Abstract
While the assessment of β-d-glucan (BDG) levels in adults improves the early diagnosis of invasive fungal disease (IFD), data on BDG levels in children are limited. We therefore assessed in a prospective cohort study the value of serial BDG screening for early detection of IFD in children undergoing allogeneic hematopoietic stem cell transplantation (HSCT). IFD was defined according to the revised European Organization for Research and Treatment of Cancer/Mycosis Study Group (EORTC/MSG) criteria, with the necessary modification that BDG was not included as a microbiological criterion. For the analysis, a total of 702 serum samples were obtained in 34 pediatric HSCT recipients. Proven IFD occurred in two patients (fusariosis and Candida sepsis, respectively), and probable invasive aspergillosis was diagnosed in four patients. Analyses including different cutoff values for BDG levels and different definitions of the onset of IFD demonstrated that the BDG assay has a relatively high sensitivity and good negative predictive value, whereas the positive predictive value has major limitations (<30%). Receiver operating characteristic analyses suggested an optimal cutoff between 60 and 70 pg/ml for different definitions of the onset of IFD. Our data show that BDG screening in pediatric HSCT recipients has a low positive predictive value and is therefore of limited usefulness.
INTRODUCTION
Despite improved supportive care strategies, the morbidity and mortality caused by invasive fungal disease (IFD) are still unacceptably high in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT) (1). Early diagnosis of IFD is usually difficult, in particular in patients suffering from mold infections, but, on the other hand, early initiation of therapy correlates with better outcome (2, 3). Significant advances in the early detection of IFD have been made with the development of serum tests for fungal antigens, such as galactomannan (GM) or (1→3)-β-d-glucan (BDG). Both biomarkers have been included as microbiological criteria in the revised definitions for IFD by the European Organization for Research and Treatment of Cancer/Mycosis Study Group (EORTC/MSG) consensus group (4), and FDA-approved assays are commercially available for the detection of these fungal antigens. Whereas the cell wall polysaccharide component GM is released by all Aspergillus species and can be detected in patients with invasive aspergillosis (IA), BDG can be detected in patients with IFD due to Aspergillus and Candida spp., Fusarium, Trichosporon, or Saccharomyces, and Pneumocystis jirovecii but also in various bacterial infections and even in healthy individuals (5). A number of factors, such as the concomitant administration of various antibiotic compounds, may result in false positivity, whereas systemic mold-active prophylaxis may increase the number of false-negative results (6, 7).
Since GM data in children favorably compare with the results of a meta-analysis for GM testing in adults (8), prospective monitoring of GM levels twice weekly in children at high risk for IFD is reasonable for an early diagnosis of IA (9, 10). Unfortunately, in contrast to GM, data on BDG testing in pediatric patients are limited and therefore, routine testing to guide clinical decisions in children is currently not recommended (9, 10). The present study is the first prospective analysis of the value of serial BDG screening for the early detection of IFD in children undergoing allogeneic HSCT.
MATERIALS AND METHODS
Patients.
After written informed consent, all consecutive patients <18 years of age and undergoing allogeneic HSCT between August 2012 and January 2014 at the Hospital for Children and Adolescents at the University of Frankfurt were included in this prospective cohort study. A minimum of three BDG samples were required to be included in the analysis. The study was approved by the local ethics committee (205/12).
Methods.
Serum samples for assessing GM were measured at least once weekly until day 100 after HSCT as part of the routine evaluation; screening of GM was continued in patients suffering from complications, such as severe graft-versus-host disease (GvHD). Concentrations of GM were assessed using the Platelia assay (Bio-Rad, Munich, Germany), according to the manufacturer's instructions, and an optical density of ≥0.7 once or 0.5 in two consecutive samples was considered positive (11). According to the discretion of the treating physician, GM assay results sometimes triggered other examinations, such as imaging studies, including a pulmonary computed tomography (CT) scan.
In parallel to GM testing, a second blood sample was obtained for BDG testing, centrifuged, and frozen at −20°C until assayed. Without knowing the results of the GM tests, the concentration of BDG was measured using the Fungitell assay (Associates of Cape Cod, MA, USA), according to the manufacturer's instructions. BDG levels of >500 pg/ml were not further diluted and assessed. The test results of the BDG assay were not available for the clinician for decision making.
