Abstract
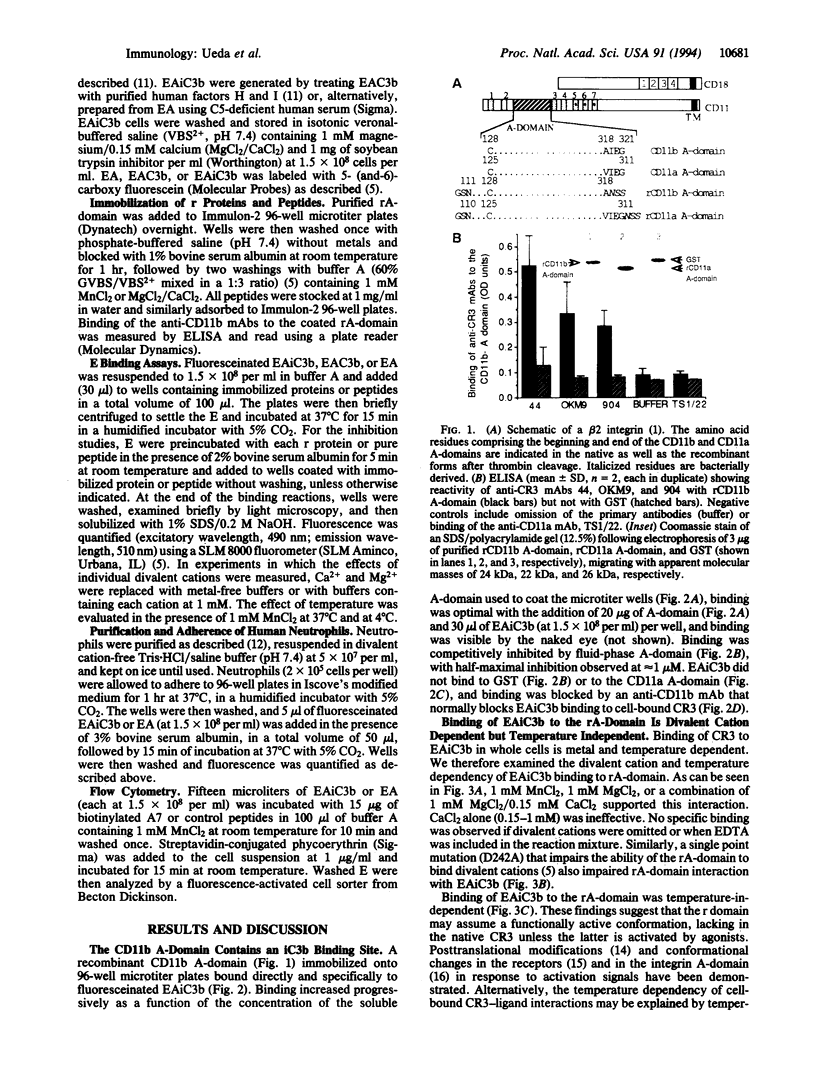
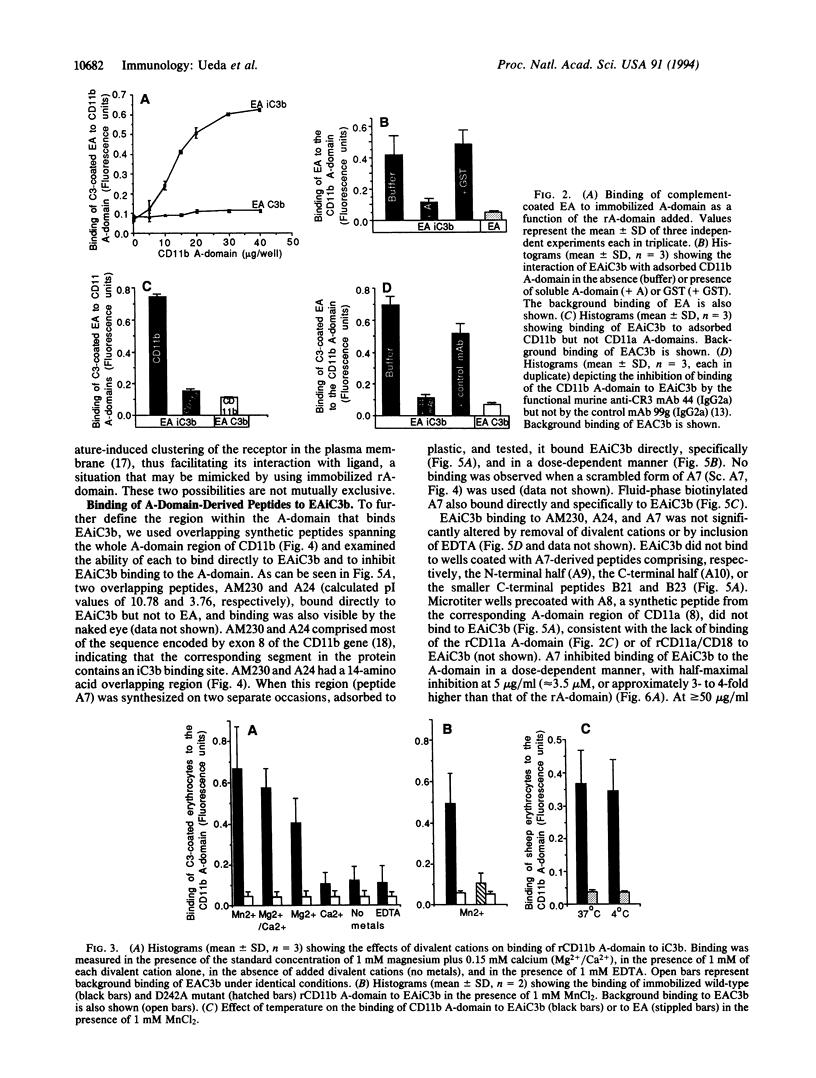
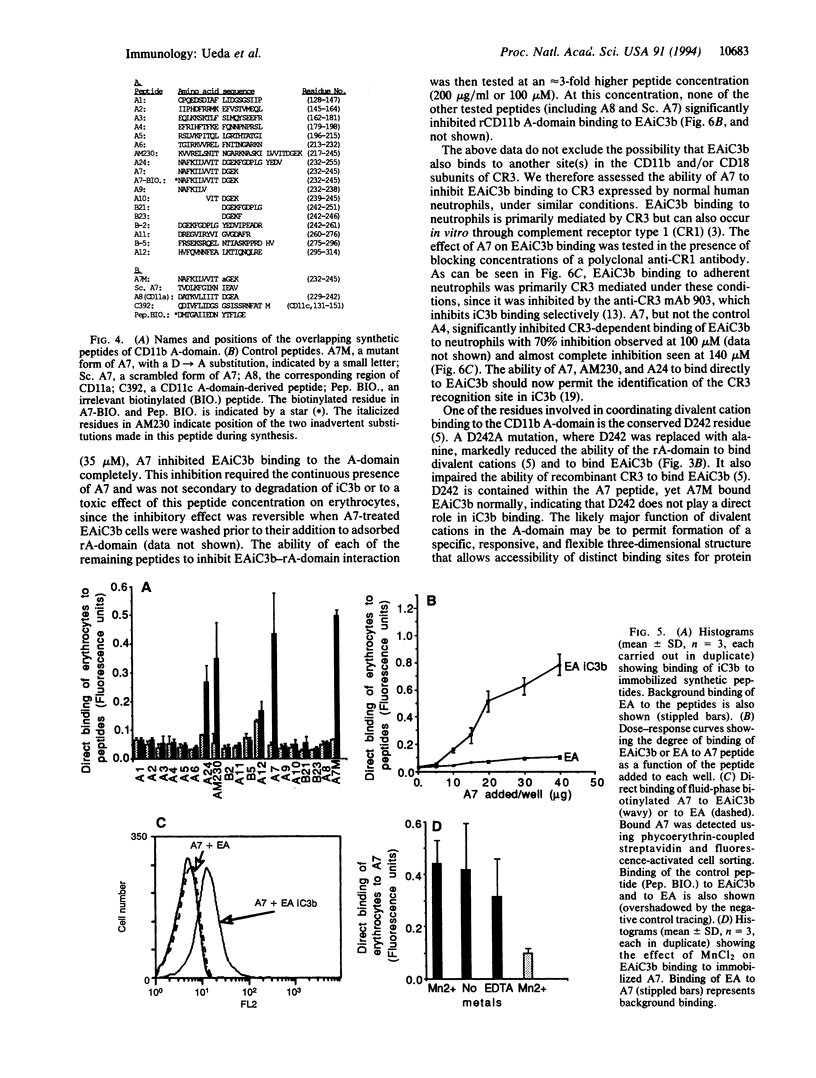
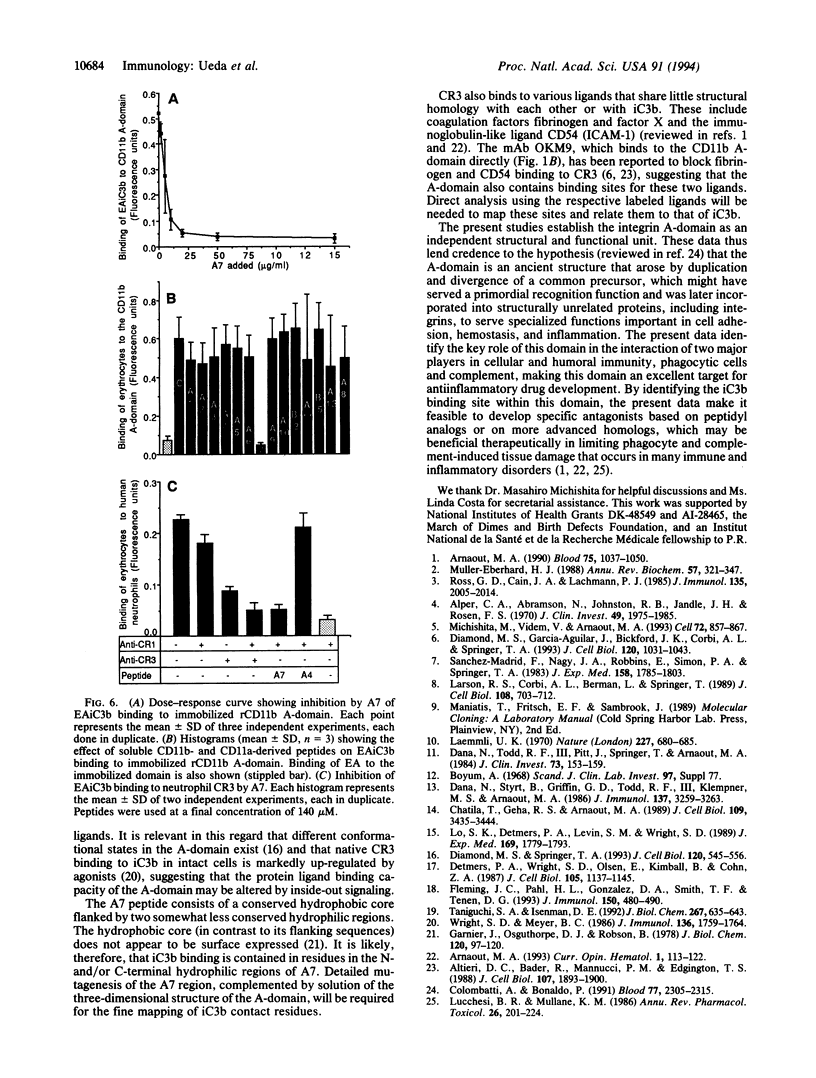
The divalent cation-dependent interaction of the beta 2 integrin CR3 (CD11b/CD18) with the major complement opsonic C3 fragment iC3b is an important component of the central role of CR3 in inflammation and immune clearance. In this investigation we have identified the iC3b binding site in CR3. A recombinant fragment representing the CR3 A-domain, a 200-amino acid region in the ectodomain of the CD11b subunit, bound to iC3b directly and in a divalent cation-dependent manner. The iC3b binding site was further localized to a short linear peptide that also bound iC3b directly and inhibited iC3b binding to the A-domain as well as to CR3 expressed by human neutrophils. These data establish a major recognition function for the integrin A-domain and have important implications for development of novel antiinflammatory therapeutics.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A., Abramson N., Johnston R. B., Jr, Jandl J. H., Rosen F. S. Studies in vivo and in vitro on an abnormality in the metabolism of C3 in a patient with increased susceptibility to infection. J Clin Invest. 