Abstract
Maslinic acid (MA) is a natural compound whose structure corresponds to a pentacyclic triterpene. It is abundant in the cuticular lipid layer of olives. MA has many biological and therapeutic properties related to health, including antitumor, anti-inflammatory, antimicrobial, antiparasitic, antihypertensive, and antioxidant activities. However, no studies have been performed to understand the molecular mechanism induced by this compound in melanoma cancer. The objective of this study was to examine the effect of MA in melanoma (B16F10) cells grown in the presence or absence of fetal bovine serum (FBS). We performed cell proliferation measurements, and the reactive oxygen species (ROS) measurements using dihydrorhodamine 123 (DHR 123) and activities of catalase, glucose 6-phosphate dehydrogenase, glutathione S-transferase, and superoxide dismutase. These changes were corroborated by expression assays. FBS absence reduced cell viability decreasing IC50 values of MA. The DHR 123 data showed an increase in the ROS level in the absence of FBS. Furthermore, MA had an antioxidant effect at lower assayed levels measured as DHR and antioxidant defense. However, at higher dosages MA induced cellular damage by apoptosis as seen in the results obtained.
1. Introduction
Olea europaea L. is an evergreen tree widely distributed in the Mediterranean countries, where they cover 8 million ha, accounting for almost 98% of the world crop [1]. It is studied for its alimentary use (the fruits and the oil are important components in the daily diet of a large part of the world's population), whereas the leaves and seeds are important for their secondary metabolites such as terpenes group.
Maslinic acid (MA), a natural pentacyclic triterpene, has attracted much interest due to its proven pharmacologic safety and its many biological activities, such as antiviral [2], antidiabetogenic [3], anti-inflammatory [4], and antimicrobial [5] functions. More recently, some studies have shown that MA has anticancer property in different types of cancer [6–12]. Moreover, MA inhibits glycogen phosphorylase in rat liver and muscle [13–15], decreases glucose in diabetogenic mouse [16], and stimulates healthy whole animal and tissue growth [17–21]. Although a recent study showed that MA induces apoptosis in several cancer cells no such effect has been reported in melanoma cells. In particular, the involvement of MA-mediated reactive oxygen species (ROS) production in apoptotic signaling in B16F10 melanoma cells remains unknown. Oxidative stress refers to a cell's state characterized by excessive production of ROS and is one of the most important regulatory mechanisms for cancer [22].
To protect from high ROS levels, living organisms possess an enzymatic antioxidant that scavenges them. In a normal situation, ROS can be detrimental when produced in high amounts in the intracellular compartments and cells generally respond to ROS by upregulating antioxidants such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione S-transferase (GST) that protect them by converting dangerous free radicals to harmless molecules. Glucose-6-phosphate dehydrogenase (G6PDH) is also involved in the antioxidant mechanism as it produces NADPH which is a direct scavenger of free radicals [23]. ROS and cellular oxidant stress have long been associated with cancer processes [24, 25]. In cancer cells, oxidative stress has been linked to the regulation of numerous cellular processes including DNA damage, proliferation, cellular adhesion, and migration and the regulation of cell survival or death [26].
Previous studies have shown that ROS induce depolarization of the mitochondrial-membrane potential as well as release of cytochrome c from the mitochondria into the cytosol. This increase triggers the activation of caspase-9 and initiates the caspase cascade, which induces apoptosis in tumor cells [6, 7, 27]. Recently, many reports have shown that components from plants such as celastrol [28, 29] and jacaranone [30] induce apoptosis of melanoma cells through production of ROS.
The aim of this study is to observe the effect of MA in the absence of fetal bovine serum (FBS) in B16F10 melanoma cells by analyzing the ROS production and the activity and expression of the main antioxidants enzymes considering that the absence of FBS during growth causes a clear situation of cellular stress.
2. Materials and Methods
2.1. Drug
MA was kindly provided by Biomaslinic S.L. Its molecular weight is 472.7 g/mol. Figure 1 shows the chemical structure of MA ((2α,3β)-2,3-dihydroxyolean-12-en-28-oic acid). The extract used is a chemically pure white powder comprising 98% MA and is stable when stored at 4°C. MA was dissolved before use at 10 mg/mL in 50% DMSO and 50% PBS. Stock solution was frozen and stored at −20°C. For treatments, this solution was diluted in cell culture medium.
Figure 1.
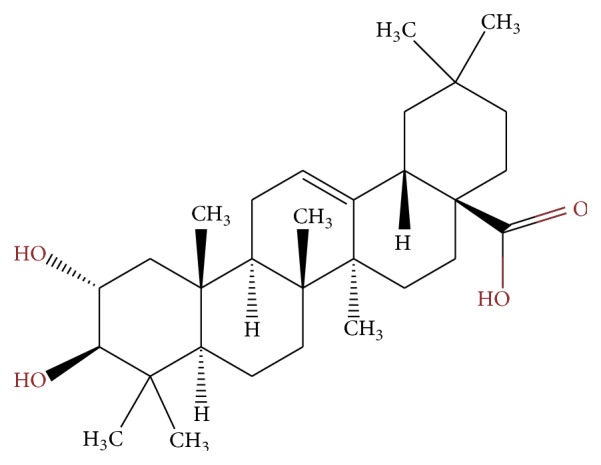
The chemical structure of maslinic acid (MA).
2.2. Cell Line and Experimental Conditions
B16F10 cells were cultured in high-glucose DMEM supplemented with 2 mM glutamine, 10% heat-inactivated FBS, 10,000 units/mL penicillin, and 10 mg/mL streptomycin. The cell line was maintained in a humidified atmosphere with 5% CO2 at 37°C. Cells were passaged at preconfluent densities by the use of a solution containing 0.05% trypsin and 0.5 mM EDTA. The B16F10 cells were seeded in the culture dishes at the desired density. After 24 h hours, when the cells are attached to the dish, the dishes were incubated with DMEM containing 0% FBS or 10% FBS for 24 h under the conditions described for cell culture. Following that, cells were incubated with MA.