Systemic antifungal prophylaxis was routinely given and consisted of liposomal amphotericin B, mold-active azoles, or micafungin, depending on comorbidity and comedications. Supportive care interventions, such as empirical antifungal therapy and therapy of proven and probable invasive fungal disease, were administered according to recently published pediatric guidelines (9, 10).
Invasive fungal disease was defined according to the revised definitions by the EORTC/MSG consensus group, with the necessary modification that BDG was not included in the microbiological criteria (4). As the optimal cutoff value for BDG levels in children is not well defined (9, 10) and the exact onset of the IFD is not clear in many cases, in particular in IA, we performed an exploratory analysis using different cutoffs (e.g., 60, 80, 100, 120, and 140 pg/ml) and different definitions of the onset of IFD (e.g., at the time of a pathological result, such as blood culture results or biopsy sample, pulmonary infiltrates, or pathological GM test result, and 2 and 4 weeks prior to these results, respectively). A single pathological GM test result, which was not associated with clinical symptoms and did not result in additional diagnostics or in a change of antifungal medication, was considered false positive. Test values, such as sensitivity (SN), specificity (SP), and positive and negative predictive value (PPV and NPV, respectively) were defined as previously described (11). For the statistical analyses, the statistical program BiAS (version 9.02; Epsilon Publishing Group) was used.
RESULTS
A total of 702 serum samples were obtained in 34 children (19 boys and 15 girls; median age, 6 years 11 months; age range, 0 to 16 years) undergoing allogeneic HSCT for a variety of underlying diseases (Table 1). The median (range) number of samples per patient was 21 (5 to 45 samples). Conditioning regimens, time of engraftment (absolute neutrophil count, ≥500/μl), and immunosuppressive therapy for prevention or treatment of GvHD, all of which influence the risk for IFD, were heterogenous and depended on a number of factors, such as underlying malignancy, comorbidity, and administered graft.
TABLE 1.
Patient characteristics
| No. | Sex/age (yr)a | Underlying malignancyb | Culture result (source) | Pathological imaging | GM (no. of samples tested/no. of positive samples)c | Maximum GM value | IFDd | BDG (no. of samples tested/no. of positive samples)f | Maximum BDG value |
|---|---|---|---|---|---|---|---|---|---|
| 2 | f/1 | ALL relapse | Fusarium spp. (palate) | − | 25/2 | 1.0 | Proven | 24/24 | >500 |
| 26 | f/16 | Thalassemia major | C. krusei (blood) | − | 5/0 | 0.1 | Proven | 5/0 | 34 |
| 1 | m/6 | AML relapse | − | Pulm CT scane | 20/5 | 2.3 | Probable | 21/6 | 113 |
| 25 | m/7 | AML relapse | − | Pulm CT scan | 45/1 | 0.7 | Probable | 46/24 | >500 |
| 3 | f/13 | AML | − | Pulm CT scan | 26/3 | 1.0 | Probable | 26/15 | >500 |
| 8 | f/13 | MDS | − | Pulm CT scan | 18/1 | 2.4 | Probable | 27/11 | >500 |
| 4 | f/2 | AML | − | − | 25/0 | 0.2 | − | 25/0 | 40 |
| 5 | m/0 | SCID | − | − | 23/0 | 0.1 | − | 24/24 | >500 |
| 6 | m/14 | Acute leukemia (bilinear) | − | − | 27/0 | 0.1 | − | 28/0 | 42 |
| 7 | m/11 | ALL relapse | − | − | 10/0 | 0.1 | − | 10/0 | 50 |
| 9 | f/7 | AML relapse | − | − | 6/0 | 0.2 | − | 6/0 | 10 |
| 10 | m/8 | AML | − | − | 15/0 | 0.1 | − | 15/3 | 244 |
| 11 | m/4 | AML | − | − | 28/0 | 0.2 | − | 28/2 | 116 |
