Abstract
Plasmacytoid melanoma is an unusual variant of malignant melanoma. The plasmacytoid morphology can be found in a variety of other malignancies including carcinomas, plasma cell neoplasms, lymphoproliferative disorders, and sarcomas. The authors report a rare case of plasmacytoid amelanotic malignant melanoma in a 78-year-old man presenting with an enlarging palpable, erythematous mass on his left posterior shoulder. A fine needle aspirate showed atypical findings with single amelanotic cells with high nuclear to cytoplasmic ratio, mono- and multi-nucleation with prominent nucleoli and intranuclear inclusions. Review of the excision and immunohistochemical analysis revealed the malignant plasmacytoid cells stained with vimentin, S-100, HMB-45, and other staining patterns consistent with melanoma. Initial evaluation was negative for other sites of disease. However, 4 months later, the patient was noted to have metastatic disease to his lungs and liver. Given that the tumor was noted to be BRAF V600R mutated, the patient was started on single agent dabrafenib. The plasmacytoid morphology can be found in a variety of malignancies. Melanoma should be considered in the differential diagnosis of any malignancy presenting with plasmacytoid features.
Key words: Amelanotic melanoma, plasmacytoid, diagnostic errors
Introduction
Malignant melanoma is histologically diverse and can present in various cutaneous and non-cutaneous sites. There are numerous morphological variants based on histopathological findings including clear cell/balloon cell, chondroid, osteoid, myxoid, small cell, signet ring cell, rhabdoid, and plasmacytoid melanoma. Plasmacytoid melanoma is an unusual variant of malignant melanoma and can present as a solitary tumor or as metastatic disease. The plasmacytoid morphology can be found in a variety of other malignancies including carcinomas, sarcomas, and plasma cell neoplasms. The infrequently encountered morphological variant can contribute to misdiagnosis if melanoma isn’t considered in the initial differential diagnosis. We report a rare case of plasmacytoid melanoma.
Case Report
A 78-year-old man, with remote history of pigmented skin lesions previously treated with liquid nitrogen, presented with an enlarging palpable, erythematous mass on his left posterior shoulder. A fine needle aspirate (FNA) showed atypical findings with single amelanotic cells with high nuclear to cytoplasmic ratio, mono- and multi-nucleation with prominent nucleoli, and intra-nuclear inclusions. Differential diagnosis at this stage favored sarcoma. A subsequent excision showed a large nodule within the dermis, composed of sheets and nests of amelanotic cells with plasmacytoid morphology (cells with abundant cytoplasm, eccentric nuclei, and pale paranuclear zones) (Figure 1A,B). Less than 5% of the nodule showed pseudopapillary architecture, lined by malignant cells (Figure 1C-E). There was no overlying melanoma in situ or any dysplasia of the epidermis. Review of the excision and immunohistochemical analysis revealed that the malignant plasmacytoid cells stained strongly and diffusely with vimentin, S-100, HMB-45, and melanoma cocktail (HMB-45 + MART-1 + tyrosinase, Biocare Medical), in keeping with melanoma. Following clinical examination (dermoscopy and ophthalmoscopy), imaging (computed tomography and magnetic resonance imaging), and investigations (esophagogastroduodenoscopy and colonoscopy) failed to reveal any other sites of disease of melanoma. A subsequent wide local excision and sentinel lymph node excision revealed a focal positive deep margin, satellite/in transit metastasis (Figure 2), and negative lymph node. The patient underwent 2 weeks of adjuvant radiotherapy to the excision site for management of the positive surgical margin. Four months following the initial excision, however, radiological imaging noted metastatic disease to the lungs and liver, and residual disease at the excision site. Molecular analysis of the melanoma revealed a BRAF V600R gene mutation. Treatment options were discussed including protein kinase inhibitors for unresectable metastatic melanoma (dabrafenib and vemurafenib) and standard chemotherapy with dacarbazine. The patient was commenced on single agent dabrafenib but unfortunately did not tolerate the treatment. He developed significant side effects and his condition deteriorated. The treatment was ceased after 10 days and the patient was transitioned to palliative care.
Figure 1.
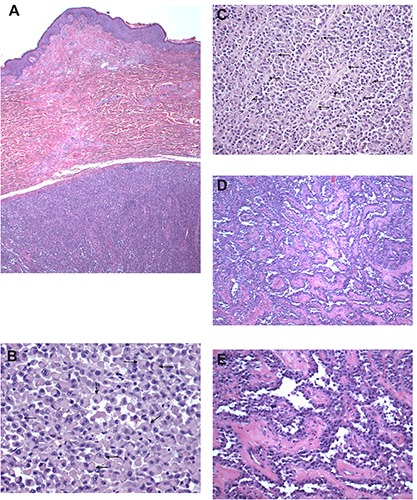
Initial excision of mass showing sheets and nests of plasmacytoid cells. A) Hematoxylin & Eosin (H&E) 20×; B) H&E 200×, and areas with pseudopapillary architecture; C-E) H&E 100×.