1970 Nov;49(11):1975–1985. doi: 10.1172/JCI106417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri D. C., Bader R., Mannucci P. M., Edgington T. S. Oligospecificity of the cellular adhesion receptor Mac-1 encompasses an inducible recognition specificity for fibrinogen. J Cell Biol. 1988 Nov;107(5):1893–1900. doi: 10.1083/jcb.107.5.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout M. A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990 Mar 1;75(5):1037–1050. [PubMed] [Google Scholar]
- Chatila T. A., Geha R. S., Arnaout M. A. Constitutive and stimulus-induced phosphorylation of CD11/CD18 leukocyte adhesion molecules. J Cell Biol. 1989 Dec;109(6 Pt 2):3435–3444. doi: 10.1083/jcb.109.6.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombatti A., Bonaldo P. The superfamily of proteins with von Willebrand factor type A-like domains: one theme common to components of extracellular matrix, hemostasis, cellular adhesion, and defense mechanisms. Blood. 1991 Jun 1;77(11):2305–2315. [PubMed] [Google Scholar]
- Dana N., Styrt B., Griffin J. D., Todd R. F., 3rd, Klempner M. S., Arnaout M. A. Two functional domains in the phagocyte membrane glycoprotein Mo1 identified with monoclonal antibodies. J Immunol. 1986 Nov 15;137(10):3259–3263. [PubMed] [Google Scholar]
- Dana N., Todd R. F., 3rd, Pitt J., Springer T. A., Arnaout M. A. Deficiency of a surface membrane glycoprotein (Mo1) in man. J Clin Invest. 