| 12 | f/2 | AML | − | − | 21/0 | 0.4 | − | 21/0 | 71 |
| 13 | m/5 | T-cell lymphoma | − | − | 25/0 | 0.2 | − | 25/2 | 185 |
| 14 | m/13 | SCID | − | − | 24/0 | 0.4 | − | 24/24 | 446 |
| 15 | m/0 | Thalassemia major | − | − | 14/0 | 0.4 | − | 14/4 | 211 |
| 16 | m/1 | ALL | − | − | 21/0 | 0.4 | − | 21/8 | >500 |
| 17 | m/13 | ALL | − | − | 17/0 | 0.2 | − | 17/2 | 221 |
| 18 | m/6 | SAA | − | − | 33/0 | 0.3 | − | 34/13 | 321 |
| 19 | f/12 | ALL | − | − | 16/0 | 0.4 | − | 16/0 | 62 |
| 20 | f/7 | Primary immunodeficiency | − | − | 24/0 | 0.2 | − | 24/1 | 201 |
| 21 | f/4 | Thalassemia major | − | − | 20/0 | 0.1 | − | 20/6 | 482 |
| 22 | f/10 | AML | − | − | 13/0 | 0.1 | − | 13/1 | 135 |
| 23 | f/4 | Neuroblastoma | − | − | 23/0 | 0.2 | − | 23/2 | 367 |
| 24 | m/16 | ALL | − | − | 20/0 | 0.2 | − | 20/3 | 160 |
| 27 | m/1 | Wiskott-Aldrich syndrome | − | − | 15/0 | 0.2 | − | 15/6 | 179 |
| 28 | m/4 | Diamond-Blackfan anemia | − | − | 21/0 | 0.2 | − | 21/6 | >500 |
| 29 | f/0 | ALL relapse | − | − | 16/0 | 0.3 | − | 16/0 | 72 |
| 30 | m/16 | ALL | − | − | 25/0 | 0.2 | − | 25/3 | 138 |
| 31 | f/15 | ALL | − | − | 20/0 | 0.1 | − | 20/1 | 121 |
| 32 | f/1 | AML | − | − | 13/0 | 0.1 | − | 13/0 | 63 |
| 33 | m/14 | CML | − | − | 26/0 | 0.3 | − | 26/2 | 129 |
| 35 | m/0 | HLH | − | − | 22/0 | 0.3 | − | 25/2 | 191 |
f, female; m, male.
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; SCID, severe combined immunodeficiency; SAA, severe aplastic leukemia; CML, chronic myeloid leukemia; HLH, hemophagocytic lymphohistiocytosis.
GM, galactomannan.
Invasive fungal disease (IFD) was defined according to the revised definitions by the EORTC/MSG consensus group, with the necessary modification that β-d-glucan (BDG) was not included in the microbiological criteria (4). None of the patients without proven or probable IFD was diagnosed with possible IFD.
Whereas the pulmonary (Pulm) CT scan showed a cavity in the left lower lobe in patient #1, well-circumscribed lesions without halo-sign were seen in patients #3, #8, and #25.
BDG was considered positive for levels of ≥80 pg/ml.
Two patients suffered from proven IFD. In one patient with fusariosis (isolated from the palate), all 5 serum samples prior to the definite diagnosis had BDG concentrations of >100 pg/ml, with maximum levels of 220 pg/ml. The other patient with proven IFD died at day 13 after transplantation due to a Candida krusei bloodstream infection. In this patient, all BDG levels measured were <40 pg/ml. Four of the patients suffered from probable IA. In three of them, BDG indicated IA earlier than GM; specifically, BDG levels turned to >200 pg/ml at 4 weeks and >100 pg/ml at 2 weeks prior to the diagnosis of probable IA (positive GM assay result and lung infiltrates) in two patients and one patient, respectively. In contrast, in one patient with probable pulmonary IA, assessment of BDG and GM levels revealed first pathological values at the same time.
According to the revised EORTC/MSG definitions, no IFD was seen in 28 patients. Out of these patients, the number of patients in whom all BDG screening tests were negative increased with higher BDG cutoff values (Fig. 1). These numbers further increased when two consecutive samples with pathological levels were required for a pathological BDG assay result (Fig. 1). In two patients in whom IFD was not suspected, all tested samples (24 each) had BDG levels of >100 pg/ml, with maximum levels of >500 and 446 pg/ml.
FIG 1.