2.3. Cell Proliferation
The assay was performed by a variation of the method described by Mosmann [31]. Samples containing 200 μL cell suspension (1.5 · 103 cells/well) were cultured in 96-well plates. Subsequent to the adherence of the cells within 24 h of incubation with and without FBS at 37°C, different MA dilutions on a scale of 10 μg/mL to 100 μg/mL were added separately. Following incubation for 24 h at 37°C in a humidified incubator with 5% CO2, MTT dissolved in PBS and medium at 5 mg/mL and sterile-filtered was added to all the wells at a final concentration of 0.5 mg/mL. Following 2 h incubation, the generated formazan was dissolved with 100 μL DMSO per well. The optical density was measured on an ELISA plate reader (ELISA, ELx800, Bio-Tek) at 550 nm. Absorbance was proportional to the number of cells. The concentrations that caused 50% of inhibition of cell growth (IC50) were calculated.
2.4. Protein Extraction
For sample preparation, cells at 70% confluence were incubated for 24 h without FBS and subsequently were incubated with IC50, IC50/2, and IC50/8 concentrations for 24 h. Following this, the cells were washed three times with PBS, scraped off with a cell scraper (Renner), and collected in 0.5 mL RIPA buffer. Immediately, cells were sonicated on ice for 5 min and maintained by moderate shaking at 4°C for 1 h. Every 15 min, the samples were moderately shaken in a vortex. The lysates were spun in a centrifuge at 10,000 g at 4°C for 15 min. The supernatants were used for enzyme activity and Western blot assays, and the protein concentrations were measured by BCA Protein Assay (Thermo Scientific, USA). For each experimental group, 2 replicates of the homogenates were made. Each replicate was made with 3 different cell populations.
2.5. Mitochondrial-Membrane Potential by DHR 123
Changes in the mitochondrial-membrane potential can be examined by monitoring the cell fluorescence after double staining with rhodamine 123 (Rh123) and propidium iodide (PI). Rh123 is a membrane-permeable fluorescent cationic dye that is selectively taken up by mitochondria directly proportional to the MMP (mitochondrial-membrane permeabilization). Around 4 × 105 cells/well were placed on 6-well plates with 2 mL of medium without FBS and treated with cytotoxic compounds for 24 h at IC50, IC50/2, and IC50/8 concentrations. Following the treatment, the medium was removed and a fresh medium with DHR, at a final concentration of 5 μg/mL, was added. After 30 min of incubation, the medium was removed and the cells were washed and resuspended in PBS with 5 μg/mL of PI. The intensity of fluorescence from Rh123 and PI was determined using an ACS flow cytometer (Coulter Corporation, Hialeah, FL, USA), at the excitation and emission wavelengths of 500 nm and 536 nm, respectively.
2.6. Enzyme Assays
All enzyme assays were carried out at 25°C using a Power Wave X microplate scanning spectrophotometer (Bio-Tek Instruments, USA) and run in duplicate in 96-well microplates. This made it necessary to adapt all the enzymatic methods described below to the microplate reader to obtain optimal activities. Adaptation of the methods was done by scaling down the reaction mixtures to a final volume of 200 μL and by adjusting both the total time for which reactions were allowed to proceed and the measurement intervals. In addition, the optimal substrate and protein concentrations for the measurement of maximal activity for each enzyme were established by preliminary assays.
The enzymatic reactions were initiated by addition of the tissue extract, except for SOD where xanthine oxidase was used. The millimolar extinction coefficients used for H2O2, NADH/NADPH, and DTNB [5,5-dithiobis (2-nitrobenzoic acid)] were 0.039, 6.22, and 13.6, respectively. The assay conditions were as follows.
Superoxide dismutase (SOD; EC 1.15.1.1) activity was measured by the ferricytochrome c method using xanthine/xanthine oxidase as the source of superoxide radicals. The reaction mixture consisted of 50 mM potassium phosphate buffer (pH 7.8), 0.1 mM EDTA, 0.1 mM xanthine, 0.013 mM cytochrome c, and 0.024 IU/mL xanthine oxidase. Activity is reported in units of SOD per milligram of protein. One unit of activity was defined as the amount of enzyme necessary to produce a 50% inhibition of the ferricytochrome c reduction rate [32].
Catalase (CAT; EC 1.11.1.6) activity was determined by measuring the decrease of hydrogen peroxide concentration at 240 nm according to Aebi [33]. The reaction mixture contained 50 mm potassium phosphate buffer (pH 7.0) and 10.6 mM of freshly prepared H2O2.
Glucose-6-phosphate dehydrogenase (G6PDH; EC 1.1.1.49) activity was determined at pH 7.6 in a medium containing 50 mM Hepes buffer, 2 mM MgCl2, 0.8 mM NADP+ and glucose 6-phosphate were used as substrate. The enzyme activity was determined by measuring the reduction of NADP+ at 340 nm as previously described by Lupiañez et al. [34] and Peragón et al. [35]. The change in absorbance at 340 nm was recorded and, after confirmation of no exogenous activity, the reaction started by the addition of substrate.
Glutathione S-transferase (GST; EC 2.5.1.18) activity was measured according to the method described by Habig et al. [36], using 1-chloro-2,4-dinitrobenzene as a substrate.
2.7. Western Blot Analysis
Polyacrylamide gel electrophoresis under denaturing conditions (SDS-PAGE) was performed in a Mini-Protean II electrophoresis system (Bio-Rad, Richmond, USA). The cell extract were mixed with a charge buffer that contained 62.5 mM Tris-HCl at pH 6.8, 20 g L−1 SDS, 100 mL L−1 glycerol, 25 g L−1 β-mercaptoethanol and 0.045 mM bromophenol blue and then heated for 5 min at 100°C. Polypeptides were separated on a 12% SDS-PAGE and subsequently transferred to polyvinylidene fluoride membranes with a semidry electroblotting system at 1.5 mA/cm2 for 45 min in a medium containing 25 mM Tris-HCl, 192 mM glycine, 200 mL L−1 methanol, and 1 g L−1 SDS. Blots were blocked for 2 h at room temperature with a Tris buffered solution (TBS) that contained 25 mM Tris-HCl, 100 mM NaCl, 2.5 mM KCl, pH 7.6, 1 mL L−1 Tween 20, and 15 g L−1 bovine serum albumin (BSA) at pH 7.6. Membranes were washed with TBS containing 1 mL L−1 Tween 20 (TBS-T) for 15 min and later incubated with specific primary antibodies: anti-glucose 6-phosphate dehydrogenase (G6PDH) 1 : 1,000 (Sigma, A9521), anti-SOD 1 : 500 (Santa Cruz Biotechnology, sc-101523), anti-GST 1 : 1,000 (Santa Cruz Biotechnology, sc-374171), anti-CAT 1 : 1,000 (Santa Cruz Biotechnology, sc-34285), anti-G6PDH 1 : 5,000 (Sigma, A9521), and anti-α-actin 1 : 1,000 (Sigma, A2668). Following three washes with TBS-T containing 10 g L−1 BSA (TBS-T-BSA) for 10 min, membranes were incubated with a HRP conjugated goat anti-rabbit antibody IgG or anti-mouse IgG (1 : 10,000). Blots were developed using the ECL-Plus Western blot detection system (GE Healthcare). The specific signals were exposed on medical film (Konica Minolta). Films were scanned with a Hewlett-Packard scanner and quantified using Multi-Gauge program (Fuji Film Europe).
