Abstract
Background
Induced sputum in children with bronchial asthma represents a non-invasive method of bronchial inflammation assessment. The main objective of our study was to analyze the cellularity of sputum in patients with bronchial asthma according to the level of disease control and the controlling therapy (with/without inhaled glucocorticoids). The second objective was to establish the correlation between sputum cellularity and other indirect parameters used to evidence bronchial inflammation (exhaled nitric oxide) and obstruction (forced expiratory volume in 1 second).
Methods
The study included children with bronchial asthma that were assessed clinically (physical exam, questionnaire on the control of bronchial asthma in children) and by medical tests (induced sputum, exhaled nitric oxide, spirometry).
Results
In patients with partially controlled asthma and those with uncontrolled asthma, the eosinophils percentage in the sputum was higher than in patients with controlled asthma (19.8±26.4% respectively 9.2±20.5% versus 4.5±14.6%, p<0.001). Higher percentage of neutrophils in the sputum was found in the partially controlled and uncontrolled asthma than in the controlled asthma (43.9±20.1% respectively 51.6±38.3% versus 35±19.7%, p=0.009). We also evidenced a direct and statistically significant correlation between the exhaled nitric oxide and the neutrophils percentage in the sputum (r=0.67, p=0.0003). Also, an indirect, moderate to good correlation (r=−0.56, p=0.005) was evidenced between the values of the forced expiratory volume in 1 second and the high eosinophils percentage in the sputum.
Conclusions
In partially controlled and uncontrolled bronchial asthma the eosinophils and neutrophils count in the sputum is significantly higher than in patients with controlled asthma. There is an indirect correlation between the high eosinophils count in the sputum and the forced expiratory volume in 1 second, as well as a direct correlation between the neutrophils count and the exhaled nitric oxide, suggesting that induced sputum should be used in combination with other indirect parameters for the evidence of bronchial inflammation.
Keywords: induced sputum, child, bronchial asthma, eosinophils, inflammation
Background and Aims
Bronchial inflammation represents one of the main pathophysiological changes in bronchial asthma (BA) [1]. The intensity of the inflammation influences the frequency and extent of symptoms, as well as the level of disease control. The controlling therapy targets the bronchial inflammation. Most tests assess bronchial inflammation indirectly. Induced sputum provides direct information about inflammation [1].
The non-invasive character and the possibility to perform this test at any disease stage, including exacerbation period, puts it in a special place in the process of evaluation of the child with bronchial asthma [2]. The method is safe, easy to perform and generally well tolerated by children, helping establish the diagnosis and the long-term management. It is also used for research purposes and may be an alternative to bronchoalveolar lavage [2,3,4].
Our study’s main objective was the analysis of the sputum cellularity in patients with BA in correlation with the level of disease control and the controlling therapy – with/without inhaled glucocorticoid (IGC). The second objective was to establish the correlation between sputum cellularity and other indirect parameters used to evidence bronchial inflammation (forced expiratory volume in 1 second – FEV1, exhaled nitric oxide – eNO).
Patients and methods
The study was carried out in a group of children diagnosed with BA, admitted to the 3rd Pediatric Clinical Hospital of Cluj-Napoca, Romania, during July 2008 – March 2012. The study was performed after obtaining informed consent of the parents, according to the regulations of the Ethics Committee of the Emergency Clinical Hospital for Children.
The inclusion criteria were:
- certified diagnosis of BA;
- age between 6 – 18 years;
- absence of airways acute infection signs in the past 6 weeks;
- patient’s ability to perform respiratory function test correctly;
- parents’ informed consent.
We excluded from our study the patients in exacerbation and who could not perform the technique of induced sputum and the spirometric test adequately.
BA diagnosis was established based on the clinical manifestations and at least one of the following criteria:
- positive bronchial reversibility: increase of FEV1 by over 12% after salbutamol administration;
- previous admissions for exacerbations;
- at least two presentations to the emergency room due to asthma exacerbations.
In children with BA included in our study, we performed induced sputum along with spirometry and determination of eNO. We also established the level of disease control.