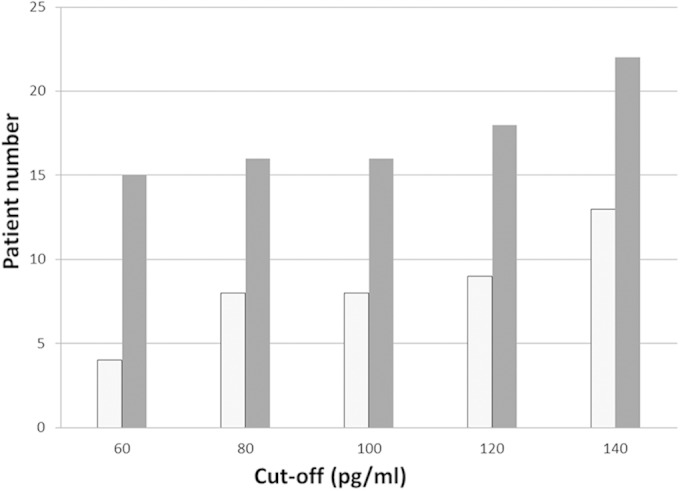
Number of patients without invasive fungal disease (n = 28) in whom all β-d-glucan (BDG) screening tests remained negative. The white columns indicate the number of patients in whom a positive BDG test was defined by a single pathological BDG assay only, and the gray columns indicate the number of patients in whom a positive BDG test was defined by two consecutive pathological BDG assays. The different cutoff values for BDG are shown on the x axis.
The analysis of sensitivity, specificity, PPV, and NPV for different cutoff values for the BDG assay and for different potential time points of the onset of IFD demonstrated that BDG has for most cutoff values a high sensitivity and good NPV, whereas the PPV has major limitations (Table 2). In addition, PPV and NPV are not considerably affected by different cutoff values or different definitions of the onset of disease, which is in contrast to the sensitivity and specificity of the assay. Receiver operating characteristic (ROC) analyses indicate optimal cutoffs for BDG levels of 61 pg/ml, 72 pg/ml, and 71 pg/ml, respectively, for the different definitions of the onset of infection (i.e., 0, 2, and 4 weeks prior to first pathological signs, such as pathological imaging or GM assay result).
TABLE 2.
Performance of β-d-glucan screening in pediatric hematopoietic stem cell transplant recipients according to different cutoff values and different definitions of onset of invasive fungal disease
| Cutoff (pg/ml) by definition of onset of IFD | No. with resulta |
Sensitivity (%) | Specificity (%) | NPVb | PPVc | |||
|---|---|---|---|---|---|---|---|---|
| TP | FN | TN | FP | |||||
| Time of first pathological signd | ||||||||
| 60e | 20 | 0 | 438 | 164 | 100 | 73 | 1 | 0.11 |
| 20 | 0 | 467 | 135 | 100 | 78 | 1 | 0.13 | |
| 80 | 18 | 2 | 472 | 130 | 90 | 78 | 1 | 0.12 |
| 16 | 4 | 483 | 100 | 80 | 82 | 0.99 | 0.13 | |
| 100 | 18 | 2 | 495 | 107 | 90 | 82 | 1 | 0.14 |
| 16 | 4 | 511 | 91 | 80 | 82 | 0.99 | 0.15 | |
| 120 | 13 | 7 | 510 | 92 | 65 | 85 | 0.99 | 0.12 |
| 11 | 8 | 525 | 78 | 58 | 87 | 0.98 | 0.12 | |
| 140 | 12 | 8 | 520 | 82 | 60 | 86 | 0.98 | 0.13 |
| 9 | 11 | 535 | 67 | 45 | 89 | 0.98 | 0.12 | |
| Time up to 2 wk prior to first pathological sign | ||||||||
| 60 | 32 | 7 | 431 | 152 | 82 | 74 | 0.98 | 0.17 |
| 32 | 7 | 460 | 123 | 82 | 79 | 0.99 | 0.21 | |
| 80 | 29 | 10 | 464 | 119 | 74 | 80 | 0.98 | 0.20 |
| 27 | 12 | 483 | 100 | 69 | 83 | 0.98 | 0.21 | |
| 100 | 26 | 13 | 484 | 99 | 67 | 83 | 0.97 | 0.21 |
| 24 | 15 | 500 | 83 | 62 | 86 | 0.97 | 0.22 | |
| 120 | 17 | 22 | 495 | 88 | 44 | 85 | 0.96 | 0.16 |
| 15 | 23 | 510 | 74 | 39 | 87 | 0.96 | 0.17 | |
| 140 | 16 | 23 | 505 | 78 | 41 | 87 | 0.96 | 0.17 |
| 13 | 26 | 520 | 63 | 33 | 89 | 0.95 | 0.17 | |
| Time up to 4 wk prior to first pathological sign | ||||||||
| 60 | 37 | 16 | 422 | 147 | 70 | 74 | 0.96 | 0.20 |
| 37 | 16 | 451 | 118 | 70 | 79 | 0.97 | 0.24 | |
| 80 | 33 | 20 | 454 | 115 | 62 | 80 | 0.96 | 0.22 |
| 31 | 22 | 473 | 96 | 58 | 83 | 0.96 | 0.24 | |
| 100 | 30 | 23 | 474 | 95 | 57 | 83 | 0.95 | 0.24 |
| 28 | 25 | 490 | 79 | 53 | 86 | 0.95 | 0.26 | |
| 120 | 21 | 32 | 485 | 84 | 40 | 85 | 0.94 | 0.20 |
| 19 | 33 | 500 | 70 | 37 | 88 | 0.94 | 0.21 | |
| 140 | 20 | 33 | 495 | 74 | 38 | 87 | 0.94 | 0.21 |
| 17 | 36 | 510 | 59 | 32 | 90 | 0.93 | 0.22 | |
