Abstract
Background and aims:
Castleman’s disease is a rare disorder situated at the boundary between reactive and neoplastic conditions. The pathogenesis is a subject of debate and the limited number of cases renders the study of the disease difficult.
In our paper we present a series of six cases of Castleman disease with emphasis on the clinical presentation, pathology examination and the use of immunohistochemistry in the final diagnosis of the cases.
Patients and method:
The classification of the disease was based on clinical, imaging and pathological assessment. Specimens were obtained by surgical excision and were routinely processed for the pathology examination.
Results:
All cases were unicentric disease. Two cases were locally extensive. The clinical symptoms were related mostly to compression effects. Five case were of the hyaline-vascular type and one was included in the plasma cell variant. One case showed angiomyoid differentiation.
Conclusions:
We strongly believe that by understanding the pathogenesis of the precursor lesions we will gain better understanding of the pathways that lead to neoplasia and that Castleman disesase is a very interesting ”natural experiment” illustrating the progression from chronic antigen stimulation to reactive lymphoid hyperplasia and finally to overt lymphoid neoplasia.
Keywords: Giant lymph node hyperplasia, Interleukin-6, Herpesvirus 8, Human, Immunohistochemistry
Background and aims
The gathering of new evidence in the field of lymphoproliferative disorders has brought to attention entities considered benign or of uncertain malignant potential. Castelman’s disease (CD) is included in this wide and poorly defined category. Described by Dr. Benjamin Castleman in 1954 and later in 1956 as a hyperplastic process involving mediastinal lymph nodes, the disease was most often mistaken for a thymoma and the clinical course was thought initially to be benign [1]. The disease has been granted many synonyms (angiofollicular lymph node hyperplasia, giant cell lymph node hyperplasia, follicular lymphoreticuloma, lymphoid hamartoma [2,3], perhaps due to the lack of evidence regarding the pathogenesis. Chronic viral stimulation, especially Herpes virus 8 (HHV-8, Kaposi sarcoma virus) infection, the IL-6 signaling cascade, angiogenesis and clonal rearrangements play a role in the pathogenesis of CD [4].
CD is rare and exact statistical data are missing [3]. In the simplest way the disease can be classified as localized (unicentric) or multicentric based on the clinical presentation and imaging examination [5,6]. Microscopically the disease has two main types: the hyaline vascular type and the plasma cell type [7]. The localized form is clinically silent and is associated commonly with the hyaline vascular type [3]. The multicentric disease is associated with general symptoms (fatigue, fever) and nonspecific laboratory findings (increased sedimentation rates, hypoalbuminemia, anemia etc) [4,8]. The rarity of the disease also influences the therapeutic approach, no unique therapeutic regimens being unanimously accepted [3,9,10,11,12]. Disease associations include the POEMS syndrome (Polyneuropathy, Organomegaly, Endocrinopathy, M-protein, and Skin changes) [13,14], Kaposi sarcoma, pemphigus, refractory anemia, nephrotic syndrome, amyloidosis [15,16,17]. Associations with lymphoid malignancies have been described and these include: diffuse large- B cell lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma and lymphoplasmacytic lymphoma, follicular lymphoma [18].
The prognosis is dependent on the disease type. The localized form has an excellent prognosis following excision or radiotherapy, but the multicentric form (especially with plasma cell histology) frequently requires systemic therapy [3,4].
We present in this paper a series of six cases, from a clinical and pathological perspective. Emphasis was placed on the clinical presentation and histology. Detailed information are presented regarding the use of immunohistochemistry (IHC) in the final diagnosis of the case.
Patients and method
The tumors were classified as unicentric by clinical and imaging criteria. Specimens were obtained by surgery and the processing was routinely performed (fixation in formalin 10% and paraffin embedding). Immunohistochemical stains were performed following the manufactures specifications. One case was referred to our department from an external source and information was limited.
Results
The disease was unicentric in all cases. The mean age was 33.5 years, with female: male ratio of 4:2. Symptoms were related mostly to the compression effects. One case showed other hematological abnormalities. Mean tumor size was of 5.5 cm. Two case (cases 3 and 6) were locally extensive.
