Abstract
Since a plant miRNA (miR168) cross-regulating a mammalian transcript was reported, miRNA-mediated cross-kingdom communication has become one of the most compelling but controversial topics. In the present study, we used silkworm and mulberry, which is a model for studies on the interactions between the insect and its host plant, to address whether miRNA-mediated cross-kingdom communication is a common phenomenon. The results of TA clone, Sanger sequencing and droplet digital PCR demonstrated that several mulberry-derived miRNAs could enter to silkworm hemolymph and multiple tested tissues. Synthetic miR166b was also detected in hemolymph and fat body. However, the ingestion of synthetic miR166b did not play roles in silkworm physiological progress, which was revealed by RNA-seq analyses, RT-PCR, and phenotypic investigations. Mulberry miRNAs are convincingly transferred to the silkworm orally and no physiological process associated with the miRNAs was demonstrable. The results provided a new aspect of cross-kingdom miRNA transfer.
miRNAs, which are a class of ~22 nt non-coding small RNA molecules, play vital roles by compounding to transcripts of target genes to inhibit translation or degrade mRNA of target genes in animals and plants1. At present, the studies of miRNAs are mainly focusing on the intracellular miRNAs functions in their own species. For example, miRNAs regulate the growth and development of root, leaf, and flower in plants2,3,4, and have important roles in the development of organs in animals5,6. Recently, released miRNA was reported to be secreted from cells to body fluid and circulate in body fluid in a surprisingly stable-state in mammals7,8. Those miRNAs could be carried by lipoprotein or other RNA-binding molecules9,10, or packed by microvesicles to deal with the harsh condition (e.g., RNase, and extreme pH)10. Circulating miRNAs in body fluid were guided to target cells to regulate the expression of target genes9,11.
Recently, miRNA-mediated cross-kingdom communication has become an interesting but controversial topic since it was first reported in 201112. Zhang et al. discovered that rice-derived miRNAs cross the mammalian gastrointestinal tract to the mouse bloodstream, liver, and other tissues, where they regulate cholesterol levels by reducing the amount of low-density lipoprotein receptor-associated protein 1 (LDLRAP1)12. These findings provide new insights for genetic regulation by food ingestion and raise the prospect of engineering food or using oral small nucleic acid to prevent or cure diseases13,14. However, in several other recent studies of mice, pigtailed macaques, or insects (Helicoverpa zea and Spodoptera frugiperda), plant-derived miRNAs were not detected after ingestion of plant-derived food13,15,16. In this context, we used silkworm, Bombyx mori, an oligophagous insect that feeds only on mulberry leaves, to study whether mulberry miRNAs can transfer to silkworm upon ingestion. TA clone, Sanger sequencing and droplet digital PCR assay were used to detect mulberry miRNAs in silkworm subset of tissues. RNA-seq was then performed to explore the physiological progresses associated with one of plant miRNA, miR166b, in silkworm.
Results
Mulberry-derived miRNAs were detected in silkworm hemolymph
We aligned silkworm hemolymph small RNA sequences (GSE48168)17 with mulberry miRNAs revealed by next generation sequencing18. To our surprise, eight mulberry miRNAs (mno-miR166c, mno-miR166b, mno-miR167e, mno-miR396b, mno-miR159a, mno-miR162, mno-miR156c, and mno-miR398) were identified in silkworm hemlolymph (Table 1). The reads of these miRNAs were relatively low, with the highest being 11. To ensure that these mulberry-derived miRNAs do not represent contamination during solexa sequencing or sample preparation, we used stem-loop PCR to clone the eight mulberry miRNAs, generating 50 TA-clones for each miRNA, and then subjected them to Sanger sequencing. All of the miRNAs except for mno-miR162 could be detected, with positive clone ratios (perfectly matched clones/total clones) ranging from 35.90% to 70.83% (Table 2, 1st sequencing Hemolymph). Positive clones with incorrect sequences could be explained by the relative low contents of these miRNAs or to homologous silkworm derived small RNAs. As control, we also cloned four mulberry miRNAs that were not identified in the silkworm hemolymph and have varied expression levels in mulberry leaves18. There were no positive clones for miR535, miR168b, and miR172a (Table 2, 1st sequencing Hemolymph), and the positive clone ratio of miR169a was 5.13%, which is much lower than other seven miRNAs (Table 2, 1st sequencing Hemolymph). These results confirm the specificity of the seven mulberry miRNAs (miR166c, miR166b, miR167e, miR396b, miR159a, miR156c, and miR398). To further confirm these results, we re-cloned miR169a, miR166b, miR166c, miR167e, and miR396b in silkworm hemolymph. The sequencing frequencies of the five miRNAs were similar to those of the first sequencing run (Table 2, 2nd sequencing Hemolymph).
Table 1. Eight mulberry miRNAs in silkworm hemolymph identified by solexa sequencing.
| miRNA name | Sequence | The reads in the 1st small RNA library | The reads in the 2nd small RNA library |
|---|---|---|---|
| mno-miR166c** | UCUCGGACCAGGCUUCAUUCC | 5 | 3 |
| mno-miR166b** | UCGGACCAGGCUUCAUUCCCC | 2 | 11 |
| mno-miR167e | UGAAGCUGCCAGCAUGAUCUG | 1 | 1 |
| mno-miR396b | UUCCACAGCUUUCUUGAACUG | 1 | – |
| mno-miR159a | UUUGGAUUGAAGGGAGCUCUG | 2 | – |
| mno-miR162 | UCGAUAAACCUCUGCAUCCAG | 1 | – |
| mno-miR156c | UUGACAGAAGAUAGAGAGCAC | – | 2 |
| mno-miR398 | UGUGUUCUCAGGUCGCCCCUG | – | 2 |
**miRNAs reported in the mulberry genome by He et al.17. “–” means the relative sequence was not detected in the hemolymph small RNA libraries.
