Abstract
Aims
VENTURE-AF is the first prospective randomized trial of uninterrupted rivaroxaban and vitamin K antagonists (VKAs) in patients with non-valvular atrial fibrillation (NVAF) undergoing catheter ablation (CA).
Methods and results
Trial size was administratively set at 250, the protocol-specified target. Events were independently and blindly adjudicated. We randomly assigned 248 NVAF patients to uninterrupted rivaroxaban (20 mg once-daily) or to an uninterrupted VKA prior to CA and for 4 weeks afterwards. The primary endpoint was major bleeding events after CA. Secondary endpoints included thromboembolic events (composite of stroke, systemic embolism, myocardial infarction, and vascular death) and other bleeding or procedure-attributable events. Patients were 59.5 ± 10 years of age, 71% male, 74% paroxysmal AF, and had a CHA2DS2-VASc score of 1.6. The average total heparin dose used to manage activated clotting time (ACT) was slightly higher (13 871 vs. 10 964 units; P < 0.001) and the mean ACT level attained slightly lower (302 vs. 332 s; P < 0.001) in rivaroxaban and VKA arms, respectively. The incidence of major bleeding was low (0.4%; 1 major bleeding event). Similarly, thromboembolic events were low (0.8%; 1 ischemic stroke and 1 vascular death). All events occurred in the VKA arm and all after CA. The number of any adjudicated events (26 vs. 25), any bleeding events (21 vs. 18), and any other procedure-attributable events (5 vs. 5) were similar.
Conclusion
In patients undergoing CA for AF, the use of uninterrupted oral rivaroxaban was feasible and event rates were similar to those for uninterrupted VKA therapy.
Name of the Trial Registry
Clinicaltrials.gov trial registration number is NCT01729871.
Keywords: Atrial fibrillation, Catheter ablation, Oral anticoagulant, Uninterrupted, Thromboembolism
Introduction
Catheter ablation (CA) of atrial fibrillation (AF) was first reported in 1996.1 It is now used routinely to establish rhythm control in selected patients.2 The traditional anticoagulation approach is to interrupt administration of an oral vitamin K antagonist (VKA) and use heparin bridging. Recent reports indicate that uninterrupted anticoagulation may be safer and more effective.3–7 The incidence of major bleeding or thromboembolic events after CA in AF patients randomly assigned to uninterrupted VKA could be as low as 0.38 and 0.25%, respectively.3 Atrial fibrillation guidelines recommend consideration of uninterrupted oral anticoagulation for patients undergoing CA. In a European Heart Rhythm Association survey, 71.6% of respondents reported using the uninterrupted VKA strategy for CA.8
Rivaroxaban is a selective oral direct factor Xa inhibitor anticoagulant approved for multiple clinical indications, including stroke risk reduction in patients with non-valvular AF (NVAF).9,10 Non-randomized studies suggest that it may be feasible to manage patients undergoing CA with rivaroxaban.11 Calls for randomized trials make this study clinically relevant.12–14 The objective of this prospective randomized study (NCT01729871) was to determine whether the number of complications associated with uninterrupted rivaroxaban are similar to those for a VKA in this setting.
Methods
The VENTURE-AF (ActiVe-controlled multi-cENTer stUdy with blind-adjudication designed to evaluate the safety of uninterrupted Rivaroxaban and uninterrupted vitamin K antagonists in subjects undergoing cathEter ablation for non-valvular Atrial Fibrillation) trial was a multinational, randomized, open-label, parallel-group phase IIIb study of patients with AF undergoing catheter-based ablation of the arrhythmia substrate. Details of the study design have been published.15 Patients scheduled for CA of AF were randomly assigned 1:1 to rivaroxaban 20 mg orally once-daily (recommended evening dosing) or to VKA [recommended international normalized ratio (INR) of 2.0–3.0].
Study population
Patients aged 18 years or older scheduled for CA of paroxysmal, persistent, or long-standing persistent NVAF were eligible. Exclusion criteria included valvular AF, defined as the presence of a prosthetic heart valve (annuloplasty with or without prosthetic ring, commissurotomy and/or valvuloplasty were permitted), haemodynamically significant mitral valve stenosis or rheumatic heart disease. This trial complied with the Declaration of Helsinki, locally or centrally appointed ethics committees approved the research protocol and informed consent was obtained from the patients (or their guardians/caregivers).