2.8. Statistical Analysis
Data are shown as mean ± the standard deviation (SD). The statistical significance of differential findings between non-FBS group and control was determined by Student's t-test. One-way ANOVA test was performed to determine the significance of the differences between MA concentrations. SPSS version 15.0 for Windows software package was used for statistical analysis. P values smaller than 0.05 were considered statistically significant.
3. Results and Discussion
3.1. FBS Deprivation and Cellular Growth
We examined the effect of MA on the proliferation of B16F10 melanoma cell lines using MTT assay under the presence and absence of FBS. Several groups have studied the effects of the absence of FBS in cancer cells. However, their focus has not been on the variation of cytotoxicity of a compound but on the synchronization of the cells in a particular phase of cell cycle as it was reported that the deprivation of FBS allows arrest of cell cultures into G0/G1 cell cycle stage [37]. It has been shown that lower concentrations of FBS (0.5% and 0.75%) caused a significant decrease in cellular quantity and this condition could induce DNA fragmentation and subsequent cell death [38]. Moreover, several studies have been focused on improving the composition of FBS in order to enhance the culture cell growth; in this sense, FBS dose dependent studies have been performed [39].
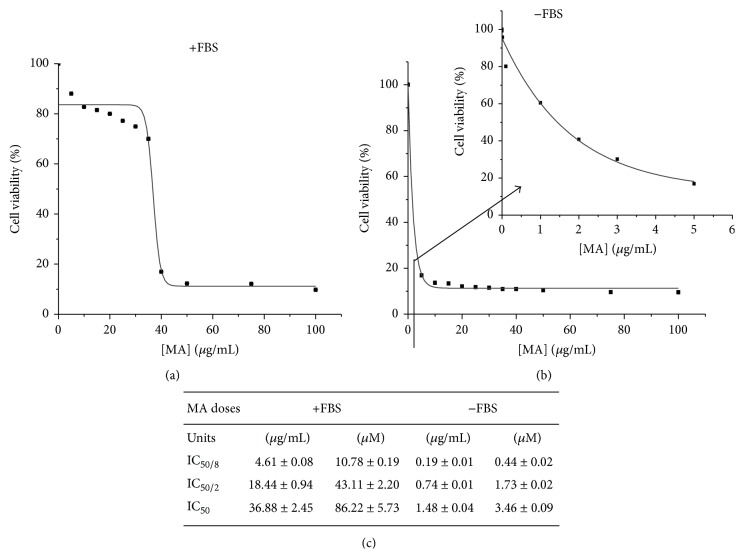
Percentage of living cells (viable formazan accumulating cells) decreased in a dose dependent manner in the presence and absence of FBS. We noticed that 50% growth inhibition values (IC50) in response to MA were different in media without FBS, compared to media supplemented with FBS; therefore, MA has an increased cytotoxic effect when cells do not have FBS. These results are reported in Figure 2.
Figure 2.
The effect of MA on B16F10 murine melanoma cell viability in the presence of FBS (a) and in the absence of FBS (b). MA cytotoxic doses are shown in (c). Cell proliferation was determined by MTT assay. Values are expressed as means ± SD.
MA dose, which reaches IC50 value in the culture medium supplemented with 10% of FBS, was 36.88 μg/mL (86 μM) and 1.48 μg/mL (3.5 μM) in a medium with 0% of FBS. These results indicate that MA has a cytotoxic effect with FBS in the cell culture, but this effect is higher in its absence. Our explanation of this is that absence of FBS modifies the cell homeostasis causing an increase of free radicals and therefore, MA presents a higher cytotoxic effect in the absence of FBS. An imbalance between free radicals and antioxidants results in a condition known as oxidative stress, which leads to metabolic malfunctions and damage to biological macromolecules [40].
MA cytotoxicity has been studied by other groups in several types of cancer cells and it has been shown that the MA quantity has a variable effect on the function of the type of cells and experimental conditions. For example, in HT29 cells the MA quantity that results in growth inhibition of 50% of the population is 30 μM [8, 10] and in Caco-2 the IC50 of MA is 10.82 μM [7], whereas in bladder cancer the IC50 value changed according to the bladder type cell lines, the values being between 20 and 300 μM [41].