Table 2. TA-cloning and Sanger sequencing results for mulberry miRNAs identified in silkworm hemolymph.
| 1st sequencing | 2nd sequencing | |||
|---|---|---|---|---|
| Name | correct/Total clones | Rate | correct/Total clones | Rate |
| mno-miR169a | 2/39 | 5.13% | 0/37 | 0.00% |
| mno-miR166b | 25/40 | 62.50% | 26/39 | 66.67% |
| mno-miR166c | 21/37 | 56.76% | 32/46 | 69.56% |
| mno-miR167e | 27/49 | 55.10% | 15/46 | 32.61% |
| mno-miR396b | 19/42 | 45.24% | 16/32 | 50.00% |
| mno-miR159a | 17/24 | 70.83% | – | – |
| mno-miR156c | 14/39 | 35.90% | – | – |
| mno-miR398 | 20/29 | 68.97% | – | – |
| mno-miR162 | 0/49 | 0.00% | – | – |
| mno-miR535 | 0/38 | 0.00% | – | – |
| mno-miR168b | 0/42 | 0.00% | – | – |
| mno-miR172a | 0/39 | 0.00% | – | – |
Results are shown for four control mulberry miRNA (mno-miR169a, mno-miR535, mno-miR168b, and mno-miR172a) and the eight mulberry miRNAs (mno-miR166b, mno-miR166c, mno-miR167e and mno-miR396b, mno-miR159a, mno-miR156c, mno-miR398, mno-miR162) in hemolymph. The TA-cloning of the five miRNAs (mno-miR169a, mno-miR166b, mno-miR166c, mno-miR167e, and mno-miR396b) was performed twice. Other miRNAs was performed once. We randomly selected 50 TA-clones for each miRNA for Sanger sequencing, but there were a number of double clones and failed sequencing reactions, so that the total clone number was less than 50. Clones were considered “correct” if the sequence of the clone was identical to the sequence of the corresponding miRNA. The “rate” indicates the sequencing frequency of correct clones relative to the total clones. “-” indicates that we did not clone the miRNA in the hemolymph.
Mulberry-derived miRNAs were detected in multiple silkworm tissues
As the silkworm is a non-vertebrate with an open circulatory system, its organs and tissues are suspended in the hemolymph. To explore whether plant miRNAs enter into the silkworm tissues, the seven mulberry miRNAs were cloned in the fat body and silk gland. The sequencing frequency of four miRNAs (miR166b, miR166c, miR396b, and miR167e) ranged from 12.50% to 73.17% in fat body, and from 13.51% to 72.22% in silk gland, which is significantly higher than frequency of the control, mno-miR169a (2.63% in fat body; 0.00% in silk gland) (Table 3, 1st sequencing Fat body and Silk gland). Repeat sequencing verified the overall trend that mno-miR166b, mno-miR166c and mno-miR396b were more abundant than the control mno-miR169a in the fat body and silk gland, although the frequencies fluctuated in the two experiments (Table 3, 2nd sequencing Fat body and Silk gland). In addition, these four mulberry miRNAs were also cloned in other seven silkworm tissues including brain, prothoracic gland, salivary gland, gut, malpighian tubule, ovary, and testis. The sequencing frequency of four miRNAs (miR166b, miR166c, miR396b, and miR167e) ranged from 25.64%–87.23% in brain, 9.76%–66.67% in prothoracic gland, 6.67%–36.84% in salivary gland, 8.11%–31.71% in gut, 10.81%–48.78% in malpighian tubule, 7.32%–34.09% in ovary, and 8.16%–37.50% in testis, which was more abundant than the control mno-miR169a (Table 3, supplementary Table S1). The results support the assertion that mulberry miRNAs can enter into silkworm tissues.
Table 3. TA-cloning and Sanger sequencing results for mulberry miRNAs identified in silkworm tissues.
| Fat body (C/T) | Silk gland (C/T) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| miRNA name | 1st sequencing | 2nd sequencing | 1st sequencing | 2nd sequencing | Brain (C/T) | Prothoracic gland (C/T) | Salivary gland (C/T) | Gut (C/T) | Malpighian tubule (C/T) | Ovary (C/T) | Testis (C/T) |
| mno-miR169a | 2.63% | 0.00% | 0.00% | 0.00% | 0.00% | 2.17% | 0.00% | 0.00% | 0.00% | 2.33% | 0.00% |
| mno-miR166b | 20.55% | 76.19% | 56.76% | 59.46% | 53.66% | 31.71% | 9.09% | 8.57% | 10.81% | 25.00% | 13.33% |
| mno-miR166c | 54.05% | 95.45% | 72.22% | 93.02% | 59.52% | 61.76% | 20.83% | 24.39% | 42.11% | 31.82% | 37.50% |
| mno-miR167e | 12.50% | 2.27% | 13.51% | 2.33% | 25.64% | 9.76% | 6.67% | 8.11% | 20.00% | 7.32% | 8.16% |
| mno-miR396b | 73.17% | 65.96% | 55.81% | 45.45% | 87.23% | 66.67% | 36.84% | 31.71% | 48.78% | 34.09% | 22.44% |
| mno-miR159a | 0.00% | – | 5.00% | – | – | – | – | – | – | – | – |
| mno-miR156c | 8.33% | – | 2.17% | – | – | – | – | – | – | – | – |
| mno-miR398 | 0.00% | – | 0.00% | – | – | – | – | – | – | – | – |
| mno-miR162 | 0.00% | – | 0.00% | – | – | – | – | – | – | – | – |
Results are shown for one control mulberry miRNA (mno-miR169a) and four mulberry miRNAs (mno-miR166b, mno-miR166c, mno-miR167e and mno-miR396b) in silkworm tissues (brain, prothoracic gland, salivary gland, gut, malpighian tubule, ovary, testis, fat body, and silk gland). The TA-cloning of the five miRNAs (mno-miR169a, mno-miR166b, mno-miR166c, mno-miR167e, and mno-miR396b) was performed twice in fat body and silk gland, once in other tissues. The percentage represents the sequencing frequency of correct clones relative to the total clones. “–” indicates that we did not clone the miRNA in the fat body, silk gland, brain, prothoracic gland, salivary gland, gut, malpighian tubule, ovary, or testis.