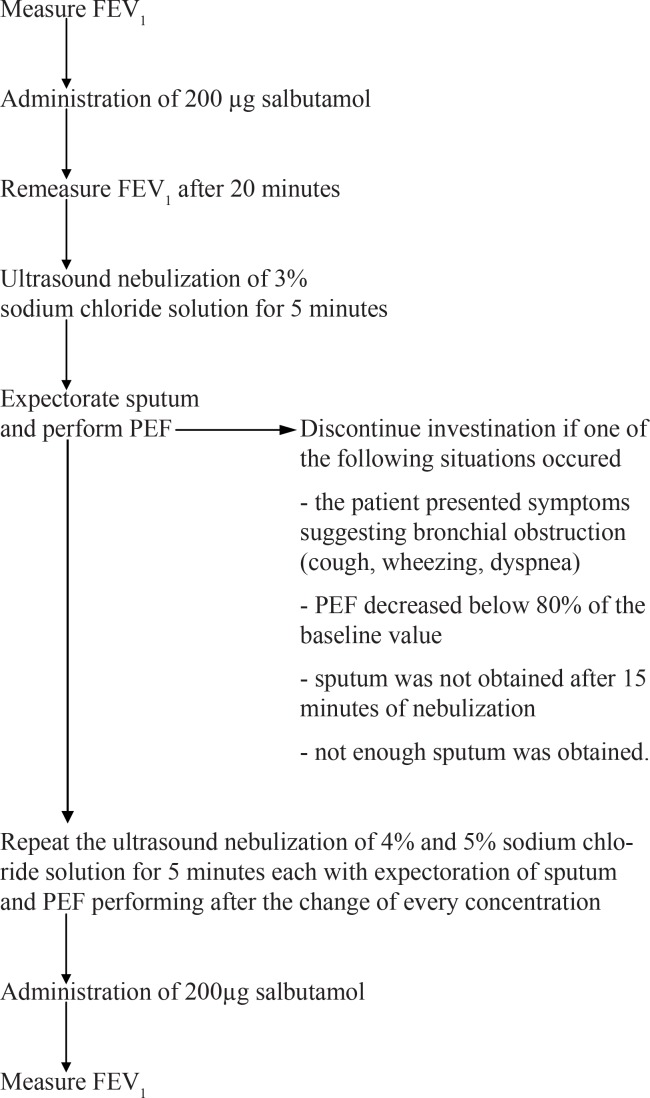
We started sputum induction with ultrasound nebulization of 3% sodium chloride solution for 5 minutes, after which the patient was asked to expectorate. If sputum was not obtained with this concentration, higher concentrated saline solutions of 4% and 5% were nebulized for another 5 minutes each. After the change of every concentration, the patient was asked to expectorate. Both before and after saline nebulization, the patients were assessed by spirometry and received 200 µg salbutamol at the beginning and at the end of the test. When changing the solutions, the patient was asked to perform the peak expiratory flow (PEF) using the peak flow meter (Figure 1).
Figure 1.
Method for sputum induction
The collected sputum was processed within maximum 2 hours from the procedure. The sputum sample was weighed and Dithiothreitol (DTT) was added in a 4:1 ratio. The sputum-DTT mixture was centrifuged (300–1500 X g) for 5 minutes, thus having two phases: the sediment (represented by cells, which was analyzed under the microscope), and the liquid phase [5].
After centrifugation, the cells from the sediment were counted with a hemocytometer, the test being valid for interpretation only if the counting showed at least 400 non-squamous cells on slides [6].
From the centrifugal sediment, a May-Grunwald-Giemsa stained smear was made. Using the optical microscope the number of eosinophils (Eo), neutrophils (N), macrophages and bronchial epithelial cells were counted, each value being expressed as percentage from the total of non-squamous cells. The sample obtained was considered unsatisfactory if the percentage of squamous cells was higher than 80%.
According to the literature, we considered the upper limit of normal 2.5% for eosinophils and 47% for neutrophils from the sputum [7].