3.2. Maslinic Acid Effect on Mitochondrial-Membrane Potential
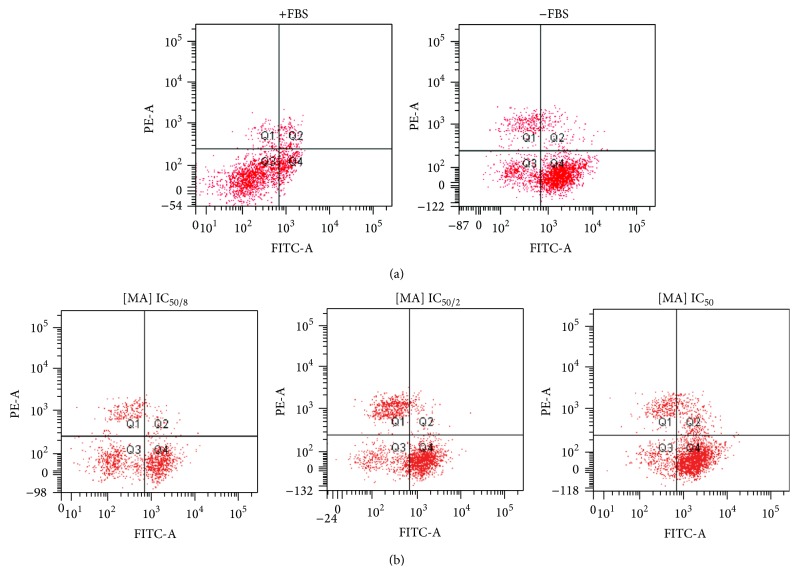
DHR 123 is a ROS assay in which this compound can be oxidized intracellularly by ROS to form the positively charged fluorescent Rh123 [42] and is accumulated into the mitochondria [43]. We have performed an assay of ROS production by DHR in the absence of FBS and using three different MA levels (IC50/8, IC50/2, and IC50) based on our cytotoxicity results. Firstly, we have shown that the absence of FBS produced an increase in ROS production due to mitochondrial disruption (Figure 3(a)). FBS composition includes proteins, growth factors, hormones, and ions [44, 45]. Cell culture without FBS forces the cell to synthesize all required compounds necessary for its normal metabolic activity. This fact involves an increase in the cellular metabolism which is reflected in a higher cellular mitochondrial activity, indirectly determined by DHR 123 assay as shown in quadrant 4 (Q4).
Figure 3.
Positive fluorescent Rh123 on B16F10 cells with or without FBS (a) and after MA treatment at different dosages without FBS (b).
Our results indicated that ROS level decreased at lower concentration of MA used (IC50/8). Increasing MA induced a direct increase in ROS level; hence, a dose dependent response was observed (Figure 3(b)). Among other properties, MA has been reported as a natural antioxidant [46–49]. In this context, the decrease in ROS level observed at MA at IC50/8 concentration could be attributed to its antioxidant property. On the other hand, MA induces apoptosis by activating intrinsic pathways that increase cellular ROS production [4, 48–52]. Mitochondria are the primary cellular site of ROS production and, under certain conditions, elevated mitochondrial ROS levels can serve as proapoptotic signals in cancer cells; as a result, drugs that induce ROS are receiving greater attention for their potential as chemotherapeutic agents [53]. In our previous studies, we have shown that MA induces apoptosis in cancer colon cells [10, 13, 54].
In the present study, the observed results on ROS production at higher MA levels are not just probably due to the absence of the antioxidant effect but due to a combination of antioxidant and apoptotic properties of MA when cells are cultured without FBS. Therefore, the increased MA levels are related to the higher apoptotic capacity of triterpene as shown by the higher ROS levels determined by DHR 123 assay (Figure 3).
3.3. Antioxidant Enzymatic Response
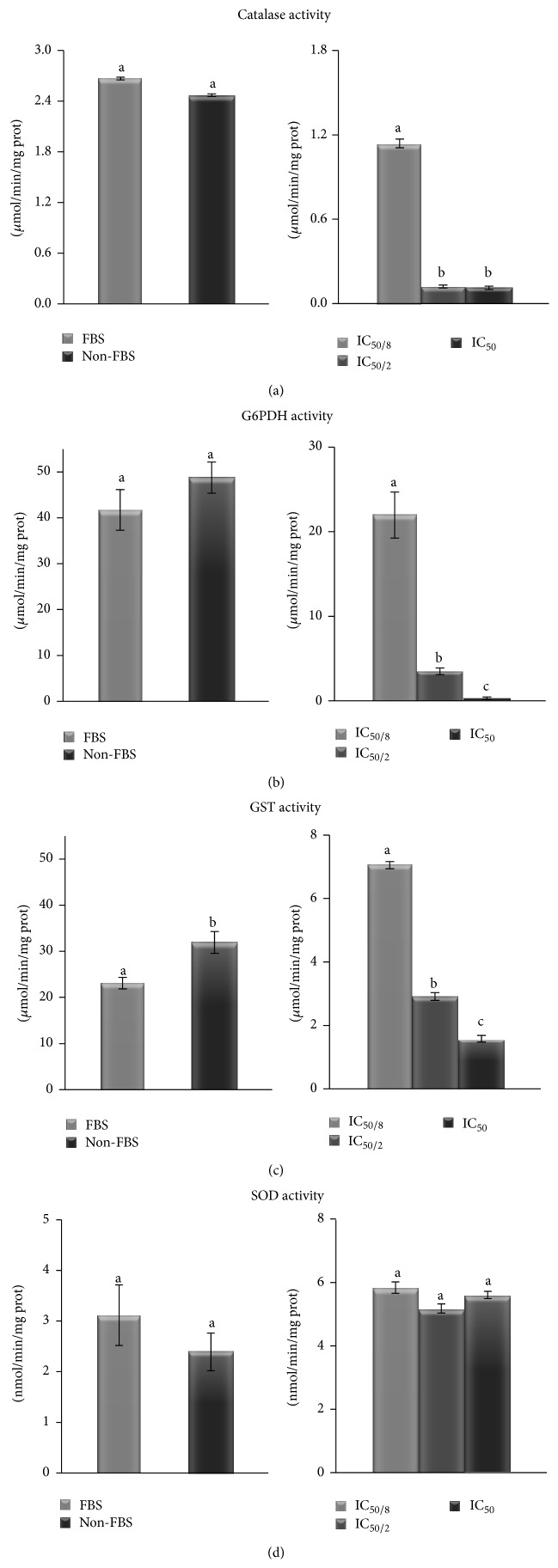
An evaluation of the activities of the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), glucose 6-phosphate dehydrogenase (G6PDH), and glutathione-S-transferase (GST) was performed under our experimental conditions (Figure 4). The results indicated that CAT, G6PDH, and SOD showed no change in their activity in the absence of FBS compared to the control with FBS, and only GST activity increased in the absence of FBS with respect to control in the presence of FBS, since this enzyme is related to detoxification of lipid peroxidation products, which are being generated under the assayed conditions.
Figure 4.
CAT (a), G6PDH (b), GST (c), and SOD (d) specific activities on B16F10 cells. On the left side, cells cultivated with and without FBS, and on the right side, cell cultivated without FBS and different MA dosages. Values are expressed as means ± SD. Different letters indicate significant differences (P < 0.05).