Synthetic miR166b was detected in silkworm hemolymph and fat body
To further investigate the potential role of miR166b, we synthesized this miRNA with modified 2’-O-methyl on its terminal nucleotide, which is characteristic of plant miRNAs19, and smeared 300 pmol on a piece of mulberry leaf to feed silkworm larvae. Droplet digital PCR was applied to detect the copy numbers of miR166b in silkworm hemolymph, fat body, and silk gland before and after ingestion. The copy number counts differed over time according to the tissue type (Fig. 1, supplementary Fig. S1 and Table S2). In hemolymph, the miR166b counts peaked at 0.5 h and 3 h, with 30 and 18 times the count at 0 h; the counts at 6 h and 12 h were similar to those at 0 h. The trend in fat body was similar to that in hemolymph. However, the trend in silk gland differed, with miR166b counts at 0.5 h, 3 h, 6 h, and 12 h slightly less than the count at 0 h. These results suggest that synthetic miR166b could enter to silkworm hemolymph and fat body, but not to silk gland. These results also suggest that it takes 0.5 h or less for synthetic miR166b to enter hemolymph and fat body and that miR166b lasts 3 hours before degradation.
Figure 1. Synthetic miR166b entry into silkworm hemolymph and fat body after silkworm ingestion as determined by droplet digital PCR.
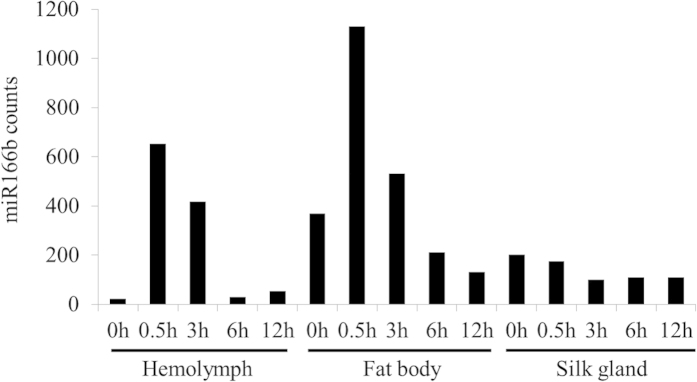
The copy number counts of miR166b in 80 ng small RNA of silkworm hemolymph, 240 ng total RNA of fat body, and 240 ng total RNA of silk gland were investigated by droplet digital PCR before (0 h) and following ingestion of 300 pmol synthetic miR166b (0.5 h, 3 h, 6 h, and 12 h).
Expression of miR166b potential target genes were not repressed by the synthetic miR166b
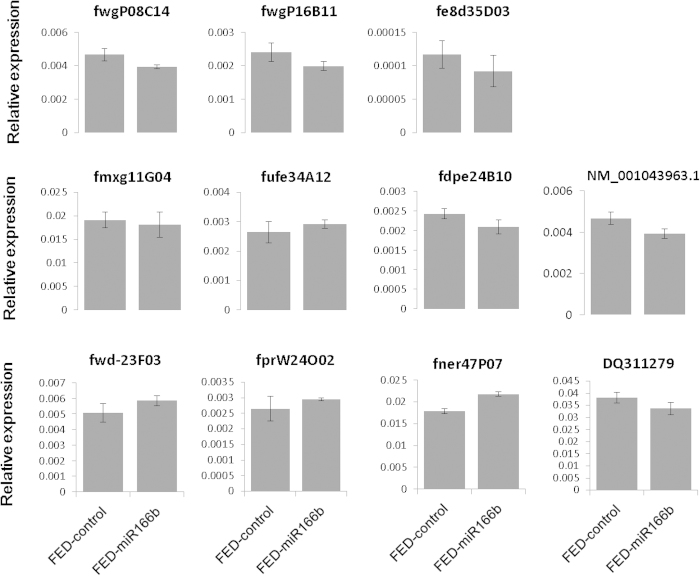
To explore the functions of miR166b in silkworm, we predicted the potential target genes for miR166b in silkworm full-length cDNA data using miranda software. With the criteria of energy lower than −25 kcal/mol and perfected “seed region” match, we identified 72 potential target genes of miR166b (data not shown). Eleven best matched target genes, whose energy were lower than −26.84 kcal/mol (supplementary Table S3) were subjected to RT-PCR verification. The results showed the expression levels of these genes were not significantly changed in silkworm fed on synthetic miR166b (Fig. 2, supplementary Fig. S2).