True-positive (TP) and false-negative (FN) results refer to all BDG values assessed at the time of the potential onset of IFD (e.g., four, two, and zero weeks prior to the first pathological sign, such as blood culture results or biopsy, pathological imaging, or galactomannan positivity); all subsequent levels of BDG were considered a response control for therapy and were not included in the analysis. The number of samples collected during the period of 0, 2, and 4 weeks prior to the first pathological sign were 20, 39, and 53, respectively. True-negative (TN) and false-positive (FP) results refer to all β-d-glucan (BDG) values assessed during episodes without indication of invasive fungal disease (IFD), e.g., during the entire screening period in patients without IFD and during the time period prior to the potential onset of IFD (e.g., four, two, and zero weeks prior to the first pathological sign, such as pathological imaging or galactomannan positivity). The number of samples collected during the period of 0, 2, and 4 weeks prior to the first pathological sign were 602, 583, and 569, respectively.
NPV, negative predictive value.
PPV, positive predictive value.
Examples of first pathological signs were culture results, finding on pathological imaging, or increased galactomannan (GM).
The first line of the results for a specific cutoff indicates the results for a single BDG level higher than the respective cutoff value, and the second line indicates the assay characteristics for two consecutive positive BDG values.
For BDG samples assessed in patients without IFD, linear regression analysis demonstrated no correlation between BDG levels and age (R2 = 0.0003; data not shown).
DISCUSSION
Clinical signs and symptoms of IFD are often nonspecific; hence, screening strategies with non-culture-based assays, such as BDG screening, can be useful for early diagnosis of infection (12). BDG is included as a mycological criterion in the revised definitions for IFD by the EORTC/MSG consensus group (4), and BDG testing has a BII recommendation for adults with hematological malignancies and HSCT recipients (13). Unfortunately, data on BDG levels in children are limited; therefore, current pediatric guidelines discourage the use of BDG screening for clinical decisions until further evidence in the pediatric age group has accumulated (9, 10). The results of the present study indicate that in the setting of children undergoing allogeneic HSCT, BDG screening has a high sensitivity and a good NPV but a very low PPV. Whereas no studies on BDG testing in pediatric HSCT recipients have been published to date, data on the assessment of BDG levels in children with hematological malignancies demonstrate highly variable findings on sensitivity, specificity, PPV, and NPV (14, 15).
The value of a diagnostic test can dramatically be affected by the cutoff value and by the definition of the onset of disease, which is particularly the case in IFD, in which the true onset of infection is rather poorly defined in many patients. There might be several febrile episodes due to IFD prior to the time of definite diagnosis, but, on the other hand, extending a true-positive episode of IFD and including prior febrile episodes might also severely bias sensitivity and specificity. In addition, the optimal cutoff for BDG levels is not well defined, particularly in children. Whereas uninfected children have higher BDG blood levels than those of healthy adults (16), our data indicate that BDG levels are age independent in children undergoing HSCT. When defining the onset of IFD between 2 and 4 weeks prior to the first pathological sign, such as pathological imaging, ROC analyses indicate an optimal cutoff value of 70 pg/ml for the BDG assay. This cutoff value is higher than the cutoff value of 60 pg/ml, which was suggested in a recent meta-analysis for adults (17), but on the other hand, it is lower than the cutoff of 80 pg/ml recommended by the manufacturer. Future studies in the pediatric age group have to validate the best cutoff value for BDG levels in the different settings.