When MA is added to the cell culture, CAT, G6PDH, and GST activities decreased below controls data. This effect was observed at all doses of MA used and in a dose dependent response pattern. However, SOD activity in the presence of MA was not affected. This variability in responses of different antioxidant enzymes may be due to linked factor combination. As previously indicated, MA induces apoptosis at higher doses. Apoptosis mechanism involves biochemical changes in the cells to achieve death. The changes observed in the first step of apoptosis imply homeostatic cellular disruption.
These alterations inhibit biosynthesis of new molecules and damaged metabolites renewal, a fact related to the low G6PDH activity observed. G6PDH is the main enzyme to produce NADPH which is essential for reductive biosynthesis and nucleic acid synthesis [55] and protects the cell against oxidants [56]. Among other biological properties of NADPH, it has been demonstrated that it protects CAT from inactivation [57–59] and plays a crucial role in maintaining the redox state of the cell through GSH regeneration from GSSG. Low NADPH levels due to the imbalance in the cellular homeostasis could be, partially, responsible for the low activity levels observed in CAT and GST enzyme activities. All these results are in accordance with DHR 123 assay.
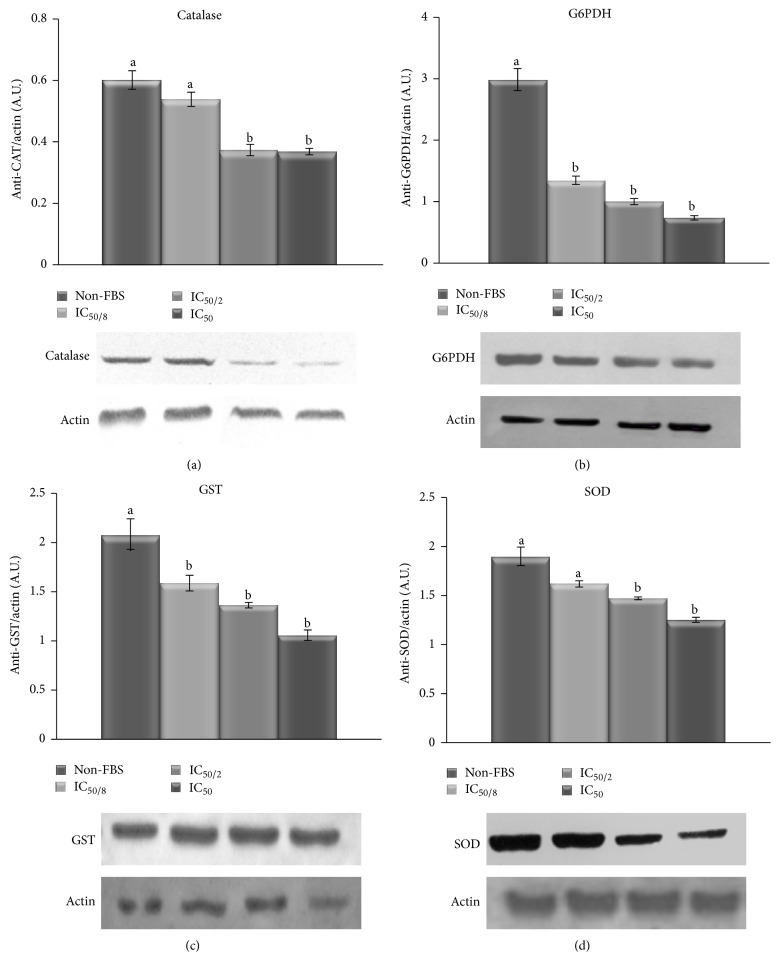
3.4. Validation by Western Blot
To corroborate the effect of different MA levels in cells without FBS in the antioxidant enzymes measured, an immune-blotting analysis was carried out to confirm the differential expression of same specific enzymes: CAT, G6PDH, GST, and SOD (Figure 5), under the same experimental condition (MA levels without FBS). Results obtained of immune-blotting analysis agree with those in the enzyme analysis, tending to decrease their activity and expression as MA concentration increases without FBS, although this pattern is not statistically significant in all enzymes.
Figure 5.
Western blots of CAT (a), G6PDH (b), GST (c), and SOD (d) on B16F10 cells cultivated without FBS at different MA dosages. The levels of specific protein expression are shown as arbitrary intensity units of each band compared to arbitrary intensity units of actin. Values are expressed as means ± SD. Different letters indicate significant differences (P < 0.05).
4. Conclusions
It has been described in the scientific literature that triterpenes and polyphenols, in physiological doses, are able to improve the intrinsic cell tolerance against oxidative stress by increasing the antioxidant potential and modulation related to proliferation/survival in cell cultures and experimentation animals. However, at supraphysiological doses, these compounds appear to induce programmed death via the activation of signals involved in apoptosis and inhibition of proliferation proteins associated with survival/cell death. This indicates that the experimental conditions (concentrations, culture conditions, cell type, duration of the treatment, etc.) have to be seriously considered as they may determine the biological activity of these natural compounds. As it is difficult to predict their effect, there is a need to understand their molecular mechanisms of action in each context. Results obtained in this paper must be considered in these experimental conditions.
Acknowledgments
This study has been supported, in part, by funds of the consolidated Research Group BIO-157, from the General Secretariat of Universities, Research and Technology of the Ministry of Economy, Innovation, Science and Employment Government of the Junta de Andalucía (Spain), and by the Research Contract no. C-3650-00 under the program FEDER-INNTERCONECTA from the Spanish Government and European Union FEDER funds. Amalia Pérez-Jiménez is a recipient of a postdoctoral research fellowship Torres-Quevedo no. PTQ 12-05739.
Conflict of Interests
Amalia Pérez-Jiménez declares having a research contract with the company Biomaslinic S.L., which owns part of the rights of international patents related to the extraction and exploitation of maslinic acid and hydroxytyrosol from different natural sources. The rest of the authors declare that there is no conflict of interests to disclose.