Gross aspect was represented by gray white tumors, with variable delimitation and on cut surface a typical multinodular appearance was present in most cases. Five cases were pathologically classified as the hyaline vascular type of Castleman’s disease and one was classified as the plasma cell type. One case (case 4) showed angiomyoid proliferation and another showed only focal changes suggestive for this particular subtype (case 5). Immunhistochemical stains confirmed the normal distribution of B and T cell compartments (CD3, CD20) and showed the distribution of blood vessels (CD31, CD34). CD21 and CD23 revealed the follicular dendritic cells in the germinal centers and in the interfollicular area. CD138 revealed plasma cells. BCL2 and Cyclin D1 staining pattern were used to exclude follicular respectively mantle cell lymphoma.
Discussion
The cases were classified as hyaline vascular (83.3%) and plasma cell variants (16.7%). Although the number of cases is low, the percentage distribution of cases is similar to the data in literature [4]. The mean age for the hyaline vascular type was 28.2 years, much younger than the patient with the plasma cell variant (number 6), also consistent with data from the literature [3,4].
The pathology findings were based on the following criteria: diffuse effacement of normal lymph node histology, proliferation of abnormal lymphoid follicles with expanded mantle zone, vascular proliferation of hylinized capillaries extending into the germinal center, expansion of the interfollicular area for the hyaline vascular type [19]. The arguments for angiomyoid differentiation were represented by spindle cell proliferation and reactivity for smooth muscle actin (SMA) [20]. For the plasma cell variant the criteria were as follows: hyperplastic lymphoid follicles, active germinal centers, interfollicular sheets of plasma cells, plasmacytoid monocytes and polyclonal immunoglobulins [19].
In the differential diagnosis we included follicular lymphoma and mantle cell lymphoma for the hyaline type. In the case of the spindle cells variant the main differential diagnosis was the dentritic cell sarcoma. For the plasma cell variant the systemic IgG4-related lymphadenopathy was strongly taken in consideration.
The histological aspect is highly suggestive of a chronic reactive process although some authors include the disease in the lymphproliferative disorders category [4]. Preservation of B and T cell compartments is a useful clue in establishing the diagnosis. Evidence regarding the pathogenesis of the disease are supporting the idea of a viral driven/endogenous driven chronic inflammatory processes mediated by Interleukin-6 (IL-6) [3,4,21].
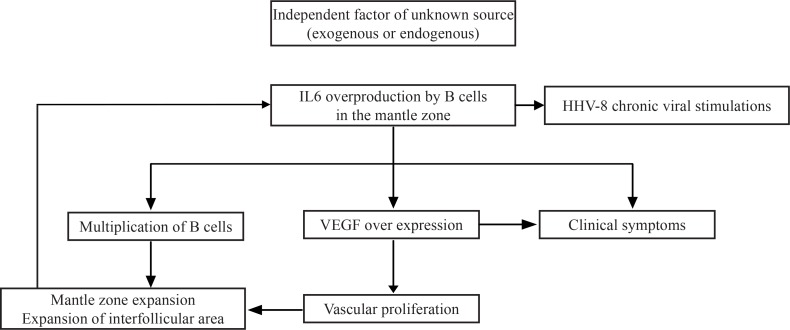
Interleukin-6 has been clearly linked to the pathogenesis, by acting as a promoter of the terminal B cell differentiation and by stimulation of plasma cell proliferation and production of immunglobulins [4,21]. Also, by stimulating the production of acute phase proteins, IL-6 is responsible for part of the clinical manifestations [21]. VEGF has been strongly related to the secretion of IL-6 [3] and acts by promoting cellular survival and angiogenesis. The increased production of VEGF has been strongly related to the manifestations of POEMS syndrome [3,22]. HHV-8 uses the same signaling pathway, due to the presence of a human IL-6 mimic [23].
However drugs targeting the IL-6 pathway, such as the anti-interleukin-6 receptor monoclonal antiboby tocilizumab, although with proven efficiency in most cases, have failed to control the symptoms in a small number of cases [3] suggesting an alternative pathway [24]. IL1, IL5, TNF, epidermal growth factor and interferon alpha [3,4] are other preeminent molecules that have been reported as elevated. The human immunodeficiency virus (HIV) is also involved in the pathogenesis, but its role is secondary to the immunodeficiency status for which it is responsible, which in turn favors the infection with HHV-8 [3]. Clonal rearrangenents in the T-cell receptor and immunoglobulins have not been constantly described [4] and they seem to play a minor role in the pathogenesis.