We found that the NPV was relatively high (>0.92) for all the cutoff values and definitions of onset of disease we used in our analysis. In contrast, the low PPV, which did not exceed 0.26, was the major limitation of the test. Although we recognize that the low number of proven and probable IFD limits the value of our analysis and may ultimately skew the results, it is important to note that corroborating results were reported using BDG screening for adult patients receiving chemotherapy for cancer (18).
The usefulness of BDG screening for IFD diagnosis was especially limited by the high rate of false-positive results. There are a number of factors reported that may lead to false-positive results of the BDG assay, such as severe mucositis (19), the administration of albumin and immunoglobulins (20), thrombocyte infusion with leukocyte-removing filters, or the administration of antibiotics, such as amoxicillin-clavulanate or piperacillin-tazobactam (6). Although some authors argue that bacteremia is an improbable cause of a positive BDG assay (21), one recent study found a significant higher rate of false-positive BDG tests in bacteremic patients, independently of Gram-positive or Gram-negative bacteremia (6). Importantly, transient candidemia has been reported as a cause of persistent false-positive BDG levels (22), and mucosal colonization with Candida species may result in a higher BDG level than the recommended cutoff (23). Since many of these circumstances that potentially cause false-positive BDG levels are regularly seen in children undergoing allogeneic HSCT, the high rate of false-positive BDG levels in our study is not surprising. In fact, it was suggested that serum BDG is not useful for HSCT patients while receiving regular immunoglobulin replacement (24). Interestingly, both patients with persistent BDG levels of >100 pg/ml had repeatedly mucosal colonization with Candida spp., and one of them, suffering from bronchiectasis, also had streptococci in expectorated sputum samples at several occasions. Unfortunately, it is impossible to prove whether one of these factors caused false-positive BDG results in our population, since we did not measure BDG levels in the agents given prior to BDG sampling. However, we are currently planning a prospective study evaluating factors associated with false-positive BDG assay results. In contrast to data from adults receiving conventional chemotherapy for hematological malignancies, we did not find a higher rate of false-positive BDG levels during time of neutropenia (data not shown) (25, 26), which might be explained by the complex immunosuppression, particularly in HSCT recipients, which affects all arms of the immune system. Unfortunately, in contrast to other reports, the diagnostic performance of the BDG assay did not significantly improve for two consecutive positive BDG tests than for a single one (Table 2) (25, 27).
In conclusion, our data indicate that BDG screening in pediatric HSCT recipients is of limited usefulness, which is mainly due to the low PPV. Although the NPV is relatively high, the cost and the specific laboratory equipment required have to be taken into account. Last, one has to recognize that using the revised EORTC/MSG criteria, the high rate of false-positive BDG values might result in an overreporting of probable IFD in children undergoing allogeneic HSCT.
ACKNOWLEDGMENT
The BDG assays (Fungitell) were kindly provided by the Associates of Cape Cod, East Falmouth, MA, USA.