1984 Jan;73(1):153–159. doi: 10.1172/JCI111186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmers P. A., Wright S. D., Olsen E., Kimball B., Cohn Z. A. Aggregation of complement receptors on human neutrophils in the absence of ligand. J Cell Biol. 1987 Sep;105(3):1137–1145. doi: 10.1083/jcb.105.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. S., Garcia-Aguilar J., Bickford J. K., Corbi A. L., Springer T. A. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993 Feb;120(4):1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. S., Springer T. A. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 1993 Jan;120(2):545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. C., Pahl H. L., Gonzalez D. A., Smith T. F., Tenen D. G. Structural analysis of the CD11b gene and phylogenetic analysis of the alpha-integrin gene family demonstrate remarkable conservation of genomic organization and suggest early diversification during evolution. J Immunol. 1993 Jan 15;150(2):480–490. [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson R. S., Corbi A. L., Berman L., Springer T. Primary structure of the leukocyte function-associated molecule-1 alpha subunit: an integrin with an embedded domain defining a protein superfamily. J Cell Biol. 1989 Feb;108(2):703–712. doi: 10.1083/jcb.108.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S. K., Detmers P. A., Levin S. M., Wright S. D. Transient adhesion of neutrophils to endothelium. J Exp Med. 1989 May 1;169(5):1779–1793. doi: 10.1084/jem.169.