Abstract
Background and aim
Sexually transmitted infections are a very frequent and under-diagnosed cause of illness worldwide. A high number of detection methods and a large range of specimens in which sexually transmitted infections can be determined are available at the moment. Polymerase chain reaction performed on first void urine offers the advantage of being non-invasive, self-collectable and has high sensitivity and specificity. We looked to determine the frequency of Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, Mycoplasma hominis, Mycoplasma genitalium and Ureaplasma urealyticum in symptomatic and asymptomatic patients.
Methods
Six sexually transmitted infections were determined in the first void urine of 15 symptomatic and asymptomatic patients by polymerase chain reaction. We used “Epicenter MasterPure™ Complete DNA and RNA Purification Kit” for the DNA purification and “Seeplex® STD6 ACE Detection” for the DNA amplification. The results were examined in UV light.
Results
A number of 5 patients had positive results for Chlamydia trachomatis or Neisseria gonorrhoeae. Sexually transmitted infections are more frequent in men between 27 and 40 years old.
Conclusions
Polymerase chain reaction is a good diagnostic tool for sexually transmitted infections because it has a high sensitivity and specificity. Chlamydia trachomatis is the most frequent sexually transmitted infection, followed by Neisseria gonorrhoeae.
Keywords: sexually transmitted infections, urine, polymerase chain reaction
Background and aims
Sexually transmitted infections (STI) are the most common cause of illness worldwide and they represent an important economic, therapeutic and social problem.
World Health Organization (WHO) statistics show that about 498.9 million new cases of Chlamydia trachomatis (CT), Neisseria gonorrhea (NG), syphilis and Trichomonas vaginalis (TV) occurred worldwide in 2008 in adults between the ages of 15 and 49 [1]. The number of patients with STIs is higher than the one reported in the statistics because of the asymptomatic form of the infection, the absence of partners testing, self-treatments and under-reporting the cases.
In women, STIs can determine cervicitis, pelvic inflammatory disease, salpingitis, endometritis, spontaneous abortion and prematurity/low birth weight [2,3,4]. Women have an over 3 times higher rate of contracting CT than do men [5]. Urethritis, epididymitis and chronic prostatitis occur in men with STIs [2,6]. In both genders, STIs can cause infertility and they increase the susceptibility to HIV infection [7,8,9,10]. In both sexes symptomatic infection appears to be the exception rather than the rule, up to 70% remain asymptomatic for a varying period of time [11,12,13].
There are a lot of laboratory methods to detect CT, NG, TV, Ureaplasma urealyticum (UU), Mycoplasma hominis (MH) and Mycoplasma genitalium (MG) that range from cell culture to nucleic acid amplification tests (NAAT) [8]. In bacterial culture only viable bacteria can be quantified, but NAATs such as polymerase chain reaction (PCR) can quantify both viable and nonviable bacteria [11]. The higher sensitivity and better specificity of NAATs are their main advantages over other tests [3,14]. PCR has been used to analyze a variety of specimen types including male and female urine, male urethral swabs, and female endocervical/vaginal swabs [15]. The invasiveness of traditional collection methods determined some individuals not to perform STIs tests [16]. Self-collectable samples such as first-void urine (FVU) and vaginal swabs have the advantage of being non-invasive and may increase the number of tested individuals and the practicability of screening programs [6].
In men, although urethral swabs turned to be more sensitive and specific than FVU when enzyme immunoassay (EIA) was performed, this difference between urethral swab and FVU was reduced with the NAAT [8].
In women, there is a strong consistency between vaginal swabs or FVU and endocervical swabs in the detection of STIs [17]. FVU in women has the advantage over endocervical swabs that it can be self-collected and does not require the presence of a doctor. Urine samples are the preferred collection method over vaginal swab [18].
FVU is a good specimen for detecting STIs in both genders.
PCR performed on FVU is able to detect as many or more infected patients than urethral swabs, endocervical swabs or semen [9]. PCR is a convenient method for clinicians because it can test multiple causative organisms simultaneously and in short time.
The aim of this study was to investigate the utility of PCR in the detection of CT, NG, TV, MH, MG and UU and to estimate the frequency of this six sexually transmitted infections.
Patients and methods
Patients
A number of 15 symptomatic and asymptomatic patients were included in the study, 13 men and 2 women. In the symptomatic group were included patients who presented to the doctor asking for medical advice for acute or chronic symptoms, including: genital discharge, dysuria, fever and abdominal pain. Patients who addressed to the doctor for a screening and partners of symptomatic patients, all having no symptoms, were included in the asymptomatic group.