Authors' Contribution
Eva E. Rufino-Palomares, Fernando J. Reyes-Zurita, and José A. Lupiáñez were responsible for conception and design of the work. Eva E. Rufino-Palomares, Khalida Mokhtari, Celeny Figuera, Amalia Pérez-Jiménez, Fernando J. Reyes-Zurita, Leticia García-Salguero, Pedro P. Medina, Juan Peragón, and José A. Lupiáñez performed data acquisition and compilation. Eva E. Rufino-Palomares, Khalida Mokhtari, Celeny Figuera, and Pedro P. Medina performed cell viability experiments. Eva E. Rufino-Palomares, Khalida Mokhtari, Celeny Figuera, and Leticia García-Salguero were responsible for rhodamine 123 fluorescence. Eva E. Rufino-Palomares, Khalida Mokhtari, Celeny Figuera, Amalia Pérez-Jiménez, Leticia García-Salguero, and Fernando J. Reyes-Zurita were responsible for enzymes activities. Eva E. Rufino-Palomares, Khalida Mokhtari, Celeny Figuera, Amalia Pérez-Jiménez, Leticia García-Salguero, Fernando J. Reyes-Zurita, Pedro P. Medina, and Juan Peragón performed Western blotting assays. Eva E. Rufino-Palomares, Amalia Pérez-Jiménez, Fernando J. Reyes-Zurita, Leticia García-Salguero, Khalida Mokhtari, Pedro P. Medina, Juan Peragón, and José A. Lupiáñez wrote and revised the paper. Eva E. Rufino-Palomares, Amalia Pérez-Jiménez, and José A. Lupiáñez were responsible for general coordination of writing. All authors have read and approved the final version of the paper. Khalida Mokhtari and Eva E. Rufino-Palomares have contributed equally to this work.
References
- 1.Pereira A. P., Ferreira I. C. F. R., Marcelino F., et al. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules. 2007;12(5):1153–1162. doi: 10.3390/12051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu H.-X., Zeng F.-Q., Wan M., Sim K.-Y. Anti-HIV triterpene acids from Geum japonicum . Journal of Natural Products. 1996;59(7):643–645. doi: 10.1021/np960165e. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Rodriguez R., Perona J. S., Herrera M. D., Ruiz-Gutierrez V. Triterpenic compounds from ‘Orujo’ olive oil elicit vasorelaxation in aorta from spontaneously hypertensive rats. Journal of Agricultural and Food Chemistry. 2006;54(6):2096–2102. doi: 10.1021/jf0528512. [DOI] [PubMed] [Google Scholar]
- 4.Aladedunye F. A., Okorie D. A., Ighodaro O. M. Anti-inflammatory and antioxidant activities and constituents of Platostoma africanum P. Beauv. Natural Product Research. 2008;22(12):1067–1073. doi: 10.1080/14786410802264004. [DOI] [PubMed] [Google Scholar]
- 5.Braca A., Morelli I., Méndez J., Battinelli L., Braghiroli L., Mazzanti G. Antimicrobial triterpenoids from Licania heteromorpha . Planta Medica. 2000;66(8):768–769. doi: 10.1055/s-2000-9601. [DOI] [PubMed] [Google Scholar]
- 6.Parra A., Martin-Fonseca S., Rivas F., et al. Solid-phase library synthesis of bi-functional derivatives of oleanolic and maslinic acids and their cytotoxicity on three cancer cell lines. ACS Combinatorial Science. 2014;16(8):428–447. doi: 10.1021/co500051z. [DOI] [PubMed] [Google Scholar]
- 7.Reyes-Zurita F. J., Rufino-Palomares E. E., Medina P. P., et al. Antitumour activity on extrinsic apoptotic targets of the triterpenoid maslinic acid in p53-deficient Caco-2 adenocarcinoma cells. Biochimie. 2013;95(11):2157–2167. doi: 10.1016/j.biochi.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Rufino-Palomares E. E., Reyes-Zurita F. J., García-Salguero L., et al. Maslinic acid, a triterpenic anti-tumoural agent, interferes with cytoskeleton protein expression in HT29 human colon-cancer cells. Journal of Proteomics. 2013;83:15–25. doi: 10.1016/j.jprot.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Tena S., Reyes-Zurita F. J., Díaz-Moralli S., et al. Maslinic acid-enriched diet decreases intestinal tumorigenesis in ApcMin/+ mice through transcriptomic and metabolomic reprogramming. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0059392.e59392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyes-Zurita F. J., Rufino-Palomares E. E., Lupiáñez J. A., Cascante M. Maslinic acid, a natural triterpene from Olea europaea L., induces apoptosis in HT29 human colon-cancer cells via the mitochondrial apoptotic pathway. Cancer Letters. 2009;273(1):44–54. doi: 10.1016/j.canlet.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 11.Martín R., Carvalho J., Ibeas E., Hernández M., Ruiz-Gutierrez V., Nieto M. L. Acidic triterpenes compromise growth and survival of astrocytoma cell lines by regulating reactive oxygen species accumulation. Cancer Research. 2007;67(8):3741–3751. doi: 10.1158/0008-5472.CAN-06-4759. [DOI] [PubMed] [Google Scholar]
- 12.Reyes F. J., Centelles J. J., Lupiáñez J. A., Cascante M. (2α,3β)-2,3-Dihydroxyolean-12-en-28-oic acid, a new natural triterpene from Olea europea, induces caspase dependent apoptosis selectively in colon adenocarcinoma cells. FEBS Letters. 2006;580(27):6302–6310. doi: 10.1016/j.febslet.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 13.Wen X., Sun H., Liu J., et al. Naturally occurring pentacyclic triterpenes as inhibitors of glycogen phosphorylase: synthesis, structure-activity relationships, and X-ray crystallographic studies. Journal of Medicinal Chemistry. 2008;51(12):3540–3554. doi: 10.1021/jm8000949. [DOI] [PubMed] [Google Scholar]