The determination of eNO was performed with an appropriate device (Niox-MiNo Sweden), the results being expressed in p.p.b. (parts per billion). For children under IGC therapy, high levels of eNO were considered to be ≥20 p.p.b. if under 12 years of age, and ≥25 p.p.b. if over 12 years. In patients not under IGC therapy, high eNO values were considered ≥5 p.p.b..
The degree of bronchial obstruction was established by spirometry. FEV1 values <80% indicated bronchial obstruction. The bronchial reversibility test was considered positive in cases in which FEV1 increased by more than 12% after salbutamol administration.
Asthma was labeled as atopic if patients presented one or more of the following: associated allergic rhinitis, positive skin test for one or several allergens, high total immunoglobulin E (IgE) concentration. We used the prick technique for allergy skin tests and the total IgE concentration was determined by ELISA.
The level of the disease control was assessed based on the GINA guidelines [8].
Statistical analysis
The obtained data were processed using the SPSS v. 15 software and Microsoft Excel. We calculated the statistically significant differences between the means of variables obtained in the study subgroups. When we had 3 subgroups we used the Kruskal-Wallis test, while for the situations in which we formed 2 subgroups the Mann-Whitney test was applied. Statistical significance of differences between groups was established at p ≤0.05. The correlation between the parameters was documented using Spearman’s correlation coefficient (direct correlation if r>0.6 and indirect correlation if r<−0.6), considered statistically significant if p<0.05.
Results
The characteristics of children with BA included in the study are shown in Table I.
Table I.
Characteristics of the patients included in the study
| Parameter | Value |
|---|---|
| Age (mean ± SD) | 10.4±3.1 (5–18 years) |
| Male (%) | 58 |
|
| |
| Allergy skin test (%) | 100 (16/16)* |
| Allergic rhinitis (%) | 48 |
|
| |
| IGC therapy | |
| Doses (budesonide equivalent) | |
| <200 µg (%) | 15 |
| 200–400 µg (%) | 31 |
| >400 µg (%) | 54 |
|
| |
| Level of disease control (GINA) | |
| Controlled BA (%) | 31 |
| Partially controlled BA (%) | 51 |
| Uncontrolled BA (%) | 18 |
of the patients examined
Sputum for cellularity assessment was obtained from 55 of the 67 patients studied (82%).
During the test only 11 children (16.4%) had mild adverse reactions: rhinorheea (7.4%), sneezing (2.9%), cough (5.9%), FEV1 decrease by more than 12% after nebulization (4.4%). The symptoms subsided completely after salbutamol administration.
Sputum cellularity evidenced the existence of statistically significant differences both for eosinophils (p=0.049) and neutrophils (p=0.004) between patients with controlled BA and those with partial or uncontrolled disease.
In patients with controlled BA the eosinophils count was significantly lower than in those with partially controlled or uncontrolled BA. Regarding neutrophils, the highest percentage was found in patients with uncontrolled BA, while the lowest count was observed in patents with controlled BA.
The analysis of the sputum’s cellularity in relation to the controlling therapy did not evidence statistically significant differences between patients on IGC therapy and those without this therapy. Higher neutrophils levels were found in patients with uncontrolled asthma, both in those under IGC therapy and those without this treatment (Table IV).
Table IV.
Sputum neutrophilia in relation to the therapy used to control the disease
| Neutrophils (%) | Controlled BA (20 patients) | Partially controlled BA (25 patients) | Uncontrolled BA (10 patients) | p |
|---|---|---|---|---|
| Netrophils ≥47% (Mean±SD) | 5 patients | 15 patients | 8 patients | |
| With IGC | 67.5±9.5 | 55.9±5.6 | 71.6±24.9 | 0.07 |
| Without IGC | - | 64.8±16.5 | 80.5±7 | 0.1 |
| Netrophils <47% (Mean±SD) | 15 patients | 10 patients | 2 patients | |
| With IGC | 25.6±11.6 | 28±16 | - | 0.07 |
| Without IGC | 25.2±5.5 | 26±17 | 10±7 | 0.19 |
Analyzing the sputum cellularity in relation to spirometric parameters, we found higher eosinophils counts in patients with FEV1 ≤80% as compared with those with normal FEV1 (mean±SD: 40.3±30.3% versus 10.6±11.9%, p=0.003) (Table V).