All patients were recruited multidisciplinarily from the dermatology, urology and infection diseases ambulatory departments in Cluj Napoca over the period between January 2014 to August 2014. From the total of 15 patients, 12 had symptoms of STIs and 3 patients were asymptomatic. The age of the enrolled patients was between 22 years and 71 years and all of them were sexually active. Patients who had a history of antibiotic use in the previous 2 weeks were excluded from the study. An informed consent was obtained from all patients enrolled in the study and they had all answered a standard questionnaire in which they were asked to provide their age, sexual status, marital status, origin, socio-economic status, symptoms, history of STIs, partner’s symptomatology and other investigations performed for STIs in order to have a complete medical history. The study was approved by the ethics committee of University of Medicine and Pharmacy from Cluj Napoca. Confidentiality was addressed on the patient information sheet.
Collection of urine
First void urine was collected from all 15 patients. In the morning, when patients had not urinated for at least 4 hours, 50 ml of urine had been self-collected in a sterile plastic container. The specimens were transported to the laboratory without added transport medium and stored at 4° C, then examined within a maximum of 7 days.
Methods
In the laboratory, 1 ml from the specimens was centrifuged in order to obtain the sediment.
DNA purification
In order to purify the DNA, we used “Epicenter MasterPure™ Complete DNA and RNA Purification Kit”. 150 μl from the sample was mixed with lysis solution, which contains Proteinase K, and then incubated at 65° C. Then, the sample was cooled at 37° C, RNase A was added and afterwards incubated at 37° C for 30 minutes. The mix was placed on ice for 5 minutes and then reagent for protein precipitation was added and vortexed vigorously in order to eliminate the proteins, afterwards it was centrifuged with Hettich mikro 200 R centifuge for 10 minutes at 14000 rotation per minute (rpm). The supernatant which contained DNA was mixed with isopropanol, then the microcentrifuge tube was inverted 30–40 times and afterwards centrifuged for 10 minutes at 14000 rpm, thus DNA would depose in the pellet. DNA was rinsed twice with 75% ethanol and re-suspended in TE Buffer. At the end, it would result 35 μl buffer with DNA.
DNA amplification
For this part, we used “Seeplex® STD6 ACE Detection”. In an Eppendorf container the following were mixed: primer, DNA polymerase, deoxynucleoside triphosphate (dNTP), internal control and a solution to prevent contamination. This mix was placed in PCR tubes, the patients DNA sample was added together with positive and negative controls. Afterwards the mix was electrophoresed in 2% agarose gel containing ethidium bromide, with a molecular weight marker.
Results analysis
The detection and examination of PCR products were performed in UV light. The distance of migration is proportional with the size in agarose gel. TV has the greatest size in agarose gel, so it migrates on a small distance. It is followed by MH, MG, CT and NG. UU has the smallest size in agarose gel, so it migrates on the longest distance.
Results
In order to analyze the collected data, we used descriptive statistics to provide essential information related to the demographic features of the patients. Thus, 86.7% of the patients were males, with an average age of 31 years old (Tab. I). Among young men aged between 27 and 40 years old, we found positive results of our tested STIs.
Tabel I.
Main clinical characteristics of the studied group.
| N (%) | Mean Age | Symptomatic (%) | Asymptomatic (%) | |
|---|---|---|---|---|
| Male | 13 (86.7%) | 31 | 11 (84.6) | 2 (15.4%) |
| Female | 2 (13.3%) | 35 | 1 (50%) | 1 (50%) |
Frequencies were conducted to provide important results related to the number of the patients that had one of the six tested STIs, which represent the categorical variables of the present study.
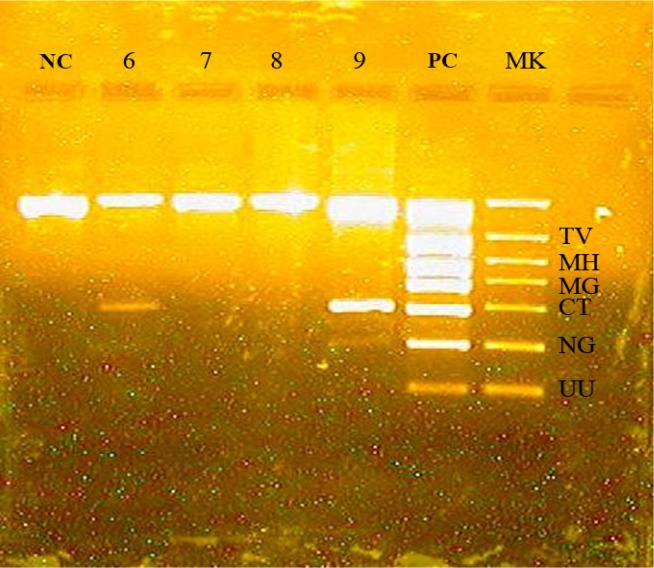
Therefore, CT was found in 4 patients and NG in 2 patients. One patient had positive result for CT and also for NG (Fig. 1). MH, MG, TV and UU were not found in this 15 patients.