Conclusions:
We presented a series of Castleman disease cases, with emphasis on the clinical aspects, pathology findings and extensive description of the IHC stains used for the diagnosis. To our best knowledge it is the only series of cases with CD presented so far in Romania, focused on the clinical-pathological findings. Our data are consistent with the ones presented in literature. We strongly believe that by understanding the pathogenesis of the precursor lesions we will gain better understanding of the pathways that lead to neoplasia and that Castleman disease is a very interesting “natural experiment” illustrating the progression from chronic antigen stimulation to reactive lymphoid hyperplasia and finally to overt lymphoid neoplasia.
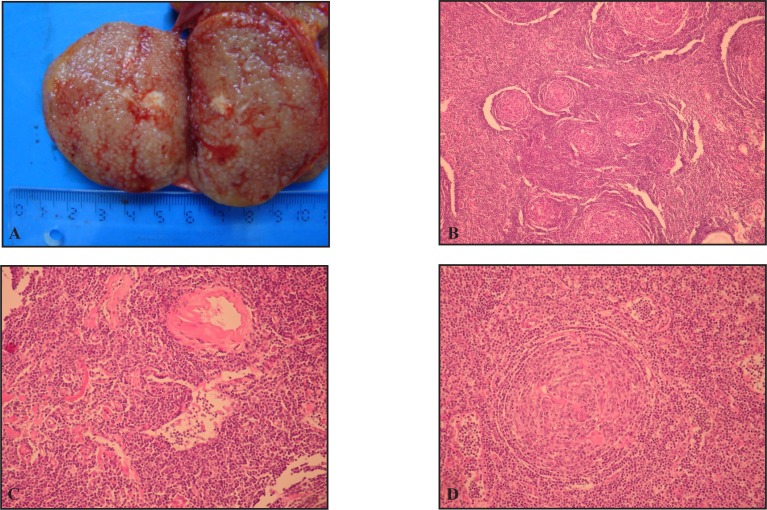
Figure 1.
Gross and microscopic aspect of Castleman disease hyaline vascular type. A. Cut section of lymph node with micronodular pattern B. Follicular hyperplasia replacing the normal architecture (large reactive follicles middle upper and lower part and atrophic follicles in the center); C. Thick walled-hyaline small diameter blood vessels. D. Lymphoid follicle with “onionskin” pattern and hyaline capillaries.
Figure 2.
Schematic representation of the pathogenesis of the disease [4].
Table I.
Clinical features of cases
| Patient Number | Age | Sex | Initial complaints/Cause(s) of discovery | Location | Size (cm) | Observations |
|---|---|---|---|---|---|---|
| 1 | 31 | F | Palpation of nodule | Above the right clavicle | 5/4/2.5 | - |
| 2 | 28 | F | Abdominal pain | Mesentery | 7/5/5.5 | Chronic refractory anemia associated |
| 3 | 17 | M | Palpation of nodule | Left lateral cervical area | 4.5/3/2.3 | - |
| 4 | 31 | F | Pain in the right flank and back | Right retroperitoneal area | 5/8 | Tumor surrounded completely the external iliac vein, partially the right the inferior vena cava and displaced the right ureter and right adnexa |
| 5 | 37 | M | - | - | - | Submitted from an external pathology department |
| 6 | 57 | F | Abdominal pain/Intestinal transit disturbances/Rectal haemorrhage | Mesentery of sigmoid colon | 3/2 | Tumor infiltrated the colonic layers |
Table II.
Summary of the histology findings
| Patient Number | Disease type | Architectural effacement | Mantle zone expansion | Aspect of follicles | Dysplastic follicular dentric cells | Interfollicular space |
|---|---|---|---|---|---|---|
| 1 | Hyaline vascular CD | Yes | Yes | Atrophic Hyaline capillaries penetrating follicles | Yes | Vascular proliferation |
| 2 | Hyaline vascular CD | Yes | Yes (focal) | Large, reactive/Atrophic Hyaline capillaries penetrating follicles Plasma cells |
No | Vascular proliferation Plasma cells |
| 3 | Hyaline vascular CD | Yes/Extension beyond capsule | Yes | Atrophic/Focal fragmentation Hyaline capillaries penetrating follicle |
Yes | Vascular proliferation with high endothelial cells |
| 4 | Hyaline vascular CD | Yes | Yes (focal) | Atrophic Hyaline capillaries penetrating follicles |
No | Expanded Spindle cells Vascular proliferation |
| 5 | Hyaline vascular CD | Yes/Extension beyond capsule | Yes | Atrophic Hyaline capillaries penetrating follicles Fibrosis of germinal centers |
No | Expanded Spindle cells Vascular proliferation Rare plasma cell and eosinophils |
| 6 | Plasma cell CD | Yes/Extension beyond capsule | Yes | Atrophic/Focal fragmentation Plasma cells with Russell bodies |
No | Plasma cells with Russell bodies Vascular proliferation |
Table III.