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchesi B. R., Mullane K. M. Leukocytes and ischemia-induced myocardial injury. Annu Rev Pharmacol Toxicol. 1986;26:201–224. doi: 10.1146/annurev.pa.26.040186.001221. [DOI] [PubMed] [Google Scholar]
- Michishita M., Videm V., Arnaout M. A. A novel divalent cation-binding site in the A domain of the beta 2 integrin CR3 (CD11b/CD18) is essential for ligand binding. Cell. 1993 Mar 26;72(6):857–867. doi: 10.1016/0092-8674(93)90575-b. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Yount W. J., Walport M. J., Winfield J. B., Parker C. J., Fuller C. R., Taylor R. P., Myones B. L., Lachmann P. J. Disease-associated loss of erythrocyte complement receptors (CR1, C3b receptors) in patients with systemic lupus erythematosus and other diseases involving autoantibodies and/or complement activation. J Immunol. 1985 Sep;135(3):2005–2014. [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi-Sidle A., Isenman D. E. Mutagenesis of the Arg-Gly-Asp triplet in human complement component C3 does not abolish binding of iC3b to the leukocyte integrin complement receptor type III (CR3, CD11b/CD18). J Biol Chem. 1992 Jan 5;267(1):635–643. [PubMed] [Google Scholar]
- Wright S. D., Meyer B. C. Phorbol esters cause sequential activation and deactivation of complement receptors on polymorphonuclear leukocytes. J Immunol. 1986 Mar 1;136(5):1759–1764. [PubMed] [Google Scholar]