Figure 2. The expression level of 11 potential miR166b target genes in whole body before and after silkworm ingested synthetic miR166b.
FED-control represents the silkworm larvae were fed on only mulberry leaves. FED-miR166b means the silkworm larvae were fed on a piece of mulberry leaf containing synthetic miR166b. Statistical significance was determined by Student’s t test (*p < 0.05).
RNA-seq analyses of silkworm fed on synthetic miR166b
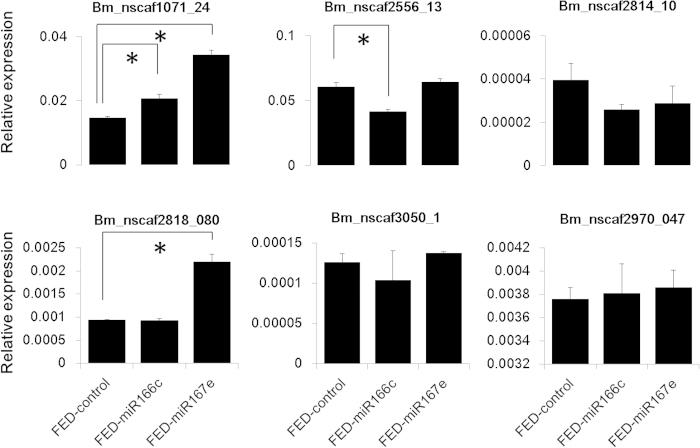
For a better understanding of the potential overall biological roles of ingested mno-miR166b in silkworms, we used RNA-seq to reveal the global expression patterns in whole body of silkworm larvae before and after feeding with synthetic miR166b for 3 hours. Compared with silkworm larvae fed on mulberry leaves, only 30 genes were differentially expressed, of which 17 were up-regulated and 13 were down-regulated in larvae feeding with synthetic miR166b. These genes can be classified into seven groups (supplementary Fig. S3, Table 4): immunity (11 genes), response to stress (1 gene), development (5 genes), cytoskeleton (1 gene), digestion (1 gene), hydrolyzing juvenile hormone (1 gene), and unknown function (10 genes). Genes involved in immunity and stress comprised about 40% of the differentially expressed genes. Thus, the ingestion of synthetic miR166b might activate the host response against non-self-molecules. We further fed silkworm larvae with two other synthetic miRNAs, miR166c and miR167e. Six genes randomly chosen from 30 DEGs (Differentially Expressed Genes) were subjected to RT-PCR analyses. The result showed that Bm_nscaf2556_13 was down-regulated by synthetic miR166c, Bm_nscaf2818_080 was up-regulated by miR167e, and Bm_nscaf1071_24 gene was up-regulated by both (Fig. 3). The results indicated that the different expression of these immunity-related genes was caused by the ingestion of nucleic acids, not specific to miR166b. Therefore, we speculate that the ingested synthetic miR166b basically did not play a significant physiological role to B. mori. Consistently, there was no significant overlap in the predicted miR166b target sites for the 30 differentially expressed genes (data not shown). In addition, the expression levels of 72 potential target genes for miR166b were not significantly changed in silkworm fed on syntetic miR166b (supplementary table S4). These results suggest that mulberry miR166b has no significant effect on the expression of silkworm genes.
Table 4. Genes that were differentially expressed before and after silkworms were fed synthetic miR166b.
| Gene ID | FED-control | FED-166b | log2ratio | Up/Down-Regulated | E-value | Annotation (nr database) | Function | Species | Refences |
|---|---|---|---|---|---|---|---|---|---|
| Bm_scaffold779_2 | 2.595 | 0.924 | −1.49 | Down | 0 | heat shock protein 68 | stress response | H. zea | Zhang and Denlinger, 201024 |
| Bm_nscaf2818_080 | 27.183 | 57.649 | 1.085 | Up | 1.00E-29 | protease inhibitor-like protein | immunity | A. mylitta | Gandhe et al. 200625 |
| Bm_nscaf3098_40 | 8.832 | 52.509 | 2.572 | Up | 1.00E-126 | gloverin 1 precursor | immunity | B. mori | Kawaoka et al. 200826 |
| Bm_nscaf3050_1 | 0.607 | 1.886 | 1.634 | Up | 0 | glucose dehydrogenase [acceptor]-like, partial | immunity | M. sexta | Diana et al. 199427 |
| Bm_nscaf2556_12 | 25.945 | 65.987 | 1.347 | Up | 2.00E-149 | attacin precursor | immunity | B. mori H. cecropia | Sugiyama et al. 199528 Carlsson et al. 199129 |
| Bm_nscaf1071_24 | 26.328 | 61.537 | 1.225 | Up | 2.00E-32 | cecropin-D precursor | immunity | B. mori | Yang et al. 199930 |
| Bm_nscaf2556_13 | 112.806 | 232.042 | 1.041 | Up | 2e-150 | attacin precursor | immunity | B. mori | Sugiyama et al. 199528 Carlsson et al. 199129 |
| Bm_nscaf2655_045 | 2.21 | 4.466 | 1.015 | Up | 1.00E-174 | division abnormally delayed protein-like | immunity | Drosophilia | Zhu and Zhang, 201331 |
| Bm_nscaf2970_047 | 4.009 | 1.501 | −1.417 | Down | 7.00E-130 | mucin-17-like | immunity | Trichoplusia ni | Wang and Granados, 199732 |