Ablation
Prior to CA, patients randomized to rivaroxaban received a once-daily dose of 20 mg orally, preferentially with the evening meal. Patients randomized to VKA received a VKA regimen based on local standards of care (recommended INR 2.0–3.0). Patients were required to receive continuous oral anticoagulation for at least 3 weeks prior to ablation (delayed CA strategy) or for 1 to 7 days (early CA strategy) if an immediate transoesophageal echocardiography (TOE) or intracardiac echocardiography (ICE) demonstrated the absence of an intra-cardiac thrombus. During CA, patients received intravenous unfractionated heparin to achieve a target activated clotting time (ACT) of 300 to 400 s. After CA, the next post-ablation rivaroxaban dose was administered at least 6 h following establishing haemostasis. The next dose of VKA was administered in accordance with the usual care. After CA, the administration of study drug was continued for 30 ± 5 days after CA and the subsequent anticoagulation regimen was determined by the patient's clinician.
Medications and procedures
The study protocol included recommendations with respect to the general management of rivaroxaban patients, anticoagulant transitions, and reversal strategies for patients with bleeding complications. Rivaroxaban non-compliance was defined by pill count as unexplained missed doses that resulted in the subject returning more than 20% of the prescribed study drug. Interruption and subsequent restarting of VKA was done in accordance with the usual care. This trial did not control for ablation catheter type, ablation parameters, or lesion sets.
Study endpoints
The invasiveness of CA and the expectation of higher rates of bleeding complications compared with thromboembolic events was the basis for the focus on bleeding events in this study. The primary endpoint was the incidence of major bleeding events in the uninterrupted rivaroxaban and uninterrupted VKA treatment groups within the first 30 ± 5 days after CA. Major secondary endpoints included the composite (and individual) events of ischemic stroke, non-central nervous system (CNS) systemic embolism, myocardial infarction (MI), and vascular death; other bleeding events; and procedure-attributable adverse events.
An adjudicated major bleeding event was based on the presence of at least one of the three following definitions: an International Society on Thrombosis and Haemostasis (ISTH) major bleed, a Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) severe/life-threatening bleed or a Thrombosis in Myocardial Infarction (TIMI) major bleed (see Appendix for detail).16,17
The mean rivaroxaban plasma concentration was extrapolated from chromogenic anti-factor Xa assay-based data, collected on the morning of the day of CA.18 The blood sample was drawn on the day of the procedure and the assay performed at a later time.
Study governance
An independent academic Steering Committee oversaw study design and conduct. Inclusion of SC members who were not performing uninterrupted CA was deemed important to ensure diversity of perspective in study design, conduct and evaluation. An independent academic Clinical Endpoint Committee (CEC) performed blinded-adjudication and classification of events. A Writing Committee drafted the initial version of the manuscript. Data management and statistical analyses complied with good clinical practice standards. All authors had full access to data.
Statistical analysis
After traditional estimation of sample size indicated an unfeasibly large number of patients needed to show a significant difference between two randomized (1:1) arms for either establishing non-inferiority or superiority, we decided that a descriptive comparison using ∼250 patients would generate clinically relevant information. We also assumed that ∼20% of patients would be discontinued from the study before undergoing CA. To minimize potentially confounding factors such as enrollment bias, a central randomization strategy was implemented. Patients receive the study drug based on a computer-generated randomization schedule, using randomly permuted blocks and stratification by country.
The intention-to-treat (ITT) analysis set included all randomized patients who met inclusion and exclusion criteria. The per-protocol analysis set was the pre-specified primary analysis set, including all randomized patients who took at least 1 dose of study drug (safety population) and underwent CA. The safety population was used for the primary safety analysis. The treatment-emergent period spanned the time from administration of the first dose until the last dose of study medication plus 2 days. Statistical analyses were descriptive. All continuous variables were expressed in terms of mean or median and standard deviations. Categorical data were expressed as numbers and percentages. Categorical group comparisons were made using the χ2-test without continuity correction. Two-sided probability values of <0.05 were considered to be statistically significant. The SAS FREQ procedure was used for confidence interval calculation. All analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient characteristics
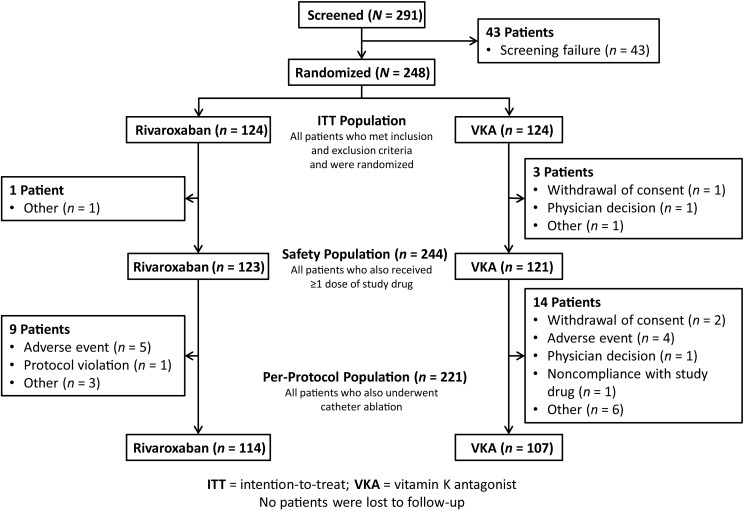
A total of 291 adult patients with paroxysmal, persistent, or long-standing persistent NVAF scheduled for elective CA at 46 sites opened for enrollment in five countries (Belgium, France, Germany, the UK, and the USA) were screened between February 2013 and September 2014. Of them, 248 were randomized at 37 sites (ITT population; mean of 6 patients per site), 244 received at least one dose of the assigned study drug (safety population), 221 also underwent CA (per-protocol population), 213 patients completed the study, and no patient was lost to follow-up (Figure 1).