Figure 2.
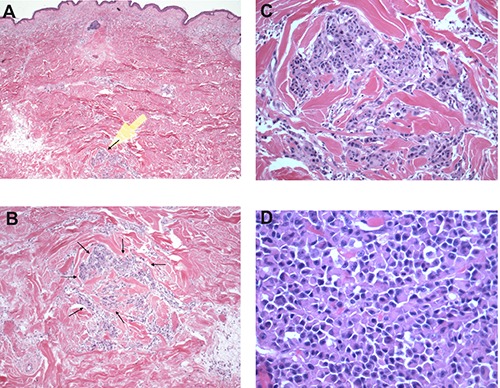
Subsequent wide excision with satellite/in transit metastasis of plasmacytoid melanoma. A) Hematoxylin & Eosin (H&E) 20×; B) H&E 40×, C) H&E 100×, D) H&E 200×.
Discussion
This case highlights the diagnostic dilemma that melanoma presents when presenting as an amelanotic lesion with unusual morphology. The initial FNA favored a diagnosis of sarcoma. Previous cases of both primary and metastatic plasmacytoid melanoma have also been misdiagnosed. The misdiagnoses have included rhabdomyosarcoma and lymphoproliferative neoplasms (e.g. plasma cell neoplasms, plasmacytoid lymphoma, plasmablastic lymphoma).1 FNA cytology of plasmacytoid melanoma has been noted in cutaneous lesions and metastases to various locations including lymph nodes, liver, and breast.2-5 The cytomorphologic characteristic of plasmacytoid morphology was shown to be useful in differentiating metastatic melanoma from primary hepatocellular carcinoma in liver FNAs.4 Similarly, plasmacytoid morphology in FNAs was found in the majority of cases (14 of 16 cases) of metastatic melanoma of the breast.5
Excisional biopsies showing plasmacytoid melanoma have been documented in primary and metastatic melanoma. Primary melanoma sites include skin, intra-nasal, intra-oral, and esophagus.1,6-9 Metastatic plasmacytoid melanoma has been reported in biopsies of lymph nodes, breast, and bladder (Table 1).1-9
Table 1.
Plasmacytoid malignant melanoma cases described in literature from both cytology and biopsy specimens.
| Primary or metastatic site | Cases in literature |
|---|---|
| Cytology | |
| Liver metastases4 | 16* |
| Breast metastases5 | 14 |
| Chest wall, amelanotic2 | 1 |
| Lymph node metastasis,3 amelanotic | 1 |
| Biopsy | |
| Oral7,8 | 14 |
| Skin,1,6 1 case amelanotic | 2 |
| Intra-nasal1 | 1 |
| Bladder metastasis9 (from esophageal primary) | 1 |
| Metastases1 | NA° |
*Parwani (2004) explains that 16 cases of metastatic melanoma to the liver were examined but does not list how many of the 16 cases had plasmacytoid morphology. The plasmacytoid morphology was listed as an abundant cytomorphologic feature in these cases and not present in cases of hepatocellular carcinoma.
°Banerjee (2000) explains that a few cases of metastatic melanoma had the plasmacytoid morphology. NA, not available.