REFERENCES
- 1.Pagano L, Caira M, Nosari A, Van Lint MT, Candoni A, Offidani M, Aloisi T, Irrera G, Bonini A, Picardi M, Caramatti C, Invernizzi R, Mattei D, Melillo L, de Waure C, Reddiconto G, Fianchi L, Valentini CG, Girmenia C, Leone G, Aversa F. 2007. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study–Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect Dis 45:1161–1170. doi: 10.1086/522189. [DOI] [PubMed] [Google Scholar]
- 2.von Eiff M, Roos N, Schulten R, Hesse M, Zuhlsdorf M, van de Loo J. 1995. Pulmonary aspergillosis: early diagnosis improves survival. Respiration 62:341–347. doi: 10.1159/000196477. [DOI] [PubMed] [Google Scholar]
- 3.Caillot D, Casasnovas O, Bernard A, Couaillier JF, Durand C, Cuisenier B, Solary E, Piard F, Petrella T, Bonnin A, Couillault G, Dumas M, Guy H. 1997. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J Clin Oncol 15:139–147. [DOI] [PubMed] [Google Scholar]
- 4.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group, National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oz Y, Kiraz N. 2011. Diagnostic methods for fungal infections in pediatric patients: microbiological, serological and molecular methods. Expert Rev Anti Infect Ther 9:289–298. doi: 10.1586/eri.10.168. [DOI] [PubMed] [Google Scholar]
- 6.Sulahian A, Porcher R, Bergeron A, Touratier S, Raffoux E, Menotti J, Derouin F, Ribaud P. 2014. Use and limits of (1-3)-β-d-glucan assay (Fungitell), compared to galactomannan determination (Platelia Aspergillus), for diagnosis of invasive aspergillosis. J Clin Microbiol 52:2328–2333. doi: 10.1128/JCM.03567-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marr KA, Balajee SA, McLaughlin L, Tabouret M, Bentsen C, Walsh TJ. 2004. Detection of galactomannan antigenemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: variables that affect performance. J Infect Dis 190:641–649. doi: 10.1086/422009. [DOI] [PubMed] [Google Scholar]
- 8.Pfeiffer CD, Fine JP, Safdar N. 2006. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis 42:1417–1427. doi: 10.1086/503427. [DOI] [PubMed] [Google Scholar]
- 9.Lehrnbecher T, Phillips R, Alexander S, Alvaro F, Carlesse F, Fisher B, Hakim H, Santolaya M, Castagnola E, Davis BL, Dupuis LL, Gibson F, Groll AH, Gaur A, Gupta A, Kebudi R, Petrilli S, Steinbach WJ, Villarroel M, Zaoutis T, Sung L, International Pediatric Fever and Neutropenia Guideline Panel. 2012. Guideline for the management of fever and neutropenia in children with cancer and/or undergoing hematopoietic stem-cell transplantation. J Clin Oncol 30:4427–4438. doi: 10.1200/JCO.2012.42.7161. [DOI] [PubMed] [Google Scholar]
- 10.Groll AH, Castagnola E, Cesaro S, Dalle JH, Engelhard D, Hope W, Roilides E, Styczynski J, Warris A, Lehrnbecher T, Fourth European Conference on Infections in Leukaemia, Infectious Diseases Working Party of the European Group for Blood Marrow Transplantation (EBMT-IDWP), Infectious Diseases Group of the European Organisation for Research and Treatment of Cancer (EORTC-IDG), International Immunocompromised Host Society (ICHS), European Leukaemia Net (ELN). 2014. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. Lancet Oncol 15:e327–e340. doi: 10.1016/S1470-2045(14)70017-8. [DOI] [PubMed] [Google Scholar]
- 11.Duarte RF, Sanchez-Ortega I, Cuesta I, Arnan M, Patiño B, Fernandez de Sevilla A, Gudiol C, Ayats J, Cuenca-Estrella M. 2014. Serum galactomannan-based early detection of invasive aspergillosis in hematology patients receiving effective antimold prophylaxis. Clin Infect Dis 59:1696–1702. doi: 10.1093/cid/ciu673. [DOI] [PubMed] [Google Scholar]
- 12.Theel ES, Doern CD. 2013. β-d-Glucan testing is important for diagnosis of invasive fungal infections. J Clin Microbiol 51:3478–3483. doi: 10.1128/JCM.01737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchetti O, Lamoth F, Mikulska M, Viscoli C, Verweij P, Bretagne S, European Conference on Infections in Leukemia (ECIL) Laboratory Working Groups. 2012. ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant 47:846–854. doi: 10.1038/bmt.2011.178. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L, Tang JY, Wang Y, Zhou YF, Chen J, Li BR, Xue HL. 2009. Value of plasma β-Glucan in early diagnosis of invasive fungal infection in children. Zhongguo Dang Dai Er Ke Za Zhi 11:905–908. (In Chinese.) [PubMed] [Google Scholar]