Figure 1.
Patient disposition during the study.
Baseline characteristics were not significantly different between the two treatment groups (Table 1). The mean age was 59.6 ± 10.2 (SD) years. The majority of patients were male, Caucasian and non-Hispanic/Latino, and had paroxysmal AF (73.4%). The mean CHA2DS2-VASc score was 1.6 ± 1.3 and 40.7% of patients had a history of having had at least 1 prior electrical cardioversion procedure and 8.9% had a history of at least 1 prior CA procedure. Approximately 50% of patients were already taking rivaroxaban (21.0%) or a VKA (29.4%) prior to randomization.
Table 1.
Baseline demographic characteristics of the ITT population
| Rivaroxaban (N = 124) | VKA (N = 124) | Total (N = 248) | P Value | |
|---|---|---|---|---|
| Mean age, years (SD) | 58.6 (9.9) | 60.5 (10.5) | 59.6 (10.2) | 0.211 |
| Age ≥75, n (%) | 5 (4.0) | 10 (8.1) | 15 (6.0) | 0.183 |
| Age 65–75 | 34 (27.4) | 41 (33.1) | 75 (30.2) | 0.183 |
| Male | 86 (69.4) | 90 (72.6) | 176 (71.0) | 0.576 |
| Caucasian | 112 (90.3) | 116 (93.5) | 228 (91.9) | 0.351 |
| Non-Hispanic/Latino | 90 (72.6) | 94 (75.8) | 184 (74.2) | 0.562 |
| Paroxysmal AF | 95 (76.6) | 87 (70.2) | 182 (73.4) | 0.250 |
| Prior cardioversion | 47 (37.9) | 54 (43.5) | 101 (40.7) | 0.366 |
| Prior catheter ablation | 11 (8.9) | 11 (8.9 | 22 (8.9) | 0.563 |
| Mean BMI, kg/m2 (SD) | 29.8 (5.7) | 28.9 (5.5) | 29.4 (5.6) | 0.231 |
| CHF | 12 (9.7) | 9 (7.3) | 21 (8.5) | 0.494 |
| Hypertension | 59 (47.6) | 57 (46.0) | 116 (46.8) | 0.799 |
| Mean systolic BP, mmHg (SD) | 133 (16) | 131 (18) | 132 (17) | 0.325 |
| Mean diastolic BP, mmHg (SD) | 81 (10) | 79 (11) | 80 (10) | 0.233 |
| Diabetes mellitus | 8 (6.5) | 14 (11.3) | 22 (8.9) | 0.180 |
| Prior Stroke/TIA/embolism | 0 | 3 (2.4) | 3 (1.2) | 0.081 |
| Vascular disease | 22 (17.7) | 25 (20.2) | 47 (19.0) | 0.627 |
| Mean CHADS2 Score (SD) | 0.7 (0.7) | 0.8 (0.9) | 0.7 (0.8) | 0.179 |
| Mean CHA2DS2-VASc Score (SD) | 1.5 (1.3) | 1.7 (1.4) | 1.6 (1.3) | 0.277 |
| Beta blocker, selective | 65 (52.4) | 61 (49.2) | 126 (50.8) | 0.611 |
| Antiarrhythmic, class IC | 51 (41.1) | 49 (39.5) | 100 (40.3) | 0.796 |
| Antiarrhythmic, class III | 30 (24.2) | 39 (31.5) | 69 (27.8) | 0.202 |
| Vitamin K antagonist | 36 (29.0) | 37 (29.8) | 73 (29.4) | 0.889 |
| Rivaroxaban | 23 (18.5) | 29 (23.4) | 52 (21.0) | 0.349 |
| Dabigatran | 12 (9.7) | 10 (8.1) | 22 (8.9) | 0.655 |
| Antiplatelet agent | 37 (29.8) | 29 (23.4) | 66 (26.6) | 0.250 |
| Proton pump inhibitor | 26 (21.0) | 18 (14.5) | 44 (17.7) | 0.184 |
Units are listed as n(%) unless otherwise indicated.