Table V.
Sputum cellularity in relation to FEV1
| Sputum cellularity (%) | FEV1 | p | |
|---|---|---|---|
| ≤80% | >80% | ||
| Eosinophilic sputum | |||
| Eosinophils ≥ 2.5% | 40.3±30.3 | 10.6±11.9 | 0.003 |
| Eosinophils<2.5% | - | 0.5±0.8 | - |
| Neutrophilic sputum | |||
| Neutrophils ≥47% | 61±10.8 | 68.2±15.04 | 0.19 |
| Neutrophils <47% | 21±15.5 | 25.3±11.9 | 0.61 |
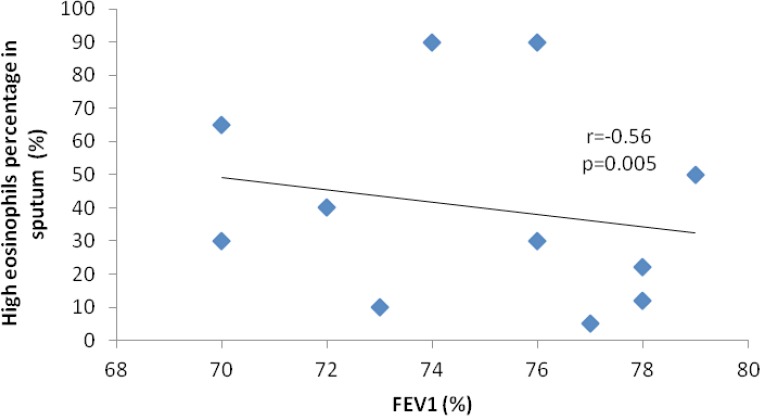
When analyzing FEV1 in patients with high eosinophils values, we observed the existence of a moderate to good indirect correlation (r=−0.56, p=0.005), but the correlation was not present for patients with normal eosinophils values (Figure 1). We could not establish a correlation between FEV1 and the neutrophils count in the sputum, whether the latter was high or normal.
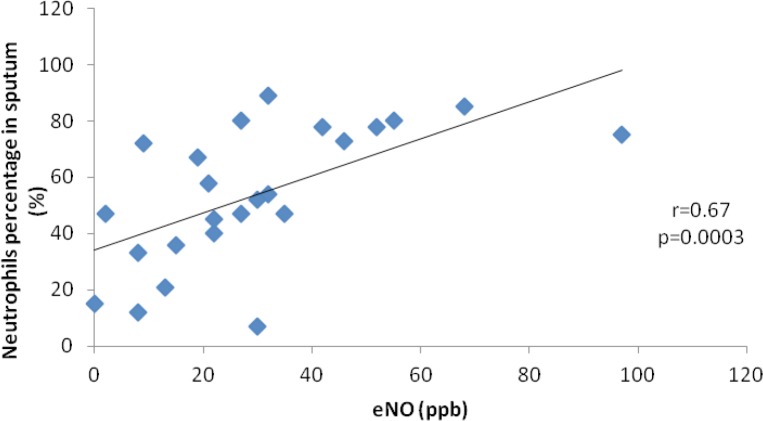
Of all 55 patients that provided sputum sample, only 24 underwent determination of eNO. Our study demonstrated the presence of a moderate to good correlation, statistically significant, between the value of eNO and the neutrophils percentage in the sputum (r=0.67, p=0.0003).
Discussion
Our study evidenced higher eosinophils and neutrophils values in the sputum of patients with partially controlled or uncontrolled BA than in those with controlled asthma. We also obtained a statistically significant correlation between the value of eNO and the neutrophils percentage in the sputum, which demonstrates the same involvement of neutrophils in the bronchial inflammatory process.