- 14.Wen X., Sun H., Liu J., et al. Pentacyclic triterpenes. Part 1: the first examples of naturally occurring pentacyclic triterpenes as a new class of inhibitors of glycogen phosphorylases. Bioorganic & Medicinal Chemistry Letters. 2005;15(22):4944–4948. doi: 10.1016/j.bmcl.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Wen X., Zhang P., Liu J., et al. Pentacyclic triterpenes. Part 2. Synthesis and biological evaluation of maslinic acid derivatives as glycogen phosphorylase inhibitors. Bioorganic and Medicinal Chemistry Letters. 2006;16(3):722–726. doi: 10.1016/j.bmcl.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Liu J., Sun H., Duan W., Mu D., Zhang L. Maslinic acid reduces blood glucose in KK-Ay mice. Biological & Pharmaceutical Bulletin. 2007;30(11):2075–2078. doi: 10.1248/bpb.30.2075. [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Navarro M., Peragón J., Amores V., de la Higuera M., Lupiáñez J. A. Maslinic acid added to the diet increases growth and protein-turnover rates in the white muscle of rainbow trout (Oncorhynchus mykiss) Comparative Biochemistry and Physiology C: Toxicology and Pharmacology. 2008;147(2):158–167. doi: 10.1016/j.cbpc.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Fernández-Navarro M., Peragón J., Esteban F. J., de la Higuera M., Lupiáñez J. A. Maslinic acid as a feed additive to stimulate growth and hepatic protein-turnover rates in rainbow trout (Onchorhynchus mykiss) Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2006;144(2):130–140. doi: 10.1016/j.cbpc.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Rufino-Palomares E. E., Reyes-Zurita F. J., García-Salguero L., Peragón J., de la Higuera M., Lupiáñez J. A. The role of maslinic acid in the pentose phosphate pathway during growth of gilthead sea bream (Sparus aurata) Aquaculture Nutrition. 2013;19(5):709–720. doi: 10.1111/anu.12018. [DOI] [Google Scholar]
- 20.Rufino-Palomares E. E., Reyes-Zurita F. J., García-Salguero L., Peragón J., De La Higuera M., Lupiañez J. A. Maslinic acid, a natural triterpene, and ration size increased growth and protein turnover of white muscle in gilthead sea bream (Sparus aurata) Aquaculture Nutrition. 2012;18(5):568–580. doi: 10.1111/j.1365-2095.2011.00933.x. [DOI] [Google Scholar]
- 21.Rufino-Palomares E. E., Reyes-Zurita F. J., García-Salguero L., Peragón J., De La Higuera M., Lupiáñez J. A. Maslinic acid and ration size enhanced hepatic protein-turnover rates of gilthead sea bream (Sparus aurata) Aquaculture Nutrition. 2012;18(2):138–151. doi: 10.1111/j.1365-2095.2011.00882.x. [DOI] [Google Scholar]
- 22.Abdal Dayem A., Choi H.-Y., Kim J.-H., Cho S.-G. Role of oxidative stress in stem, cancer, and cancer stem cells. Cancers. 2010;2(2):859–884. doi: 10.3390/cancers2020859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirsch M., de Groot H. NAD(P)H, a directly operating antioxidant? The FASEB Journal. 2001;15(9):1569–1574. doi: 10.1096/fj.00-0823hyp. [DOI] [PubMed] [Google Scholar]
- 24.Ogasawara N., Oguro T., Sakabe T., et al. Hemoglobin induces the expression of indoleamine 2,3-dioxygenase in dendritic cells through the activation of PI3K, PKC, and NF-κB and the generation of reactive oxygen species. Journal of Cellular Biochemistry. 2009;108(3):716–725. doi: 10.1002/jcb.22308. [DOI] [PubMed] [Google Scholar]
- 25.Waris G., Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. Journal of Carcinogenesis. 2006;5, article 14 doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storz P. Targeting the alternative NF-κB pathway in pancreatic cancer: a new direction for therapy? Expert Review of Anticancer Therapy. 2013;13(5):501–504. doi: 10.1586/era.13.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu Z.-L., Pio F., Xie Z., et al. A novel enhancer of the Apaf1 apoptosome involved in cytochrome c-dependent caspase activation and apoptosis. The Journal of Biological Chemistry. 2001;276(12):9239–9245. doi: 10.1074/jbc.m006309200. [DOI] [PubMed] [Google Scholar]
- 28.Kim J. H., Lee J. O., Lee S. K., et al. Celastrol suppresses breast cancer MCF-7 cell viability via the AMP-activated protein kinase (AMPK)-induced p53-polo like kinase 2 (PLK-2) pathway. Cellular Signalling. 2013;25(4):805–813. doi: 10.1016/j.cellsig.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Lee J.-H., Won Y.-S., Park K.-H., et al. Celastrol inhibits growth and induces apoptotic cell death in melanoma cells via the activation ROS-dependent mitochondrial pathway and the suppression of PI3K/AKT signaling. Apoptosis. 2012;17(12):1275–1286. doi: 10.1007/s10495-012-0767-5. [DOI] [PubMed] [Google Scholar]
- 30.Massaoka M. H., Matsuo A. L., Figueiredo C. R., et al. Jacaranone induces apoptosis in melanoma cells via ROS-mediated downregulation of akt and p38 MAPK activation and displays antitumor activity in vivo . PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0038698.e38698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 32.McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) The Journal of Biological Chemistry. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 33.Aebi H. [13] Catalase in vitro . Methods in Enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 34.Lupiañez J. A., Adroher F.-J., Vargas A. M., Osuna A. Differential behaviour of glucose 6-phosphate dehydrogenase in two morphological forms of Trypanosoma cruzi . International Journal of Biochemistry. 1987;19(11):1085–1089. doi: 10.1016/0020-711x(87)90310-7. [DOI] [PubMed] [Google Scholar]
- 35.Peragón J., Aranda F., García-Salguero L., Corpas F. J., Lupiáñez J. A. Stimulation of rat-kidney hexose monophosphate shunt dehydrogenase activity by chronic metabolic acidosis. Biochemistry International. 1989;18(5):1041–1050. [PubMed] [Google Scholar]
- 36.Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. The Journal of Biological Chemistry. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 37.Khammanit R., Chantakru S., Kitiyanant Y., Saikhun J. Effect of serum starvation and chemical inhibitors on cell cycle synchronization of canine dermal fibroblasts. Theriogenology. 2008;70(1):27–34. doi: 10.1016/j.theriogenology.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Gatehouse H. S., Poulton J., Markwick N. P., et al. Changes in gene expression in the permissive larval host light brown apple moth (Epiphyas postvittana, Tortricidae) in response to EppoNPV (Baculoviridae) infection. Insect Molecular Biology. 2009;18(5):635–648. doi: 10.1111/j.1365-2583.2009.00904.x. [DOI] [PubMed] [Google Scholar]