BMI, body mass index; BP, blood pressure; CHF, congestive heart failure; ITT, intention-to treat; SD, standard deviation.
Anticoagulation management
The mean estimated compliance rate with rivaroxaban was 99.9 ± 7.1% (SD; n = 123; minimum = 57%). Only one patient had a mean estimated compliance rate of <60%, none were 60–79%, and values for the remaining patients were >80%. The mean rivaroxaban plasma concentration was 151 ± 115 µg/L (n = 103 patients in the rivaroxaban arm of the study). After CA (i.e. during the primary endpoint period), the majority of patients (79.8%) in the VKA treatment group achieved therapeutic anticoagulation as defined by an average INR value of 2.0 to 3.0 (the guideline-recommended and protocol-preferred range). Most patients in the VKA treatment group (87.2%) had an average after-ablation INR value within a range that is likely more reflective of real-world clinical practice (i.e. 1.8 to 3.2). On the day of ablation, the majority of patients had average INR values of 2.0 to 3.0 or 1.8 to 3.2 (52.6 and 64.9%, respectively).
All patients (100%) received heparin on the day of CA (Table 2). The mean total units of heparin administered to achieve the target ACT range was 26% higher for patients in the rivaroxaban treatment group compared with those in the VKA arm (13 871 ± 6516 and 10 964 ± 5912, respectively; P < 0.001). The mean ACT level achieved was 9% lower for patients in the rivaroxaban arm compared with patients in the VKA treatment group (302 ± 49 and 332 ± 58, respectively; P < 0.001).
Table 2.
The practical management of activated clotting time on the day of catheter ablation in the per protocol population
| Rivaroxaban | VKA | Total | P Value | |
|---|---|---|---|---|
| N | 114 | 107 | 221 | |
| Patients heparinized, n (%) | 114 (100) | 107 (100) | 221 (100) | |
| N | 113 | 107 | 221 | |
| Total units of heparin, mean (SD) | 13 871 (6516) | 10 964 (5912) | 12 457 (6383) | <0.001 |
| N | 111 | 106 | 218 | |
| ACT level, mean (SD) | 302 (49) | 332 (58) | 317 (55) | <0.001 |
| N | 114 | 107 | 221 | |
| Protamine for heparin reversal, n (%) | 32 (28.1) | 27 (25.2) | 59 (26.7) | 0.634 |
One total heparin dose value, recorded as 195 000, is not included.
Multiple ACT values were measured for each subject on ablation procedure day. Minimum, median, maximum of ACT values were calculated first for each subject. Summary statistics were then calculated for the minimum, median, and maximum of ACT values. The mean and standard deviation (SD) of the median ACT values is shown. One ACT level exceeded 999 and is not included. Because the system did not accept the ACT value greater than 999, the number 999 was entered in the database for this subject.
ACT, activated clotting time; SD, standard deviation.
Outcomes
There was a similar number (26 vs. 25) of CEC-adjudicated events during the study period among patients in the rivaroxaban and VKA treatment groups (Table 3). For the combined/composite efficacy endpoint, one ischemic stroke and one vascular death were observed in separate patients. Both events occurred in the VKA treatment group at 27 and 14 days after CA, respectively. Throughout the study period, the number of major bleeding events was very low in both treatment groups and consistent across the three definitions of major bleeding events used in this study. There was one ISTH major bleeding event in the VKA treatment group vs. none in the rivaroxaban treatment group. The number of non-major bleeding events (21 vs. 17) was comparable between the rivaroxaban and VKA arms. These events were primarily comprised of procedure-attributable complications, the most frequent of which was haematoma (8 events for rivaroxaban and 10 events for VKA).
Table 3.