- 15.Badiee P, Alborzi A, Karimi M, Pourabbas B, Haddadi P, Mardaneh J, Moieni M. 2012. Diagnostic potential of nested PCR, galactomannan EIA, and beta-d-glucan for invasive aspergillosis in pediatric patients. J Infect Dev Ctries 6:352–357. [DOI] [PubMed] [Google Scholar]
- 16.Smith PB, Benjamin DK Jr, Alexander BD, Johnson MD, Finkelman MA, Steinbach WJ. 2007. Quantification of 1,3-beta-d-glucan levels in children: preliminary data for diagnostic use of the beta-glucan assay in a pediatric setting. Clin Vaccine Immunol 14:924–925. doi: 10.1128/CVI.00025-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He S, Hang JP, Zhang L, Wang F, Zhang DC, Gong FH. 28 July 2014. A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-beta-d-glucan for invasive fungal infection: focus on cutoff levels. J Microbiol Immunol Infect doi: 10.1016/j.jmii.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Racil Z, Kocmanova I, Lengerova M, Weinbergerova B, Buresova L, Toskova M, Winterova J, Timilsina S, Rodriguez I, Mayer J. 2010. Difficulties in using 1,3-{beta}-d-glucan as the screening test for the early diagnosis of invasive fungal infections in patients with haematological malignancies–high frequency of false-positive results and their analysis. J Med Microbiol 59:1016–1022. doi: 10.1099/jmm.0.019299-0. [DOI] [PubMed] [Google Scholar]
- 19.Ellis M, Al-Ramadi B, Finkelman M, Hedstrom U, Kristensen J, Ali-Zadeh H, Klingspor L. 2008. Assessment of the clinical utility of serial beta-d-glucan concentrations in patients with persistent neutropenic fever. J Med Microbiol 57:287–295. doi: 10.1099/jmm.0.47479-0. [DOI] [PubMed] [Google Scholar]
- 20.Koo S, Bryar JM, Page JH, Baden LR, Marty FM. 2009. Diagnostic performance of the (1→3)-beta-d-glucan assay for invasive fungal disease. Clin Infect Dis 49:1650–1659. doi: 10.1086/647942. [DOI] [PubMed] [Google Scholar]
- 21.Furfaro E, Mikulska M, Del Bono V, Guolo F, Minetto P, Gobbi M, Ghiso A, Bacigalupo A, Viscoli C. 2014. Bloodstream infections are an improbable cause of positive serum (1,3)-beta-d-glucan in hematology patients. Clin Vaccine Immunol 21:1357–1359. doi: 10.1128/CVI.00214-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naselli A, Faraci M, Lanino E, Morreale G, Cangemi G, Bandettini R, Castagnola E. 2015. Persistence of high-level (1,3)-β-d-glucan after candidemia following autologous peripheral SCT in a pediatric patient. Bone Marrow Transplant 50:137–138. doi: 10.1038/bmt.2014.206. [DOI] [PubMed] [Google Scholar]
- 23.Mokaddas E, Burhamah MH, Khan ZU, Ahmad S. 2010. Levels of (1→3)-β-d-glucan, Candida mannan and Candida DNA in serum samples of pediatric cancer patients colonized with Candida species. BMC Infect Dis 10:292. doi: 10.1186/1471-2334-10-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffner U, Abdel-Mageed A, Dahl K, Fogg G, Hester J. 2012. Serum (1→3)-beta-d-glucan levels (Fungitell assay) is not useful as a screening test for recipients of an allogeneic HSCT while on immunoglobulin replacement. Bone Marrow Transplant 47:151–152. doi: 10.1038/bmt.2011.24. [DOI] [PubMed] [Google Scholar]
- 25.Odabasi Z, Mattiuzzi G, Estey E, Kantarjian H, Saeki F, Ridge RJ, Ketchum PA, Finkelman MA, Rex JH, Ostrosky-Zeichner L. 2004. Beta-d-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis 39:199–205. doi: 10.1086/421944. [DOI] [PubMed] [Google Scholar]
- 26.Kami M, Tanaka Y, Kanda Y, Ogawa S, Masumoto T, Ohtomo K, Matsumura T, Saito T, Machida U, Kashima T, Hirai H. 2000. Computed tomographic scan of the chest, latex agglutination test and plasma (1AE3)-β-d-glucan assay in early diagnosis of invasive pulmonary aspergillosis: a prospective study of 215 patients. Haematologica 85:745–752. [PubMed] [Google Scholar]
- 27.Lamoth F, Cruciani M, Mengoli C, Castagnola E, Lortholary O, Richardson M, Marchetti O, Third European Conference on Infections in Leukemia (ECIL-3). 2012. β-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin Infect Dis 54:633–643. doi: 10.1093/cid/cir897. [DOI] [PubMed] [Google Scholar]