Figure 1.
Example of PCR result. NC - negative control. 6, 7, 8, 9 - samples. PC - positive control. MK - marker of molecular weight. Sample 6 - positive for CT. Sample 9 - positive for CT and NG.
The 3 asymptomatic patients were negative for all these six STIs. From the 12 symptomatic patients, 5 had positive results for CT or NG and 7 were negative for the tested STIs. One woman enrolled in this study was symptomatic and 1 was asymptomatic, but both had negative results for the six STIs tested. Of the 13 men enrolled in the study, 2 were asymptomatic and had negative results and 11 were symptomatic, out of which 5 had positive results.
Discussion
Although other diagnostic tools, such as culture, antigen detection and serology are available for the diagnostic of STIs, molecular testing seems to be highly sensitive and specific [4,8,9,10].
This is to our knowledge the first study in our area using PCR as a diagnostic tool for STIs.
Our data is consistent with other studies reporting CT as the most prevalent bacterial STI [3,4,8,12]. In 2008, from a total of 46.8 million new cases of CT, NG, syphilis and TV reported by WHO for the European Region, 20.6 million were new cases of CT [1].
The second most common STI in our prospective study was gonorrhea, which is the second most commonly reported sexually disease in United States [19]. In the WHO statistics for the European Region, in 2008, there were 3.4 million new cases of NG [1], but in this statistics syphilis was also included, a fact that placed gonorrhea on the third place after chlamydiasis and syphilis.
Co-infection with CT and NG seems to be frequent, 10–30% of people infected with NG are also co-infected with CT [19,20]. In the United States, empiric treatment of CT concurrently with gonococcal treatment is recommended if diagnostic testing for CT was not performed [20]. In this study we found one patient co-infected with NG and CT, thus another advantage of PCR as a diagnostic tool for STIs would be to help the clinicians to decide whether it is appropriate to give co-treatment for chlamydial infection if they have a positive result for NG. As it can be observed in figure 1, there is a low positive result for NG compared with other positive results, which is due to the small amount of DNA detected in the patient’s FVU. Correlating the low positive result with the patient’s medical history, we have concluded that there is an incipient gonorrhea associated with chlamydiasis.
According to WHO global estimation, in 2008 there were 22.6 million new cases of TV in the European region [1]. We did not detect TV in our 15 cases. This can be explained by the fact that only 2 women were included in the study and trichomoniasis is more frequent in women [7].
MH, MG and UU were not found in our study, probably due to the small number of tested patients and to the fact that they affect a large number of infertile couples [9].
One of the most important limitations of the current study is the reduced number of cases which could only provide descriptive data. TV, MH, MG and UU were not detected and the asymptomatic group has also reduced number of patients. However, these are important preliminary results that provide essential insights for further extended research studies.
Conclusions
PCR performed on FVU seems to be the best choice to detect STIs because it is more sensitive and specific than previously available diagnostic tests. This method of detection uses noninvasively collected samples, such as FVU, and it has the advantage of being able to test for multiple causative organisms simultaneously.
Chlamydia trachomatis is the most frequent sexually transmitted infection, followed by Neisseria gonorrhoeae.
In the future, PCR is expected to be used as a standard diagnostic test for STIs. Because it can perform analysis on hundreds of samples in a short time, PCR is a good diagnostic tool for screening programs.
Acknowledgments
This paper was published under the frame of European Social Found, Human Resources Development Operational Programme 2007–2013, project no. POSDRU/159/1.5/138776.