The number of CEC-adjudicated outcomes reported during the study period (the number of patients is also shown)
| Rivaroxaban | VKA | Total | |
|---|---|---|---|
| Any CEC-adjudicated event | 26 | 25 | 51 |
| n = 124 | n = 124 | n = 248 | |
| Any thromboembolic events (Composite)a | 0 | 2 | 2 |
| Ischemic stroke | 0 | 1 | 1 |
| Vascular death | 0 | 1 | 1 |
| n = 123 | n = 121 | n = 244 | |
| Any bleeding eventsb | 21 | 18 | 39 |
| Major bleeding event | |||
| Vascular pseudoaneurysm | 0 | 1 | 1 |
| Non-major bleeding events | |||
| Arteriovenous fistula | 0 | 1 | 1 |
| Catheter/puncture site haemorrhage | 1 | 1 | 2 |
| Contusion | 1 | 1 | 2 |
| Ecchymosis | 0 | 1 | 1 |
| Epistaxis | 2 | 1 | 3 |
| Eye haemorrhage (non-intraocular) | 1 | 0 | 1 |
| Gingival bleeding | 1 | 0 | 1 |
| Haematoma/vessel puncture site haematoma | 8 | 10 | 18 |
| Haematuria | 2 | 0 | 2 |
| Haemorrhagic stomatitis | 0 | 1 | 1 |
| Mouth haemorrhage | 1 | 0 | 1 |
| Urinary tract infection | 1 | 0 | 1 |
| Vascular pseudoaneurysm | 3 | 1 | 4 |
| n = 114 | n = 107 | n = 221 | |
| Any other procedure-attributable eventsc | 5 | 5 | 10 |
| Atonic seizures | 0 | 1 | 1 |
| Catheter site pain | 1 | 0 | 1 |
| Chest discomfort | 1 | 0 | 1 |
| Fluid overload | 0 | 1 | 1 |
| Local swelling | 1 | 0 | 1 |
| Musculoskeletal discomfort | 1 | 0 | 1 |
| Pericardial effusion without tamponade | 0 | 1 | 1 |
| Postprocedural complication/nausea | 1 | 1 | 2 |
| Pyrexia | 0 | 1 | 1 |
The eye haemorrhage was not an intraocular bleed (i.e. not a major bleeding event). The two thromboembolic events occurred in separate patients. A 73-year-old male patient died while on a VKA after being hospitalized for a mild episode of cardiac decompensation 11 days after ablation that was resolved 12 days after ablation. The INR was 2.3 on the day of ablation. The patient died suddenly 14 days after ablation. No autopsy was performed. A 71-year-old male on a VKA experienced an ischaemic stroke event 27 days after ablation. The INR was 2.24 on the day of ablation and the prothrombin time was 24.6 on the day after the event. A 62-year-old female on a VKA experienced as vascular pseudoaneurysm CEC-classified as an ISTH major bleeding event 1 day after ablation. The INR was 2.86 on the day of ablation. Fourteen days after the end of the trial, 1 death occurred in a 53-year-old male patient who had been randomly assigned to the rivaroxaban treatment group during the study and then transitioned to VKA and continued on VKA after study-end. On the day before this event, the INR was 5.2. The autopsy indicated that the death was associated with intracranial haemorrhage and hypertension. Major bleeding events were CEC-adjudicated using GUSTO, ISTH, and TIMI criteria (see Appendix for criteria). Events were not counted if they were classified as ‘not a bleeding event’ by the CEC using all three classification methods. The numbers of events for the ITT and safety populations are equal.
aITT population; this total also represents the pre-specified combined/composite efficacy endpoint; non-zero values are listed individually below composite thromboembolic values; there was 1 CEC-adjudicated cardiac event in the VKA arm that the CEC could not further characterize due to absence of sufficient documentation surrounding the event.
bSafety population; the odds ratio (OR) for any bleeding events was 1.178 and 95% confidence interval (95% CI) was 0.593–2.341; for haematoma/vessel puncture site haematoma events, the OR was 0.772 and the 95% CI was 0.294–2.028.
cPer-protocol population; this category includes other non-thromboembolic and non-bleeding procedure-attributable events; for any other procedure-attributable events, the OR was 0.936 and the 95% CI was 0.263–3.328.
Procedure-attributable events not adjudicated as thromboembolic or bleeding events were infrequent and similar (5 vs. 5) among patients in the rivaroxaban treatment group compared with those in the VKA arm. Other procedure-attributable complications included, for example, one reported episode of chest pain in the rivaroxaban arm and one occurrence of pericardial effusion without tamponade in the VKA arm.
Serious adverse events
The number of SAEs leading to drug discontinuation were very low and similar in the rivaroxaban and VKA treatment groups [1(0.8%) vs. 3(2.5%), respectively]. The number of SAEs leading to hospitalization were similarly low in the rivaroxaban treatment group [11(8.9%)] and in the VKA treatment group [17(14.0%)].