Additionally, other patterns that can be seen include fascicles, whorls, trabeculae, nests, sheets, and pseudopapillary/pseudoglandular structures.1 The small component of pseudopapillary architecture observed in our case has also been documented in FNAs of metastatic melanoma.10 Plasmacytoid dendritic cells (pDC) may also be found in malignant melanoma and should not be confused for tumor cells due to their morphology. The tumor cells produce various immunosuppressive cytokines that act on the tumor-infiltrating pDCs and suppress their potential anti-tumor activity.11 Studies investigating potential therapeutic pathways to restore pDC activity in order to induce tumor regression are currently underway.12
The presence of melanin pigment is highly valuable in aiding in the diagnosis of melanoma. The absence of melanin, therefore, makes the diagnosis of melanoma even more challenging, as in the present case. Amelanotic melanoma has been documented in primary and metastatic melanoma at numerous sites of disease across the head and neck, trunk, and both upper and lower extremities. Amelanotic melanomas have generally been regarded as having a worse prognosis than pigmented melanomas due to the advanced stage at diagnosis for amelanotic tumors. A recent population-based comparison of survival between patients with histopathologically amelanotic melanoma and pigmented melanoma was published by Thomas et al.13 The authors found that of the 3486 primary invasive cutaneous melanomas that were included in the study 8% were amelanotic. Comparison of the 2 groups found that there was poorer overall survival for the amelanotic melanoma group than for the pigmented melanoma group. This was attributed entirely to a higher American Joint Committee on Cancer tumor stage at the time of diagnosis. The lack of melanin pigment may contribute to this delay in diagnosis. The crucial ancillary test that aids in the accurate diagnosis of malignant melanoma is immunohistochemical staining. The majority of reported cases described malignant plasmacytoid cells that were positive for the immunohistochemical stains S-100, HMB-45, and MelanA. Occasional cases also reported plasmacytoid melanoma staining with additional melanocytic markers such as MART-1, tyrosinase, and vimentin. Although immunohistochemistry can be very useful in diagnosing plasmacytoid melanoma it can also be misleading if melanocytic markers are not considered and only plasma cell markers are examined. The plasma cell marker CD138 is commonly used in the diagnosis of plasma cell neoplasms such as multiple myeloma and extra-osseous plasmacytomas but has also been noted to be positive in occasional cases of plasmacytoid melanoma.6,9
Conclusions
The plasmacytoid morphology can be found in a variety of malignancies. In order to prevent potential misdiagnoses of this aggressive malignancy, thorough histological examination should be aided by an appropriate immunohistochemical panel to confirm melanoma and exclude erroneous differentials. Melanoma should be considered in the differential diagnosis of malignancy presenting with plasmacytoid features.
References
- 1.Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology 2000;36:387-402. [DOI] [PubMed] [Google Scholar]
- 2.Siddaraju N, Yaranal PJ, Mishra MM, et al. Fine needle aspiration cytology in recurrent amelanotic melanoma: a case report. Acta Cytol 2007;51:829-32. [DOI] [PubMed] [Google Scholar]
- 3.Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med 2012;136:237-9. [DOI] [PubMed] [Google Scholar]
- 4.Parwani AV, Chan TY, Mathew S. Metastatic malignant melanoma in liver aspirate: cytomorphologic distinction from hepatocellular carcinoma. Diagn Cytopathol 2004;30:247-50. [DOI] [PubMed] [Google Scholar]
- 5.Ribu DL, Shield PW, Bligh JF. The varied presentation of metastatic melanoma in fine needle aspiration cytology of the breast. Cytopathology 2012;23:256-62. [DOI] [PubMed] [Google Scholar]
- 6.Lehmer LM, Ragsdale BD, Frost MV, et al. Large neglected ulcerated melanoma mimicking extramedullary plasmacytoma. Am J Dermatopathol 2011;33:e94-8. [DOI] [PubMed] [Google Scholar]
- 7.Ortega KL, Soares de Araujo N, de Souza FB, et al. Primary malignant melanoma of the oral cavity: a case report. International Society of Dermatology 2004;43:750-2. [DOI] [PubMed] [Google Scholar]
- 8.de-Andrade BAB, Toral-Rizo VH, Leon JE, et al. Primary oral melanoma: a histopathological and immunohistochemical study of 22 cases of Latin America. Med Oral Patol Oral Cir Bucal 2012;17:383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charfi S, Ellouze S, Mnif H, et al. Plasmacytoid melanoma of the urinary bladder and lymph nodes with immunohistochemical expression of plasma cell markers revealing primary esophageal melanoma. Case Rep Pathol 2012;2012:916256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasiell K, Tani E, Skoog L. Fine needle aspiration cytology and immunocytochemistry of metastatic melanoma. Cytopathology 1991;2:137-47. [DOI] [PubMed] [Google Scholar]
- 11.Di Domizio J, Demaria O, Gilliet M. Plasmacytoid dendritic cells in melanoma: can we revert bad into good? J Invest Dermatol 2014;134:1797-800. [DOI] [PubMed] [Google Scholar]
- 12.Castelli C, Triebel F, Rivoltini L, et al. Lymphocyte activation gene-3 (LAG-3, CD223) in plasmacytoid dendritic cells (pDCs): a molecular target for the restoration of active antitumor immunity. Oncoimmunology 2014;3:e967146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas NE, Kricker A, Waxweiler WT, et al. Comparison of clinicopathologic features and survival of histopathologically amelanotic and pigmented melanomas: a population-based study. JAMA Dermatol 2014;150:1306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