The indirect correlation between the high eosinophils percentage and FEV1 and the direct correlation between neutrophils and eNO point to the value of induced sputum test when it is associated with other indirect parameters used in the assessment of bronchial inflammation.
The success rate of induced sputum test in our study was 82%, similar to the reports in literature [2,9,10,11,12]. During the test we recorded adverse reactions in 16.4% of the patients, similar to other studies [2]. In 4.4% of the patients in which cough was induced, FEV1 decreased by more than 12% after saline nebulization, but it was restored after salbutamol administration.
The highest percent of eosinophils was observed in patients with partially controlled asthma (18±25.2), comparing with patients with uncontrolled asthma (4.9±14.4). This finding is not according to most of the studies from literature and the explanation for this result can be the relatively small number of paient included in our study, especially by those with uncontrolled asthma.
Induced sputum is considered to be one of the most accurate method of assessment of bronchial inflammation. The technique is non-invasive, cheap and may be performed even in patients during a bronchial obstruction episode. The method is successfully used not only in patients with BA, but also in other diseases, such as chronic obstructive pulmonary disease, cystic fibrosis, chronic cough [13,14,15].
Though most studies have considered the eosinophils count in the sputum to be relevant for bronchial inflammation, more recent data suggest the same relevance regarding neutrophils. Various studies evidenced high values of neutrophils in the sputum of patients with BA, especially uncontrolled asthma [9,16,17,18].
A highly increased neutrophils count was evidenced in the sputum of patients during an exacerbation episode, triggered by a viral infection or in which respiratory symptoms were persistent [16,17,18]. In the case of these patients, the eosinophils count was generally low, while the response to IGC therapy was poor. This explanation is unlikely to be valid for our study, as we excluded patients with signs of acute respiratory infections in the 6 weeks prior the study.
Various studies analyzing the response to controlling therapy in patients with a high neutrophils level evidenced a lack of response to IGC [19,20,21].
The lack of studies on the role of neutrophils in the pathogenesis of BA may be explained by the fact that many researchers did not investigate or report all cell types from the sputum, focusing only on eosinophils [10,11,22]. Another explanation may be related to the method of sputum collection, many authors analyzing only the samples rich in mucus and not the whole amount of sputum obtained [23,24,25,26].
Our study supports the theory according to which BA should be also classified according to the inflammatory phenotype of sputum cellularity. The literature describes 3 phenotypes according to sputum cellularity: eosinophilic, neutrophilic and mixed. Certain studies mention the neutrophilic type more frequently in severe asthma, refractory to IGC therapy [27,9].
Conclusions
Although our study showed that in uncontrolled asthma patients the predominant sputum cells are neutrophils and not eosinophils like we used to know, there are some recent ones that demonstrated the importance of this type of cells. This studies highlights that in uncontrolled asthmatic patients that do not respond to the glucocorticoid therapy, the explanation rises from the presence of a high percent of neutrophils in sputum. The results have clinical implications because in this patients the therapy needs to be changed and this is a motive for more studies to be made.
Regardless the contradictory results, we all agree in the importance of induced sputum test, especially when it is associated with other indirect parameters used in the assessment of bronchial inflammation.
Figure 2.
Correlation between FEV1 and high percentage of eosinophils
Figure 3.
Correlation between sputum neutrophils percentage and the value of eNO
Table II.
Sputum cellularity in relation to the level of disease control
| Controlled BA (n=20) | Partially controlled BA (n=25) | Uncontrolled BA (n=10) | p | |
|---|---|---|---|---|
| Eosinophils (%) | 4.5±14.6 | 18±25.2 | 4.9±14.4 | 0.049 |
| Neutrophils (%) | 35±19.7 | 46.2±20.1 | 64.6±30.7 | 0.004 |
| Macrophages (%) | 11.2±13.6 | 5.2±7.6 | 1±0.5 | 0.02 |
| Epithelial cells (%) | 46.8±29 | 26.9±24.2 | 20.1±10.8 | 0.02 |
Table III.
