibodies against TMUV (8). TMUV antibody titers are expressed as percent inhibition (PI) of blocking of MAb 1F5 at a test serum dilution of 1:10. The selected cutoff PI value for positive serum was 18.4%. All serum samples were prepared and tested as described previously (8). In October and December 2011, only 11.8% of the farms investigated (2/17) were TMUV seropositive in Shandong Province, and none of the 8 farms investigated in Hebei Province were seropositive (Fig. 1A). Surprisingly, 3 to 4 months later (February and March 2012), 53.7% (22/41) and 50.0% (6/12) of the farms investigated in Shandong and Hebei Provinces became TMUV seropositive. During October and December 2011, the mean rates of seropositivity of the serum samples collected were 11.7 and 0% in Shandong and Hebei Provinces, respectively, which increased to 41.0 and 54.5% during February and March 2012. At most (78.1%) of the TMUV-positive farms, the seropositivity rate was 100% and the TMUV antibody titers ranged from 57 to 70% PI values, which are similar to those of experimentally infected ducks (Fig. 1B and F). These results indicate that TMUV can spread efficiently during the winter, when ambient temperatures are below freezing and mosquitoes are not active. This strongly suggests that another route is involved in TMUV transmission.
FIG 1.
Epidemiological survey and animal experiment results of TMUV in ducks. (A) Percentages of TMUV-seropositive duck farms in Shandong and Hebei Provinces from October 2011 to March 2012. Shown above each column is the number of TMUV-seropositive farms/total number of farms. (B) Average TMUV antibody titers (PI values) of serum samples collected at duck farms in Shandong and Hebei Provinces from October 2011 to March 2012. (C) Percentages of experimental ducks having viremia at different time points postinfection or -contact. (D) TMUV RNA amounts in serum samples from experimental ducks at different time points postinfection or -contact. (E) Percentages of seroconverted ducks at different time points postinfection or -contact. (F) TMUV antibody titers determined by a blocking ELISA in experimental ducks at different time points postinfection or -contact. Titers are expressed as the PI of blocking of MAb 1F5 at a test serum dilution of 1:10.
To determine the alternative route of transmission TMUV in ducks, a direct-contact and airborne transmission study of TMUV was conducted. Briefly, 25 8-week-old ducks (Tadorna) seronegative for TMUV were used. Five ducks were inoculated intramuscularly (i.m.) with 3.5 log10 50% egg lethal doses of TMUV strain FX2010 (5) and housed in one isolator. Five naive ducks were introduced into the same isolator later that day. Another 10 naive ducks were housed in a separate clean isolator that was connected with the infected isolator by an air pipe, which allowed air to flow from the infected isolator to the clean isolator (see Fig. S2 in the supplemental material). Five mock control ducks were housed in a different isolator with a separate air supply. Serum samples were collected from all of the ducks at 0, 2, 4, 6, 9, and 12 days postinfection or -contact (dpi or dpc, respectively) to quantify viremia and the rate of seroconversion. Real-time reverse transcription-PCR was used to quantify TMUV RNA in the serum samples. Briefly, RNA was extracted from serum with the RNeasy minikit (Qiagen Inc., Valencia, CA). First-strand cDNA was then synthesized by using avian myeloblastosis virus reverse transcriptase (TaKaRa Biotechnology, Dalian, China) according to the manufacturer's instructions. A TaqMan-based real-time PCR assay targeting the E gene of TMUV was conducted as described previously (9). The duck studies were approved by the Animal Care and Use Committee of the Shanghai Veterinary Research Institute.
Depression and diarrhea were observed in the infected ducks at 2 dpi, and no infected ducks died in the whole study. Viremia occurred in all i.m. infected ducks at 2 dpi, with the viral RNA amount ranging from 2.24 to 5.64 log10 copies/μl in serum (Fig. 1C and D). Viral RNAs were also detected in the serum of two of the direct-contact ducks at 2 dpc. Four out of five direct-contact ducks were viral RNA positive at 4 dpc, with a peak viral load of 4 log10 copies/μl in serum (Fig. 1C and D). In the airborne transmission group, viral RNA was detected in 2 out of 10 ducks at 4 dpc, and 5 and 3 ducks showed viremia at 6 and 9 dpc, respectively (Fig. 1C and D). All i.m. infected and direct-contact ducks seroconverted at 4 dpi and 6 dpc, respectively. The earliest specific antibody response against TMUV was observed in one duck in the airborne transmission group at 6 dpc. All of the ducks in the airborne transmission group subsequently seroconverted at 12 dpc. All of the ducks that seroconverted produced high levels of TMUV-specific antibody (Fig. 1E and F). Neither viremia nor seroconversion was observed in mock control ducks. The data presented indicate that TMUV causes viremia quickly in i.m. infected ducks and infects naive ducks by airborne transmission. To confirm the airborne transmission of TMUV in ducks, we reconducted the airborne transmission study by using the same strategy with a filter added between the inoculated and airborne transmission groups, which can stop the movement of mites or lice between isolators. In addition, oropharyngeal swabs, cloacal swabs, and fecal samples were collected from the inoculated ducks at 0, 2, 3, 4, and 5 dpi. All of the direct-contact ducks and airborne transmission ducks seroconverted at 6 and 12 dpc, which is consistent with the first study. The data presented confirmed the airborne transmission of TMUV in ducks. In the inoculated ducks, virus was detected in oropharyngeal swabs at 2 to 3 dpi and in cloacal swabs at 3 dpi (data not shown), which is in line with our previous study (9). However, no virus was detected in fecal samples. Our data suggest that TMUV could be transmitted to ducks by aerosol produced by infected ducks. To the best of our knowledge, the present study is the first to demonstrate efficient airborne transmission of TMUV in ducks. This may explain the rapid spread of TMUV in ducks during winter in China. However, vector-borne transmission is the major mode for most flaviviruses and mosquitoes play an important role in the spread of TMUV (6, 10). It is possible that multiple transmission modes are involved in the spread of TMUV in ducks. Full understanding of the vector-borne transmission of TMUV needs further studies. Recently, TMUVs were also found in chickens and geese in China (2, 11). Despite the absence of clinical signs in TMUV-seropositive chickens and geese, TMUV may become virulent in these species after adaptation.