| Bm_nscaf2814_10 | 2.787 | 1.096 | −1.347 | Down | 0 | serine protease inhibitor 19 precursor | immunity | M. sexta | An et al. 201133 |
| Bm_nscaf1071_25 | 6.426 | 18.529 | 1.528 | Up | 9.00E-31 | antibacterial peptide enbocin 2 precursor | immunity | B. mori | Kim et al. 199834 Kaneko et al. 200735 |
| Bm_nscaf2883_004 | 2.746 | 7.882 | 1.521 | Up | 4.00E-58 | golgi-specific brefeldin A-resistance guanine nucleotide exchange factor 1-like | immunity | a cell line | Panda D, 201136 |
| Bm_nscaf2847_032 | 7.823 | 15.792 | 1.013 | Up | 1.00E-120 | maltase 1-like | digestion | A. gambiae A. aegypti | Zheng et al. 199537 James et al. 198938 |
| Bm_nscaf3032_113 | 0.859 | 2.096 | 1.287 | Up | 0 | juvenile hormone esterase-like | hydrolyzing JH, transporting JH | H. virescens | Hanzlik et al. 198939 |
| Bm_nscaf2954_01 | 2.511 | 0.762 | −1.72 | Down | 0 | protein odd-skipped-like | development | Drosophilia | Gao et al. 201140 |
| Bm_nscaf3045_44 | 1.965 | 0.59 | −1.735 | Down | 5.00E-73 | c-Cbl-associated protein isoform A (CAP) | development | B. mori | Georgomanolis et al. 200941 |
| Bm_nscaf2865_098 | 1.047 | 0.309 | −1.762 | Down | 0 | LOW QUALITY PROTEIN: voltage-dependent T-type calcium channel subunit alpha-1G-like | development | Drosophilia | Dason et al. 200942 |
| Bm_nscaf2993_283 | 2.588 | 1.053 | −1.298 | Down | 0 | synaptotagmin I | development | Drosophilia | Yoshihara et al. 200243 |
| Bm_nscaf2886_33 | 0.974 | 2.736 | 1.49 | Up | 0 | kinesin-like protein unc-104-like | development | Drosophilia | Kern et al. 201344 |
| Bm_nscaf2883_108 | 1.58 | 0.358 | −2.14 | Down | 0 | WD repeat-containing protein 67-like | cytoskeleton | Saccharomyces cerevisiae | Walter et al. 200345 |
| Bm_nscaf2795_061 | 66.485 | 21.505 | −1.628 | Down | 0 | low molecular mass 30 kDa lipoprotein 19G1-like | unknown | – | – |
| Bm_nscaf2795_060 | 453.713 | 146.829 | −1.628 | Down | 0 | low molecular 30 kDa lipoprotein PBMHP-12-like | unknown | – | – |
| Bm_nscaf2795_062 | 16.964 | 5.728 | −1.566 | Down | 0 | low molecular mass 30 kDa lipoprotein 21G1-like | unknown | – | – |
| Bm_nscaf2795_064 | 7.011 | 2.058 | −1.769 | Down | 0 | low molecular mass 30 kDa lipoprotein 19G1-like precursor | unknown | – | – |
| Bm_nscaf3003_019 | 2.588 | 9.563 | 1.885 | Up | 3.00E-75 | actin cytoskeleton-regulatory complex protein PAN1-like | unknown | – | – |
| Bm_nscaf2903_06 | 2.987 | 6.29 | 1.075 | Up | 2.00E-158 | uncharacterized protein LOC101737815 | unknown | – | – |
| Bm_nscaf2529_069 | 1.619 | 0.663 | −1.288 | Down | 0 | uncharacterized protein LOC101738767 | unknown | – | – |
| Bm_scaffold700_2 | 2.896 | 6.168 | 1.091 | Up | 6.00E-141 | neuroligin-2-like | unknown | – | – |
| Bm_nscaf2575_112 | 0.001 | 0.714 | 9.479 | Up | 1.00E-159 | Bardet-Biedl syndrome 1 protein-like | unknown | – | – |
| Bm_nscaf3063_090 | 3.78 | 12.138 | 1.683 | Up | 5.00E-25 | myotubularin-related protein 3-like | unknown | – | – |
Gene expression levels (reads per kb per million reads, RPKM) were determined prior to feeding (FED-control) or after feeding of synthetic miR166b (FED-miR166b). The “FED-control” and “FED-166b” represents FED-control-RPKM and FED-166b-RPKM, respectively. The “log2ratio” means log2 (FED-166b-RPKM/FED-control-RPKM). The “Up/down-regulated” means Up-Down-Regulation (FED_166b/FED_control). H. zea, A.mylitta, B. mori, M. sexta, H. cecropia, A. gambiae, A. aegypti, H. virescens, and S. cerevisiae represent Helicoverpa zea, Antheraea mylitta, Bombyx mori, Manduca sexta, Hyalophora cecropia, Anopheles gambiae, Aedes aegypti, Heliothis virescens, and Saccharomyces cerevisiae, respectively.
Figure 3. The expression level of 6 differentially expressed genes in silkworm ingested synthetic miR166c and miR167e.
Differentially expressed genes were identified by transcriptome analysis. FED-control represents the silkworm larvae were fed on only mulberry leaves. FED-miR166c and FED-miR167e means the silkworm larvae were fed on a piece of mulberry leaf containing synthetic miR166c and miR167e, respectively. Statistical significance was determined by Student’s t test (*p < 0.05).