References:
- 1.Global incidence and prevalence of selected curable sexually transmitted infections – 2008 [Internet] Geneva: World Health Organization; 2012. [cited 2014 Nov 21]. Available from: http://apps.who.int/iris/bitstream/10665/75181/1/9789241503839_eng.pdf?ua=1. [Google Scholar]
- 2.Korte JE, Baseman JB, Cagle MP, Herrera C, Piper JM, Holden AE, et al. Cervicitis and genitourinary symptoms in women culture positive for Mycoplasma genitalium. Am J Reprod Immunol. 2006;55:265–275. doi: 10.1111/j.1600-0897.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- 3.Abusarah EA, Awwad ZM, Charvalos E, Shehabi AA. Molecular detection of potential sexually transmitted pathogens in semen and urine specimens of infertile and fertile males. Diagn Microbiol Infect Dis. 2013;77:283–6. doi: 10.1016/j.diagmicrobio.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Krolov K, Frolova J, Tudoran O, Suhorutsenko J, Lehto T, Sibul H, et al. Sensitive and rapid detection of Chlamydia trachomatis by recombinase polymerase amplification directly from urine samples. J Mol Diagn. 2014;16:127–135. doi: 10.1016/j.jmoldx.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Madkan VK, Giancola AA, Sra KK, Tyring SK. Sex differences in the transmission, prevention, and disease manifestations of sexually transmitted diseases. Arch Dermatol. 2006;142:365–370. doi: 10.1001/archderm.142.3.365. [DOI] [PubMed] [Google Scholar]
- 6.Ostergaard L. Diagnosis of urogenital Chlamydia trachomatis infection by use of DNA amplification. APMIS Suppl. 1999;89:5–36. [PubMed] [Google Scholar]
- 7.Shipitsyna E, Zolotoverkhaya E, Chen CY, Chi KH, Grigoryev A, Savicheva A, et al. Evaluation of polymerase chain reaction assays for the diagnosis of Trichomonas vaginalis infection in Russia. J Eur Acad Dermatol Venereol. 2013;27:e217–223. doi: 10.1111/j.1468-3083.2012.04593.x. [DOI] [PubMed] [Google Scholar]
- 8.Eley A. How to detect Chlamydia trachomatis in males? J Androl. 2011;32:15–22. doi: 10.2164/jandrol.110.010363. [DOI] [PubMed] [Google Scholar]
- 9.Gdoura R, Kchaou W, Ammar-Keskes L, Chakroun N, Sellemi A, Znazen A, et al. Assessment of Chlamydia trachomatis, Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis, and Mycoplasma genitalium in semen and first void urine specimens of asymptomatic male partners of infertile couples. J Androl. 2008;29:198–206. doi: 10.2164/jandrol.107.003566. [DOI] [PubMed] [Google Scholar]
- 10.Domeika M, Zhurauskaya L, Savicheva A, Frigo N, Sokolovskiy E, Hallen A, et al. Guidelines for the laboratory diagnosis of trichomoniasis in East European countries. J Eur Acad Dermatol Venereol. 2010;24:1125–1134. doi: 10.1111/j.1468-3083.2010.03601.x. [DOI] [PubMed] [Google Scholar]
- 11.Choe HS, Lee DS, Lee SJ, Hong SH, Park DC, Lee MK, et al. Performance of AnyplexTM II multiplex real-time PCR for the diagnosis of seven sexually transmitted infections: comparison with currently available methods. Int J Infect Dis. 2013;17:e1135–1140. doi: 10.1016/j.ijid.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Noren L, von Krogh G, Bondesson L, Nohlgard C, Grillner L. Potential public health benefits from testing with Chlamydia trachomatis PCR technique on first void urine in men. Acta Derm Venereol. 1998;78:63–66. doi: 10.1080/00015559850135887. [DOI] [PubMed] [Google Scholar]
- 13.Rose SB, Bromhead C, Lawton BA, Zhang J, Stanley J, Baker MG. Access to chlamydia testing needed for high-risk groups: patterns of testing and detection in an urban area of New Zealand. Aust N Z J Public Health. 2012;36:343–350. [Google Scholar]
- 14.Aguilera-Arreola MG, Gonzalez-Cardel AM, Tenorio AM, Curiel-Quesada E, Castro-Escarpulli G. Highly specific and efficient primers for in-house multiplex PCR detection of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma hominis and Ureaplasma urealyticum. BMC Res Notes. 2014;7:433. doi: 10.1186/1756-0500-7-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumamoto Y, Matsumoto T, Fujisawa M, Arakawa S. Detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urogenital and oral specimens using the cobas® 4800, APTIMA Combo 2® TMA, and ProbeTec™ ET SDA assays. Eur J Microbiol Immunol (Bp) 2012;2:121–127. doi: 10.1556/EuJMI.2.2012.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Byrne P. Self-directed sexually transmitted infection testing: providing noninvasive sexual health services. Appl Nurs Res. 2011;24:17–21. doi: 10.1016/j.apnr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Fang J, Husman C, DeSilva L, Chang R, Peralta L. Evaluation of self-collected vaginal swab, first void urine, and endocervical swab specimens for the detection of Chlamydia tracomatis and Neisseria gonorrhoeae in adolescent females. J Pediatr Adolesc Gynecol. 2008;21:355–360. doi: 10.1016/j.jpag.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tebb KP, Paukku MH, Pai-Dhungat MR, Gyamfi AA, Shafer MAB. Home STI Testing: The adolescent female’s opinion. J Adolesc Health. 2004;35:462–467. doi: 10.1016/j.jadohealth.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Rosen T. Gonorrhea, Mycoplasma, and vaginosis. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick’s dermatology in general medicine. 8th edition. New York: Mc Graw Hill; 2012. pp. 2514–2519. [Google Scholar]
- 20.Stary A, Stary G. Sexually transmitted infection. In: Callen JP, Cerroni L, Heymann WR, Hruza G, Mancini AJ, Patterson JW, et al., editors. Dermatology. 3rd edition. Elsevier; 2012. pp. 1379–1383. [Google Scholar]