This study demonstrates the novel airborne and direct-contact routes of transmission in ducks of TMUV, which poses a significant risk to the duck industry and a potential risk for other types of poultry in China.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (31172332, 31472205, and 31402150), the Shanghai Science and Technology Program for Agriculture (2012-2-6), the Innovation Action Plan Key Basic Research Project of the Shanghai Science and Technology Commission (12JC1410600), the Innovation Action Plan Key Scientific and Technological Projects in the Field of Modern Agriculture of the Shanghai Science and Technology Commission (13391901601), and the Special Fund for Central Nonprofit Research Institutes Fundamental Research (2011JB02).
We thank Aaron Balogh for proofreading the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00770-15.
REFERENCES
- 1.Cao Z, Zhang C, Liu Y, Ye W, Han J, Ma G, Zhang D, Xu F, Gao X, Tang Y, Shi S, Wan C, He B, Yang M, Lu X, Huang Y, Diao Y, Ma X. 2011. Tembusu virus in ducks, China. Emerg Infect Dis 17:1873–1875. doi: 10.3201/eid1710.101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu M, Chen S, Chen Y, Liu C, Yin X, Li G, Zhang Y. 2012. Adapted Tembusu-like virus in chickens and geese in China. J Clin Microbiol 50:2807–2809. doi: 10.1128/JCM.00655-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu P, Lu H, Li S, Moureau G, Deng YQ, Wang Y, Zhang L, Jiang T, de Lamballerie X, Qin CF, Gould EA, Su J, Gao GF. 2012. Genomic and antigenic characterization of the newly emerging Chinese duck egg-drop syndrome flavivirus: genomic comparison with Tembusu and Sitiawan viruses. J Gen Virol 93:2158–2170. doi: 10.1099/vir.0.043554-0. [DOI] [PubMed] [Google Scholar]
- 4.Su J, Li S, Hu X, Yu X, Wang Y, Liu P, Lu X, Zhang G, Liu D, Li X, Su W, Lu H, Mok NS, Wang P, Wang M, Tian K, Gao GF. 2011. Duck egg-drop syndrome caused by BYD virus, a new Tembusu-related flavivirus. PLoS One 6:e18106. doi: 10.1371/journal.pone.0018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan P, Zhao Y, Zhang X, Xu D, Dai X, Teng Q, Yan L, Zhou J, Ji X, Zhang S, Liu G, Zhou Y, Kawaoka Y, Tong G, Li Z. 2011. An infectious disease of ducks caused by a newly emerged Tembusu virus strain in mainland China. Virology 417:1–8. doi: 10.1016/j.virol.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Platt GS, Way HJ, Bowen ET, Simpson DI, Hill MN, Kamath S, Bendell PJ, Heathcote OH. 1975. Arbovirus infections in Sarawak, October 1968–February 1970 Tembusu and Sindbis virus isolations from mosquitoes. Ann Trop Med Parasitol 69:65–71. [DOI] [PubMed] [Google Scholar]
- 7.Simpson DI, Way HJ, Platt GS, Bowen ET, Hill MN, Kamath S, Bendell PJ, Heathcote OH. 1975. Arbovirus infections in Sarawak, October 1968–February 1970: Getah virus isolations from mosquitoes. Trans R Soc Trop Med Hyg 69:35–38. doi: 10.1016/0035-9203(75)90008-5. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Li G, Teng Q, Yu L, Wu X, Li Z. 2012. Development of a blocking ELISA for detection of serum neutralizing antibodies against newly emerged duck Tembusu virus. PLoS One 7:e53026. doi: 10.1371/journal.pone.0053026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan L, Yan P, Zhou J, Teng Q, Li Z. 2011. Establishing a TaqMan-based real-time PCR assay for the rapid detection and quantification of the newly emerged duck Tembusu virus. Virol J 8:464. doi: 10.1186/1743-422X-8-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Y, Diao Y, Chen H, Ou Q, Liu X, Gao X, Yu C, Wang L. 2015. Isolation and genetic characterization of a Tembusu virus strain isolated from mosquitoes in Shandong, China. Transbound Emerg Dis 62:209–216. doi: 10.1111/tbed.12111. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Wang S, Li Z, Lin F, Cheng X, Zhu X, Wang J, Huang M, Zheng M. 2014. Isolation and characterization of a Chinese strain of Tembusu virus from Hy-Line Brown layers with acute egg-drop syndrome in Fujian, China. Archives of virology 159:1099–1107. doi: 10.1007/s00705-013-1931-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.