Number of patients in relation to sputum celullarity and the level of disease control
|
Sputum celullarity
|
Eosinophils | Neutrophils | ||
|---|---|---|---|---|
| Level of disease control | ≥2.5% | <2.5% | ≥47% | <47% |
|
| ||||
| Controlled BA | 3 | 17 | 5 | 15 |
| Partially controlled BA | 20 | 5 | 15 | 10 |
| Uncontrolled BA | 1 | 9 | 8 | 2 |
References
- 1.National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Araujo L, Moreira A, Palmares C, Beltrao M, Fonseca J, Delgado L. Induced sputum in children: Success determinants, safety, and cell profiles. J Investig Allergol Clin Immunol. 2011;21(3):216–221. [PubMed] [Google Scholar]
- 3.Nocker RET, Out TA, Weller FR, De Riemer MJ, Jansen HM, van der Zee JS. Induced sputum and bronchoalveolar lavage as tools for evaluating the effects of inhaled corticosteroids in patients with asthma. J Lab Clin Med. 2000;136(1):39–49. doi: 10.1067/mlc.2000.107305. [DOI] [PubMed] [Google Scholar]
- 4.Rutgers SR, Timens W, Kaufmann HF, van der Mark TW, Koeter GH, Postma DS. Comparison of induced sputum with bronchial wash, bronchoalveolar lavage and bronchial biopsies in COPD. Eur Respir J. 2000;15(1):109–115. doi: 10.1183/09031936.00.15110900. [DOI] [PubMed] [Google Scholar]
- 5.Scheicher ME, Terra Filho J, Oliveira Vianna E. Sputum induction: review of literature and proposal for a protocol. Sao Paulo Med J. 2003;121(5):213–219. doi: 10.1590/S1516-31802003000500008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson PG, Wlodarczyk JW, Hensley MJ, Gleeson M, Henry RL, Cripps AW, et al. Epidemiological association of airway inflammation with asthma symptoms and airway hyperresponsiveness in childhood. Am J Respir Crit Care Med. 1998;158(1):36–41. doi: 10.1164/ajrccm.158.1.9705031. [DOI] [PubMed] [Google Scholar]
- 7.Cai Y, Carty K, Henry RL, Gibson PG. Persistence of sputum eosinophilia in children with controlled asthma when compared with healthy children. Eur Respir J. 1998;11(4):848–853. doi: 10.1183/09031936.98.11040848. [DOI] [PubMed] [Google Scholar]
- 8.Global Strategy for Asthma Management and Prevention. [21 July 2014]. Available from: http://www.ginasthma.org.
- 9.Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol. 1994;95(4):843–852. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 10.Gibson PG, Henry RL, Thomas P. Noninvasive assessment of airway inflammation in children: induced sputum, exhaled nitric oxide, and breath condensate. Eur Respir J. 2000;16:1008–1015. [PubMed] [Google Scholar]
- 11.Wilson NM, Bridge P, Spanevello AW, Silverman M. Induced sputum in children: feasibility, repeatability, and relation of findings to asthma severity. Thorax. 2000;55(9):768–774. doi: 10.1136/thorax.55.9.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pin I, Gibson PG, Kolendowicz R, Girgis-Gabardo A, Denburg JA, Hargreave FE, et al. Use of induced sputum cell counts to investigate airwy inflammation in asthma. Thorax. 1992;47:25–29. doi: 10.1136/thx.47.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pizzichini E, Pizzichini MMM, Gipson P, Parameswaran K, Gleich GJ, Berman L, et al. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med. 1998;158:1511–1517. doi: 10.1164/ajrccm.158.5.9804028. [DOI] [PubMed] [Google Scholar]