- 39.Brunner D., Frank J., Appl H., Schöffl H., Pfaller W., Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. ALTEX. 2010;27(1):53–62. doi: 10.14573/altex.2010.1.53. [DOI] [PubMed] [Google Scholar]
- 40.Leung P. S., de Gasparo M. Involvement of the pancreatic renin-angiotensin system in insulin resistance and the metabolic syndrome. Journal of Cardiometabolic Syndrome. 2006;1(3):197–203. doi: 10.1111/j.1559-4564.2006.05460.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang S., Ding D., Zhang X., Shan L., Liu Z. Maslinic acid induced apoptosis in bladder cancer cells through activating p38 MAPK signaling pathway. Molecular and Cellular Biochemistry. 2014;392(1-2):281–287. doi: 10.1007/s11010-014-2038-y. [DOI] [PubMed] [Google Scholar]
- 42.Rothe G., Oser A., Valet G. Dihydrorhodamine 123: a new flow cytometric indicator for respiratory burst activity in neutrophil granulocytes. Naturwissenschaften. 1988;75(7):354–355. doi: 10.1007/bf00368326. [DOI] [PubMed] [Google Scholar]
- 43.Emaus R. K., Grunwald R., Lemasters J. J. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochimica et Biophysica Acta (BBA)—Bioenergetics. 1986;850(3):436–448. doi: 10.1016/0005-2728(86)90112-x. [DOI] [PubMed] [Google Scholar]
- 44.Lindl T. Zell-und Gewebekultur. 5th. Heidelberg, Germany: Spektrum Akademischer; 2002. [Google Scholar]
- 45.Gstraunthaler G., Lindl T., van der Valk J. A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology. 2013;65(5):791–793. doi: 10.1007/s10616-013-9633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mkhwanazi B. N., Serumula M. R., Myburg R. B., Van Heerden F. R., Musabayane C. T. Antioxidant effects of maslinic acid in livers, hearts and kidneys of streptozotocin-induced diabetic rats: effects on kidney function. Renal Failure. 2014;36(3):419–431. doi: 10.3109/0886022x.2013.867799. [DOI] [PubMed] [Google Scholar]
- 47.Qin X., Qiu C., Zhao L. Maslinic acid protects vascular smooth muscle cells from oxidative stress through Akt/Nrf2/HO-1 pathway. Molecular and Cellular Biochemistry. 2014;390(1-2):61–67. doi: 10.1007/s11010-013-1956-4. [DOI] [PubMed] [Google Scholar]
- 48.Allouche Y., Warleta F., Campos M., et al. Antioxidant, antiproliferative, and pro-apoptotic capacities of pentacyclic triterpenes found in the skin of olives on MCF-7 human breast cancer cells and their effects on DNA damage. Journal of Agricultural & Food Chemistry. 2011;59(1):121–130. doi: 10.1021/jf102319y. [DOI] [PubMed] [Google Scholar]
- 49.Montilla M. P., Agil A., Navarro M. C., et al. Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea . Planta Medica. 2003;69(5):472–474. doi: 10.1055/s-2003-39698. [DOI] [PubMed] [Google Scholar]
- 50.Siewert B., Csuk R. Membrane damaging activity of a maslinic acid analog. European Journal of Medicinal Chemistry. 2014;74:1–6. doi: 10.1016/j.ejmech.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 51.Huang B., Luo J., Han Y., Li S., Liu F., Wu L. Protective effect of maslinic acid preconditioning against oxygen-glucose deprivation-induced injuries in embryonic rat cortical neurons. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33(3):322–331. [PubMed] [Google Scholar]
- 52.Qian Y., Guan T., Tang X., et al. Maslinic acid, a natural triterpenoid compound from Olea europaea, protects cortical neurons against oxygen-glucose deprivation-induced injury. European Journal of Pharmacology. 2011;670(1):148–153. doi: 10.1016/j.ejphar.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 53.Lakshmi S. V. V., Padmaja G., Kuppusamy P., Kutala V. K. Oxidative stress in cardiovascular disease. Indian Journal of Biochemistry & Biophysics. 2009;46(6):421–440. [PubMed] [Google Scholar]
- 54.Reyes-Zurita F. J., Pachón-Peña G., Lizárraga D., Rufino-Palomares E. E., Cascante M., Lupiáñez J. A. The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNK-p53-dependent mechanism. BMC Cancer. 2011;11, article 154 doi: 10.1186/1471-2407-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuo W.-Y., Lin J.-Y., Tang T. K. Human glucose-6-phosphate dehydrogenase (G6PD) gene transforms NIH 3T3 cells and induces tumors in nude mice. International Journal of Cancer. 2000;85(6):857–864. doi: 10.1002/(SICI)1097-0215(20000315)85:6&lt;857::AID-IJC20>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 56.Bianchi D., Bertrand O., Haupt K., Coello N. Effect of gluconic acid as a secondary carbon source on non-growing L-lysine producers cells of Corynebacterium glutamicum. Purification and properties of 6-phosphogluconate dehydrogenase. Enzyme and Microbial Technology. 2001;28(9-10):754–759. doi: 10.1016/s0141-0229(01)00310-6. [DOI] [PubMed] [Google Scholar]
- 57.Bañuelos-Vargas I., López L. M., Pérez-Jiménez A., Peres H. Effect of fishmeal replacement by soy protein concentrate with taurine supplementation on hepatic intermediary metabolism and antioxidant status of totoaba juveniles (Totoaba macdonaldi) Comparative Biochemistry and Physiology B—Biochemistry and Molecular Biology. 2014;170(1):18–25. doi: 10.1016/j.cbpb.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Morales A. E., Pérez-Jiménez A., Hidalgo M. C., Abellán E., Cardenete G. Oxidative stress and antioxidant defenses after prolonged starvation in Dentex dentex liver. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2004;139(1–3):153–161. doi: 10.1016/j.cca.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Kirkman H. N., Rolfo M., Ferraris A. M., Gaetani G. F. Mechanisms of protection of catalase by NADPH: kinetics and stoichiometry. The Journal of Biological Chemistry. 1999;274(20):13908–13914. doi: 10.1074/jbc.274.20.13908. [DOI] [PubMed] [Google Scholar]