Discussion
VENTURE-AF is the first randomized prospective comparative trial of an uninterrupted NOAC vs. VKA therapy in patients with AF undergoing CA. Due to expected low event rates, VENTURE-AF was intentionally designed as an exploratory study and thus no formal statistical superiority or non-inferiority analysis was planned. Major outcome rates were low, validating our design assumptions. The incidence of primary safety events was low in the general population (0.4%; one event in a VKA patient), with no such event being reported in rivaroxaban patients. Similarly low was the overall incidence of composite thromboembolic events (0.8%; two events, in separate VKA patients). The overall complication rate was 20.6%. Bleeding and thromboembolic events represent the most common complication in patients undergoing CA of AF.19–21
The use of vitamin K antagonist peri-CA has become the practice standard.2,22–24 The use of NOACs is increasing among AF patients undergoing CA.25 Results of non-randomized studies using various dose-timing protocols suggest that the use of an NOAC may be feasible in such patients.3,11,21,25–28 In contrast, one retrospective study of uninterrupted VKA or dabigatran vs. a bridged VKA strategy reported a higher complication rate in the uninterrupted cohort.29
The observation that a slightly higher total dose of heparin may be needed to achieve target ACT range among patients in the rivaroxaban arm compared with those in the VKA treatment group is consistent with all NOACs as reported in a retrospective cohort study from a prospective AF ablation registry maintained at a large, academic medical centre.30 Although the mechanistic basis for this observation may not be known, evidence in this study confirms the clinician's ability to successfully and practically manage ACT levels in this setting.
There are several limitations in this study. The VENTURE-AF exploratory sample size reflects the infeasibility of conducting a large, fully-powered study based on standard calculations. Although the low incidence of events likely represents a population of highly skilled electrophysiologists performing CA of AF, this element could reduce the sensitivity of the read-out. The open-label design can also introduce bias related to knowledge of treatment allocation.
Despite these limitations, several findings substantiate the strength of the results of VENTURE-AF. First, this study used a randomized multicentre international trial design. Secondly, blinded-adjudication of events reduced potential reporting bias. Thirdly, the sample size is similar in magnitude to the largest reported studies in this setting. Additionally, the provision of detailed outcomes data added meaningful robustness. Finally, the number of bleeding events was generally consistent across three different sets of criteria, further supporting the consistency of study findings.
Conclusions
The use of uninterrupted rivaroxaban in patients undergoing CA for NVAF is feasible and the number of events was low and similar to that for uninterrupted dose-adjusted VKA.
Funding
This study was sponsored and funded by Janssen Scientific Affairs LLC, a Johnson and Johnson Company and by Bayer HealthCare Pharmaceuticals. Funding to pay the Open Access publication charges for this article was provided by Janssen Scientific Affairs LLC, a Johnson and Johnson Company.
Conflict of interest: All authors received research grant support from the sponsors of the trial (Johnson & Johnson and Bayer). G.V.N. is a consultant for Glaxo-Smith-Kline, Pfizer, Xention, Sanofi, Bristol Myers Squibb, Merck, Biosense-Webster, Janssen, Otsuka, Daiichi-Sankyo, and Boehringer-Ingelheim. R.C. Biosense Webster, St Jude Medical, and Bard. S.H.H. consulting and lecture fees from Boehringer Ingelheim, BMS, Bayer, Pfizer, and Sanofi-aventis. F.E.M. consulting and lecture fees from Biosense Webster, Medtronic, St Jude Medical and Cardioinsight. D.J.W. received lectures and consulting fees from Biosense Webster, Medtronic, St Jude, Cardioinsight and research support from Biosense Webster, Medtronic. C.M. received no further disclosures. D.W.D. is a consultant for Boston Scientific, Janssen, Medtronic, and Rhythmia. A.N. Biosense Webster, Boston Scientific, Janssen, St Jude Medical, Medtronic, and Biotronik. D.S.W. Speaking/Honorarium/Consulting-Medtronic; Speaking/Honorarium-Bristol Myers Squibb. B.J.H. received lecture fees from Bayer Healthcare Pharmaceuticals. J.V. has no personal conflicts of interest; the division of Electrophysiology received research grants from Medtronic and St Jude Medical. C.D.C. gave a lecturer for St Jude Medical, Medtronic, Biotronik, and MEDA PHARMA. L.E.F. is an employee of Johnson & Johnson at Janssen Scientific Affairs, LLC. J.Xiang, PhD: is an employee of Johnson & Johnson at Janssen Research & Development, LLC. Susanne Hess, MD: is an employee of Bayer at Bayer HealthCare Pharmaceuticals.