- 14.Brightling CE, Monteiro W, Ward R, Parker D, Morgan MD, Wardlaw AJ, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomized controlled trial. Lancet. 2000;356(9240):1480–1485. doi: 10.1016/S0140-6736(00)02872-5. [DOI] [PubMed] [Google Scholar]
- 15.Gibson PG, Dolovich J, Denburg J, Ramsdale EH, Hargreave FE. Chronic cough: eosinophilic bronchitis without asthma. Lancet. 1989;1(8651):1346–1348. doi: 10.1016/s0140-6736(89)92801-8. [DOI] [PubMed] [Google Scholar]
- 16.Tillie-Leblond I, Thorette C. Neutrophils and severe asthma attacks. Revue francaise d’allergologie et d’immunologie clinique. 2005;45:63–67. [Google Scholar]
- 17.Sur S, Croty TB, Kephart GM, Hyma BA, Colby TV, Reed CE, et al. Sudden-onset fatal asthma: a distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am Rev Respir Dis. 1993;148(3):713–719. doi: 10.1164/ajrccm/148.3.713. [DOI] [PubMed] [Google Scholar]
- 18.Fujisawa T, Kephart GM, Gray BH, Gleich GJ. The neutrophil and chronic airway inflammation. Am Rev Respir Dis. 1990;141:689–697. doi: 10.1164/ajrccm/141.3.689. [DOI] [PubMed] [Google Scholar]
- 19.Basyigit I, Yildiz F, Ozkara SK, Boyaci H, Ilgazli A. Inhaled corticosteroid effects both eosinophilic and non-eosinophilic inflammation in asthmatic patients. Mediators Inflamm. 2004;13(4):285–291. doi: 10.1080/09629350400003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uribe Echevarria EM, Maldonado CA, Uribe Echevarría AM, Aoki A. Neutrophil predominance in induced sputum from asthmatic patients. Therapeutic implications and role of clara cell 16-KD protein. Medicina (B Aires) 2011;71(4):343–349. [PubMed] [Google Scholar]
- 21.Leuppi JD, Salome CM, Jenkins CR, Koskela H, Brannan JD, Anderson SD, et al. Markers of airway inflammation and airway hyperresponsiveness in patients with well-controlled asthma. Eur Respir J. 2001;18:444–450. doi: 10.1183/09031936.01.00058601. [DOI] [PubMed] [Google Scholar]
- 22.Profita M, Sala A, Bonanno A, Riccobono L, Siena L, Melis MR, et al. Increased prostagladin E2 concentrations and cyclooxygenase-2 expression in asthmatic subjects with sputum eosinophilia. J Allergy Clin Immunol. 2003;112(4):709–716. doi: 10.1016/s0091-6749(03)01889-x. [DOI] [PubMed] [Google Scholar]
- 23.Popov TA, Pizzichini MM, Pizzichini E, Kolendowicz R, Punthakee Z, Dolovich J, et al. Some technical factors influencing the induction of sputum for cell analysis. Eur Respir J. 1995;8:559–565. [PubMed] [Google Scholar]
- 24.Fahy JV, Liu J, Wong H, Boushey HA. Cellular and biochemical analysis of induced sputum from asthmatic and from healthy subjects. Am Rev Respir Dis. 1993;147:1126–1131. doi: 10.1164/ajrccm/147.5.1126. [DOI] [PubMed] [Google Scholar]
- 25.Spanevello A, Beghe B, Bianchi A, Migliori GB, Ambrosetti M, Neri M, et al. Comparison of two methods of processing induced sputum: selected versus entire sputum. Am J Respir Crit Care Med. 1998;157(2):665–668. doi: 10.1164/ajrccm.157.2.9705095. [DOI] [PubMed] [Google Scholar]
- 26.Louis R, Shute J, Goldring K, Perks B, Lau LCK, Radermecker M, et al. The effect of processing on inflammatory markers in induced sputum. Eur Respir J. 1999;13:660–667. doi: 10.1183/09031936.99.13366099. [DOI] [PubMed] [Google Scholar]
- 27.Tseliou E, Bessa V, Hillas G, Delimpoura V, Papadaki G, Roussos C, et al. Exhaled nitric oxide and exhaled breath condensate pH in severe refractory asthma. Chest. 2010;138:107–113. doi: 10.1378/chest.09-1257. [DOI] [PubMed] [Google Scholar]