Phenotypic investigation of silkworms fed on synthetic miR166b
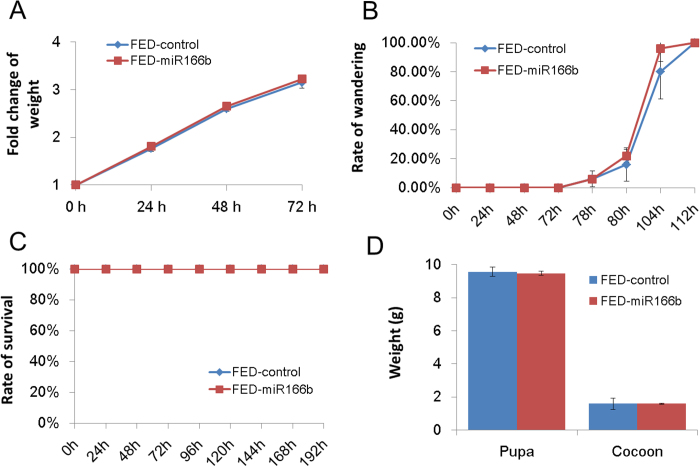
We also investigated the phenotypic changes of silkworm feeding on mulberry leaves with and without synthetic miR166b. As shown in Fig. 4, neither the weight (Fig. 4A) nor their wandering rate (Fig. 4B) showed significant difference between two groups. Similar observations had been made in lethality (Fig. 4C). The fat body and silk gland are two major organs in silkworm. The developmental growth of silk gland and fat body can be reflected by the weight of cocoon and pupae, respectively. Since the synthetic miR166b was detected in fat body and not silk gland, we then measured the pupa and cocoon weight at 10 days after ingestion of synthetic miR166b. Results showed that there was no significant difference of pupa and cocoon weight between two groups (Fig. 4D).
Figure 4. The phenotypic investigation of silkworm larvae fed on mulberry leaves with/without synthetic miR166b.
The phenotypic investigation including larva weight (A), rate of wandering (B), rate of survival (C), pupa and cocoon weight (D) were measured before (0 h) and after silkworm ingested synthetic miR166b (24 h, 48 h, 72 h, 96 h, 120 h, 144 h, 168 h, and 192 h). Fold change of weight means the ratio of lavae weight after silkworm ingested synthetic miR166b (24 h, 48 h, 72 h) divided the larvae weight at 0 h. Rate of wandering indicates the percentage of silkworm developed into the wandering stage. FED-control represents the silkworm larvae were fed only on mulberry leaves. FED-miR166b means the silkworm larvae were fed on a piece of mulberry leaf containing synthetic miR166b. Statistical significance was determined by Student’s t test (*p < 0.05).
Discussion
Using T-A clone and Sanger sequencing assay, we revealed that mulberry-derived miRNAs not only transferred to silkworm hemolymph, but also to other tissues such as fat body and silk gland. In addition, droplet digital PCR assay showed that synthetic miR166b also could enter to silkworm hemolymph and fat body after orally feeding. However, RNA-seq analyses, RT-PCR verification, and phenotypic investigation showed that no physiological process in silkworm associated with the ingestion of one tested mulberry miRNA (miR166b). Zhang et al. (2012) analyzed small RNA libraries of several species, and they concluded that low reads of plant-derived miRNA detected in animals were from contamination of large-scale sequencing and sample preparation16. Dickinson et al. (2013) applied solexa method to sequence the serum and liver of mice fed with three synthetic food containing different amount of rice13. They also emphasized those low reads of plant-derived miRNA in mammal tissues were from contamination13. However, the above two studies did not use different methods to detect the plant-derived miRNAs in animals. The methods used in this study are much more rigorous than those of previous studies and the data proved that the mulberry-derived miRNAs detected in silkworm did not result from contamination as following reasons. (1) TA-cloning and Sanger sequencing can rule out the possibility that the mulberry-derived miRNAs in silkworm were from solexa sequencing contamination; (2) Mulberry miRNAs with high expression levels were used as controls and were not detected in the silkworm hemolymph; (3) The identification of synthetic miR166b was confirmed in silkworm tissues after ingestion.
Our results demonstrated that both of mulberry-derived and synthetic plant miRNA were convincingly transferred to silkworm tissues. Zhang et al. and Zhou et al. recently reported that rice and honeysuckle-derived miRNAs could be detected in mammalian tissues12,20. Taken their results and our data together, it is undoubtedly believed that plant-derived miRNAs entering to recipient animal organisms is a conserved phenomenon. But nevertheless there are some differences between our observation and Zhang et al.’ report12. One is the plant-derived miRNAs detected in silkworm were less than those reported by Zhang et al. in mammal12. A total of seven miRNAs were identified in the silkworm hemolymph and only two plant-derived miRNA (mno-miR166b and mno-miR156c) are common in silkworm and mammals, suggesting that mammals and insects may have different selection encountered the varied plant-derived miRNAs from food. The other is the reads of xenomes from silkworm hemolymph were lower than those from mammals12. According to the current studies on miRNAs, the functions of miRNAs appear to be associated with their abundances21. Gavriel et al. reported only the most abundant miRNAs in a cell mediated significant target suppression21. Thus, there was a question whether miRNA with low abundance could play roles in physiological processes. Based on our data, the answer is no. Zhang et al. found rice miR168 regulated the level of cholesterol by targeting to LDLRAP112. Zhou et al. reported honeysuckle miR2911 inhibited H1N1-PB2 and AS1 to suppress viral infection20. The abundances of the above two miRNAs (miR168 and miR2911) were very high in mice tissues after ingestion, which may provide the basis for the miRNAs playing roles in mice. Therefore, we can speculate that cross-kingdom miRNA transfer is a conserved phenomenon, however, not all plant-derived miRNAs have influence on the physiological progress of recipient animal organism. It is still unclear how plant-derived miRNAs resist to herbivore RNAses. The differential stability or resistance to RNAses in recipient animal tissues might be crucial for their functions. Further analysis will be required to clarify the underlying mechanism.