Immunohistochemical (IHC) profile of cases
| Patient Number | IHC profile |
|---|---|
| 1 | CD20 (predominant in follicular areas, scattered cells in interfollicular area), CD3 (predominant in interfollicular area), CD31 highlight interfollicular capillaries some extending to the germinal center, CD23 and S100 positive on the dendritic follicular areas, AE1/AE3 negative. |
| 2 | CD20 (predominant in follicular areas, scattered cells in interfollicular area), CD3 (predominant in interfollicular area), CD21 highlight follicular dendtritic cell hyperplasia limited to the germinal center, CD10 and BCL-6 were expressed in the germinal center, CD4 and CD4 variable positivity in the intefollicular area, CyclinD1 excluded a mantle cell lymphoma; isolated cells were positive for CD30 in the germinal center and close vicinity, but negative for CD15. Ki-67 was high only in the germinal centers. |
| 3 | CD20 (predominant in follicular areas, scattered cells in interfollicular area), CD3 (predominant in interfollicular area), CD34 highlight intefollicular capillaries some extending to the germinal center; CD21 highlight follicular dendritic cell hyperplasia; Isolated cells were positive for CD30; B cells express k and λ chains |
| 4 | CD20 (predominant in follicular areas, scattered cells in interfollicular area), CD3 (predominant in interfollicular area); CD21 positive on follicular dendritic cells and negative on the spindle cells in the interfollicular area; SMA positive on spindle cells showing myoid differentiation; KI-67 low on spindle in the interfollicular. |
| 5 | CD20 (predominant in follicular areas, scattered cells in interfollicular area), CD3 (predominant in interfollicular area); CD21 and CD23 positive on follicular dendritic cells and negative on the spindle cells in the interfollicular; Spindle cells are negative for S100, CD1a, LCA, CD31; CD30 positive/CD15 negative cells are present in reduced number in the germinal center and its close vicinity; Ki-67 low in the interfollicular area |
| 6 | CD20 (predominant in follicular areas, scattered cells in interfollicular area), CD3 (predominant in interfollicular area); CD23 highlighted the foliicular dendritic cell are; BCL-2 staining pattern excluded a follicular limfoma; Ki-67 had low values on the interfollicular area; Cyclin D1 excluded a mantle cell lymphoma |
References:
- 1.Castleman B, Iverson L, Menendez VP. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer. 1956;9(4):822–830. doi: 10.1002/1097-0142(195607/08)9:4<822::aid-cncr2820090430>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe ES, Lee Harris N, Vardiman JW, Campo E, Arber DA. Hematopathology. 1st ed. Philadelphia: Saunders/Elsevier; 2011. pp. 124–4. [Google Scholar]
- 3.Roca B. Castleman’s Disease. A Review. AIDS Rev. 2009;11(1):3–7. [PubMed] [Google Scholar]
- 4.Casper C. The aetiology and management of Castleman disease at 50 years: translating pathophysiology to patient care. Br J Haematol. 2005;129(1):3–17. doi: 10.1111/j.1365-2141.2004.05311.x. [DOI] [PubMed] [Google Scholar]
- 5.Martino G, Cariati S, Tintisona O, Veneroso S, De Villa F, Vergine M, et al. Atypical lymphoproliferative disorders: Castleman’s disease. Case report and review of the literature. Tumori. 2004;90(3):352–355. doi: 10.1177/030089160409000319. [DOI] [PubMed] [Google Scholar]
- 6.Fajgenbaum DC, van Rhee F, Nabel CS. HHV-8-negative, idiopathic multicentric Castleman disease: novel insights into biology, pathogenesis, and therapy. Blood. 2014;123(19):2924–2933. doi: 10.1182/blood-2013-12-545087. [DOI] [PubMed] [Google Scholar]
- 7.Shahidi H, Myers JL, Kvale PA. Castleman’s disease. Mayo Clin Proc. 1995;70(10):969–977. doi: 10.4065/70.10.969. [DOI] [PubMed] [Google Scholar]
- 8.Sato Y, Kojima M, Takata K, Morito T, Asaoku H, Takeuchi T, et al. Systemic IgG4-related lymphadenopathy: a clinical and pathologic comparison to multicentric Castleman’s disease. Mod Pathol. 2009;22(4):589–599. doi: 10.1038/modpathol.2009.17. [DOI] [PubMed] [Google Scholar]