Acknowledgements
The VENTURE-AF Steering Committee thanks participants and their caregivers and families, VENTURE-AF investigators and their teams; the VENTURE-AF Core Integrated Team and colleagues.
References
- 1.Haïssaguerre M, Jaïs P, Shah DC, Gencel L, Pradeau V, Garrigues S, Chouairi S, Hocini M, Le Métayer P, Roudaut R, Clémenty J. Right and left atrial radiofrequency catheter therapy of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 1996;7:1132–1144. [DOI] [PubMed] [Google Scholar]
- 2.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 3.Di Biase L, Burkhardt JD, Santangeli P, Mohanty P, Sanchez JE, Horton R, Gallinghouse GJ, Themistoclakis S, Rossillo A, Lakkireddy D, Reddy M, Hao S, Hongo R, Beheiry S, Zagrodzky J, Rong B, Mohanty S, Elayi CS, Forleo G, Pelargonio G, Narducci ML, Dello Russo A, Casella M, Fassini G, Tondo C, Schweikert RA, Natale A. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation 2014;129:2638–2644. [DOI] [PubMed] [Google Scholar]
- 4.Gopinath D, Lewis WR, Di Biase L, Natale A. Pulmonary vein antrum isolation for atrial fibrillation on therapeutic coumadin: special considerations. J Cardiovasc Electrophysiol 2011;22:236–239. [DOI] [PubMed] [Google Scholar]
- 5.Page SP, Siddiqui MS, Finlay M, Hunter RJ, Abrams DJ, Dhinoja M, Earley MJ, Sporton SC, Schilling RJ. Catheter ablation for atrial fibrillation on uninterrupted warfarin: can it be done without echo guidance? J Cardiovasc Electrophysiol 2011;22:265–270. [DOI] [PubMed] [Google Scholar]
- 6.Hakalahti A, Uusimaa P, Ylitalo K, Raatikainen MJ. Catheter ablation of atrial fibrillation in patients with therapeutic oral anticoagulation treatment. Europace 2011;13:640–645. [DOI] [PubMed] [Google Scholar]
- 7.Gautam S, John RM, Stevenson WG, Jain R, Epstein LM, Tedrow U, Koplan BA, McClennen S, Michaud GF. Effect of therapeutic INR on activated clotting times, heparin dosage, and bleeding risk during ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:248–254. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Todd DM, Hocini M, Larsen TB, Bongiorni MG, Blomström-Lundqvist C; Scientific Initiative Committee, European Heart Rhythm Association. Current periprocedural management of ablation for atrial fibrillation in Europe: results of the European Heart Rhythm Association survey. Europace 2014;16:378–381. [DOI] [PubMed] [Google Scholar]
- 9.http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm278646.htm (accessed 6 April 2015).
- 10.http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022406s009lbl.pdf (accessed 6 April 2015).
- 11.Lakkireddy D, Reddy YM, Di Biase L, Vallakati A, Mansour MC, Santangeli P, Gangireddy S, Swarup V, Chalhoub F, Atkins D, Bommana S, Verma A, Sanchez JE, Burkhardt JD, Barrett CD, Baheiry S, Ruskin J, Reddy V, Natale A. Feasibility and safety of uninterrupted rivaroxaban for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol 2014;63:982–988. [DOI] [PubMed] [Google Scholar]
- 12.Bin Abdulhak AA, Khan AR, Wimmer AP. Dabigatran in catheter ablation of atrial fibrillation: a call for a randomized control trial. Amer J Cardiol 2014;113:2087–2088. [DOI] [PubMed] [Google Scholar]
- 13.Weitz JI, Healey JS, Skanes AC, Verma A. Periprocedural management of new oral anticoagulants in patients undergoing atrial fibrillation ablation. Circulation 2014;129:1688–1694. [DOI] [PubMed] [Google Scholar]
- 14.Naccarelli GV, Gonzalez MD. Catheter ablation of atrial fibrillation: the need for studies to assess the efficacy and safety of novel anticoagulants. J Interv Cardiol Electrophysiol 2013;36:3–4. [DOI] [PubMed] [Google Scholar]
- 15.Naccarelli GV, Cappato R, Hohnloser SH, Marchlinski FE, Wilber DJ, Xiang J, Ma C, Hess S, Davies DW, Fields LE, Natale A; VENTURE-AF Investigators. Rationale and design of VENTURE-AF: a randomized, open-label, active-controlled multicenter study to evaluate the safety of rivaroxaban and vitamin K antagonists in subjects undergoing catheter ablation for atrial fibrillation. J Interv Card Electrophysiol 2014;41:107–116. [DOI] [PubMed] [Google Scholar]