Material and Methods
Identification of mulberry-derived miRNAs in silkworm hemolymph
The hemolymph small RNA data of silkworm (GSE48168) was downloaded from NCBI (http://www.ncbi.nlm.nih.gov/). Mulberry-derived miRNAs identified from mulberry leaves small RNA library18 were aligned with silkworm hemolymph small RNA sequences.
Preparations of the silkworm hemolymph and tissue samples
Silkworm larvae were fed mulberry leaves under a 12 h–light/12 h-dark cycle at 25 °C with relative humidity of 75%. Silkworm hemolymph was collected from fifth instar day-5 larvae using sterile tubes containing a few crystals of phenylthiourea. The silkworm larvae were dissected. The brain, prothoracic gland, salivary gland, gut, malpighian tubule, ovary, testis, fat body, and silk gland samples were collected and washed three times with cold 0.75% NaCl. Hemolymph samples were centrifuged at 3000 × g for 10 min at 4 °C. The supernatants of hemolymph, tissue samples were stored at −80 °C until use.
Tissues from silkworms fed synthetic miR166b
300 pmol of synthetic miR166b (UCGGACCAGGCUUCAUUCCCC) with a 2’-O-methyl modified terminal nucleotide (RIBOBIO, China) was daubed on ~1 cm2 pieces of mulberry leaf. The synthetic miR166b was dissolved in RNase-free water. A single piece of leaf was fed to each silkworm larvae in the fifth instar day-5 after the miRNA liquid dried. The hemolymph, fat body, and silk gland were collected at 0.5 h, 3 h, 6 h and 12 h after the larvae ate the whole piece of leaf. Control (0 h) silkworms were only fed mulberry leaves. Each group contained five larvae.
RNA extraction, T-A cloning and Sanger sequencing
Total RNA from the fat body and silk gland was extracted using RNAiso Plus (D9108A, Takara, China) according to the manufacturer’s instructions. The small RNA was purified using mirVana™ PARIS™ Kit (AM1556, Ambion, USA) and eluted in 25 μl non-nuclease water. Total RNA from fat body and silk gland and small RNA from hemolymph were reverse transcribed to cDNA in 10 μl reactions using M-MLV (D2641B, Takara, China) and specific reverse transcriptional primers for each miRNA. Stem-loop PCR was performed using miRNA specific forward and reverse primers (supplementary Table S5). The PCR products were cloned into pMD19-T vector (D102A, Takara, China). We randomly picked 60 monoclones for each miRNA, and of these, chose 50 clones that could be verified by bacterial PCR for Sanger sequencing with M13 primer.
Measurement of miRNA copy number counts by droplet digital PCR
To standardize the amount of tissue at five time points, we reverse transcribed the same amount of total RNA from the silk gland and fat body, or small RNA from hemolymph at each time point. Total RNA (2.4 μg) from silkworm fat bodies or silk glands, or small RNA (800 ng) from hemolymph before or after oral synthetic miR166b at each of five time points was reverse transcribed in 15 μl reactions using the TaqMan microRNA reverse transcription kit (43366596, ABI, USA). ddPCR was performed using the QX100 droplet generator and droplet reader. Each PCR reaction was carried out in a 20 μl volume containing 10 μl 2X ddPCR supermix (Bio-rad, USA), 1.5 μl cDNA, 1 μl miR166b-specific hydrolysis probe assay (4427975, Life Technologies, USA), and 7.5 μl non-nuclease water. The 20 μl reaction was divided by the droplet generator and detected by the droplet readers according to the manufacturer’s instructions.
Materials for RNA-seq
The 450 pmol synthetic miR166b (UCGGACCAGGCUUCAUUCCCC) with 2’-O-methyl modification on its terminal nucleotide (RIBOBIO, China) was daubed on a ~1 cm2 piece of mulberry leaf. Piece of leaf were fed to silkworm larvae in the fifth instar day-2 after the miRNA liquid dried. Whole silkworm larvae were collected at 3 h after ingestion of an entire piece leaf, and this experimental group (FED-miR166b) was stored at −80 °C until use. A control group of silkworm (FED-control) was only fed mulberry leaves. Each group contained three larvae. Total RNA from whole larvae were extracted by RNAiso Plus (D9108A, Takara, China) according to manufacturer’s instructions. Agilent 2100 was used to ensure the integrity of total RNA. cDNA libraries were constructed using the TruSeq RNA Sample Preparation v2 Guide (Illumina), and then sequenced by Illumina HiSeqTM 2000. The obtained raw data from solexa sequencing were deposited on the Short Read Archive of NCBI (http://www.ncbi.nlm.nih.gov/sra/) with accession number SRP051555.
Transcriptome analysis before and after silkworm larvae were fed synthetic miR166b
The quality of raw data in the FED-control and FED-miR166b transcriptome was determined by CLC Genomics Workbench 5.5 following the manufacturer’s instructions. After filtering the low quality, the clean reads were obtained that matched the silkworm genome and silkworm genes (http://www.silkdb.org/silkdb/doc/download.html) by SOAP222,23. The distribution and coverage of clean reads on the silkworm genome and genes were analyzed using CLC Genomics Workbench 5.5. Expression analysis of each gene in two transcriptomes was performed as follows. Briefly, the expression level of each gene was normalized using the reads per kb per million reads (RPKM) method24. P value < 0.05 and false discovery rate (FDR) < 0.001 were used as thresholds for gene expression. Genes with absolute values of the log2 of the ratio of RPKMs (FED-miR166b/FED-control) that were more than 1 were regarded as a differentially expressed. Values of more than 1 indicated up-regulated genes, and values of less than −1 indicated down-regulated genes. The annotations of differentially expressed genes were performed by aligning against the non-redundant protein sequences (nr) database using BLASP (http://blast.st-va.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome).