- 9.Kim MS, Ju JK, Kim Y. Surgical Management of Unicentric Castleman’s Disease in the Abdomen. Ann Coloproctol. 2014;30(2):97–100. doi: 10.3393/ac.2014.30.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gholam D, Vantelon JM, Al-Jijakli A, Bourhis JH. A case of multicentric Castleman’s disease associated with advanced systemic amyloidosis treated with chemotherapy and anti-CD20 monoclonal antibody. Ann Hematol. 2003;82(12):766–768. doi: 10.1007/s00277-003-0718-x. [DOI] [PubMed] [Google Scholar]
- 11.Bower M, Powles T, Williams S, Davis TN, Atkins M, Montoto S, Orkin C, et al. Brief communication: rituximab in HIV-associated multicentric Castleman disease. Ann Intern Med. 2007;147(12):836–839. doi: 10.7326/0003-4819-147-12-200712180-00003. [DOI] [PubMed] [Google Scholar]
- 12.Bower M, Veraitch O, Szydlo R, Charles P, Kelleher P, Gazzard B, et al. Cytokine changes during rituximab therapy in HIV-associated multicentric Castleman disease. Blood. 2009;113(19):4521–4524. doi: 10.1182/blood-2008-12-197053. [DOI] [PubMed] [Google Scholar]
- 13.Bélec L, Mohamed AS, Authier FJ, Hallouin MC, Soe AM, Cotigny S, et al. Human herpesvirus 8 infection in patients with POEMS syndrome-associated multicentric Castleman’s disease. Blood. 1999;93(11):3643–3653. [PubMed] [Google Scholar]
- 14.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO Classification of Tumours of the Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. pp. 212–213. [Google Scholar]
- 15.Choh NA, Qayoom S, Shaheen F, Malik RA, Rabbani I, Gojwari T. Retroperitoneal Castlemans disease associated with paraneoplastic pemphigus. Hematol Oncol Stem Cell Ther. 2014;7(2):93–96. doi: 10.1016/j.hemonc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Zeng M, Zhang M, Hu W, Kong Q, Sang H, Xu X. A case of paraneoplastic pemphigus associated with Castleman’s disease. An Bras Dermatol. 2013;88(6 Suppl 1):11–14. doi: 10.1590/abd1806-4841.20132332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaduputi V, Tariq H, Badipatla K, Ihimoyan A. Systemic Reactive Amyloidosis Associated with Castleman’s Disease. Case Rep Gastroenterol. 2013;7(3):476–481. doi: 10.1159/000356825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larroche C, Cacoub P, Soulier J, Oksenhendler E, Clauvel JP, Piette JC, Raphael M. Castleman’s disease and lymphoma: report of eight cases in HIV-negative patients and literature review. Am J Hematol. 2002;69(2):119–126. doi: 10.1002/ajh.10022. [DOI] [PubMed] [Google Scholar]
- 19.Ioachim HL, Medeiros LJ. Ioachim’s Lymph Node Pathology. 4th ed. Philadelphia: Lippincott Williams Wilkins; 2008. pp. 228–236. [Google Scholar]
- 20.Izumi M, Mochizuki M, Kuroda M, Iwaya K, Mukai K. Angiomyoid proliferative lesion: an unusual stroma-rich variant of Castleman’s disease of hyaline-vascular type. Virchows Arch. 2002;441(4):400–405. doi: 10.1007/s00428-002-0611-3. [DOI] [PubMed] [Google Scholar]
- 21.Yoshizaki K, Matsuda T, Nishimoto N, Kuritani T, Taeho L, Aozasa K, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood. 1989;74(4):1360–1367. [PubMed] [Google Scholar]
- 22.Nishi J, Arimura K, Utsunomiya A, Yonezawa S, Kawakami K, Maeno N, et al. Expression of vascular endothelial growth factor in sera and lymph nodes of the plasma cell type of Castleman’s disease. Br J Haematol. 1999;104(3):482–485. doi: 10.1046/j.1365-2141.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- 23.Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274(5293):1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 24.Fajgenbaum DC, van Rhee F, Nabel CS. HHV-8-negative, idiopathic multicentric Castleman disease: novel insights into biology, pathogenesis, and therapy. Blood. 2014;123(19):2924–2933. doi: 10.1182/blood-2013-12-545087. [DOI] [PubMed] [Google Scholar]