- 16.Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 17.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 18.Samama MM, Contant G, Spiro TE, Perzborn E, Guinet C, Gourmelin Y, Le Flem L, Rohde G, Martinoli JL; Rivaroxaban Anti-Factor Xa Chromogenic Assay Field Trial Laboratories. Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost 2012;107:379–387. [DOI] [PubMed] [Google Scholar]
- 19.Pothineni NV, Deshmukh A, Pant S, Patel NJ, Badheka A, Chothani A, Shah N, Mehta K, Savani GT, Singh V, Grover P, Bhalara V, Patel N, Arora S, Rathod A, Viles-Gonzalez J, Paydak H. Complication rates of atrial fibrillation ablations: comparison of safety outcomes from real world to contemporary randomized control trials. Int J Cardiol 2014;175:372–373. [DOI] [PubMed] [Google Scholar]
- 20.Gupta A, Perera T, Ganesan A, Sullivan T, Lau DH, Roberts-Thomson KC, Brooks AG, Sanders P. Complications of catheter ablation of atrial fibrillation: a systematic review. Circ Arrhythm Electrophysiol 2013;6:1082–1088. [DOI] [PubMed] [Google Scholar]
- 21.Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta K, Grover P, Singh V, Vallurupalli S, Savani GT, Badheka A, Tuliani T, Dabhadkar K, Dibu G, Reddy YM, Sewani A, Kowalski M, Mitrani R, Paydak H, Viles-Gonzalez JF. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation 2013;128:2104–2112. [DOI] [PubMed] [Google Scholar]
- 22.Verma A, Cairns JA, Mitchell LB, Macle L, Stiell IG, Gladstone D, McMurtry MS, Connolly S, Cox JL, Dorian P, Ivers N, Leblanc K, Nattel S, Healey JS; CCS Atrial Fibrillation Guidelines Committee. 2014 Focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol 2014;30:1114–1130. [DOI] [PubMed] [Google Scholar]
- 23.Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, Sinnaeve P, Camm AJ, Kirchhof P; European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013;15:625–651. [DOI] [PubMed] [Google Scholar]
- 24.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 25.Aryal MR, Ukaigwe A, Pandit A, Karmacharya P, Pradhan R, Mainali NR, Pathak R, Jalota L, Bhandari Y, Donato A. Meta-analysis of efficacy and safety of rivaroxaban compared with warfarin or dabigatran in patients undergoing catheter ablation for atrial fibrillation. Am J Cardiol 2014;114:577–582. [DOI] [PubMed] [Google Scholar]
- 26.Winkle RA, Mead RH, Engel G, Kong MH, Patrawala RA. Peri-procedural interrupted oral anticoagulation for atrial fibrillation ablation: comparison of aspirin, warfarin, dabigatran, and rivaroxaban. Europace 2014;16:1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dillier R, Ammar S, Hessling G, Kaess B, Pavaci H, Buiatti A, Semmler V, Kathan S, Hofmann M, Lennerz C, Kolb C, Reents T, Deisenhofer I. Safety of Continuous Periprocedural Rivaroxaban for Patients Undergoing Left Atrial Catheter Ablation Procedures. Circ Arrhythm Electrophysiol 2014;7:576–582. [DOI] [PubMed] [Google Scholar]
- 28.Providência R, Marijon E, Albenque JP, Combes S, Combes N, Jourda F, Hireche H, Morais J, Boveda S. Rivaroxaban and dabigatran in patients undergoing catheter ablation of atrial fibrillation. Europace 2014;16:1137–1144. [DOI] [PubMed] [Google Scholar]
- 29.Arshad A, Johnson CK, Mittal S, Buch E, Hamam I, Tran T, Shaw RE, Musat D, Preminger M, Sichrovsky T, Herweg B, Shivkumar K, Hummel J, Steinberg JS. Comparative safety of periablation anticoagulation strategies for atrial fibrillation: data from a large multicenter study. Pacing Clin Electrophysiol 2014;37:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armbruster HL, Lindsley JP, Moranville MP, Habibi M, Khurram IM, Spragg DD, Berger RD, Calkins H, Marine JE. Safety of novel oral anticoagulants compared with uninterrupted warfarin for catheter ablation of atrial fibrillation. Ann Pharmacother 2015;49:278–284. [DOI] [PubMed] [Google Scholar]