Target site prediction for 30 differentially expressed genes and silkworm full-length cDNA for miR166b
Silkworm full-length cDNA sequences were downloaded from KAIKObase (http://sgp.dna.affrc.go.jp/FLcDNA/). Target sites prediction for 30 differentially expressed genes and silkworm full-length cDNA for miR166b was performed using Miranda software (http://www.microrna.org/microrna/getDownloads.do). The criteria were set as follows. (1) The “seed region” of miR166b (containing the 2nd to 8th nucleotides of the mature miRNA from 5’ to 3’) should match the target genes (30 differentially expressed genes) with perfect complementary. (2)The free energy of the hybrid should be less than −25 kcal/mol.
RT-PCR detection of potential target genes
The 450 pmol synthetic miR166c (UCUCGGACCAGGCUUCAUUCC) and synthetic miR167e (UGAAGCUGCCAGCAUGAUCUG) with 2’-O-methyl modification on their terminal nucleotides (RIBOBIO, China) were fed to silkworm larvae as above described. Each group contained three larvae. The larvae in control group (FED-control) were only fed on mulberry leaves. Total RNA of FED-miR166b, FED-miR166c, FED-miR167e, and FED-control from whole larvae were extracted by RNAiso Plus (D9108A, Takara, China) according to manufacturer’s instructions. Total RNA were reverse transcribed to cDNA by using PrimeScript RT reagent Kit with gDNA Eraser (RR047A, Takara, China). The cDNA was then diluted four times and used as the template to perform the RT-PCR with gene specific forward and reverse primers, as listed in supplementary Table S6. The PCR reactions were performed in ABI Step One Plus (Applied Biosystems, USA) using SYBR Premix Ex TaqTM II (RR820A, TakaRa, China) as the following conditions: 95 °C for 30 s, 40 cycles of 95 °C for 5 s, and 60 °C for 30 s. The translation initiation factor 4 A was used as an inner control. All reactions were assayed in triplicated. The relative expression level of genes was calculated using 2−∆∆Ct method.
Phenotypic investigation of silkworm larvae fed on mulberry leaves with/without synthetic miR166b
The FED-miR166 and FED-control groups contained five sub-groups, separately, and each sub-group contained 10 larvae. The weight of larva, rate of wandering, and rate of survival in each groups were investigated. The weight of pupa and cocoon were measured at 10 days after ingestion of synthetic miR166b. Student’s t test (*p < 0.05) was performed to determine the statistical significance according to the graphpad software (http://www.graphpad.com/quickcalcs/ttest1.cfm).
Additional Information
How to cite this article: Jia, L. et al. Nonfunctional ingestion of plant miRNAs in silkworm revealed by digital droplet PCR and transcriptome analysis. Sci. Rep. 5, 12290; doi: 10.1038/srep12290 (2015).
Supplementary Material
Acknowledgments
This project was funded by the research grants from the National Hi-Tech Research and Development Program of China (No. 2013AA100605-3), the Fundamental Research Funds for the Central Universities (No. 2362014xk05), the “111” Project (B12006), and the Science Fund for Distinguished Young Scholars of Chongqing (Grant No. cstc2011jjjq0010).
Footnotes
Author Contributions J.L. and H.N.J. conceived and designed the study. J.L. and Z.D.Y. performed all the experiments. J.L., X.Z.H. and H.N.J. analyzed all the experiments. J.L. and H.N.J. wrote the manuscript with support from all authors.
References
- Bartel D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. cell 116, 281–297 (2004). [DOI] [PubMed] [Google Scholar]
- Khan G. A. et al. MicroRNAs as regulators of root development and architecture. Plant Mol Biol 77, 47–58 (2011). [DOI] [PubMed] [Google Scholar]
- Kidner C. A. The many roles of small RNAs in leaf development. J Genet Genomics 37, 13–21 (2010). [DOI] [PubMed] [Google Scholar]
- Luo Y., Guo Z. & Li L. Evolutionary conservation of microRNA regulatory programs in plant flower development. Dev Biol 380, 133–144 (2013). [DOI] [PubMed] [Google Scholar]
- Ryazansky S., Mikhaleva E. & Olenkina O. Essential functions of microRNAs in animal reproductive organs. Mol Biol 48, 319–331 (2014). [PubMed] [Google Scholar]
- Waldron J. & Newbury S. The roles of miRNAs in wing imaginal disc development in Drosophila. Biochem Soc T 40, 891 (2012). [DOI] [PubMed] [Google Scholar]
- Chim S. S. et al. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem 54, 482–490 (2008). [DOI] [PubMed] [Google Scholar]
- Mitchell P. S. et al. Circulating microRNAs as stable blood-based markers for cancer detection. P Natl Acad Sci USA 105, 10513–10518 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers K. C., Palmisano B. T., Shoucri B. M., Shamburek R. D. & Remaley A. T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13, 423–433 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javidi M. A. et al. Cell-free microRNAs as cancer biomarkers: the odyssey of miRNAs through body fluids. Med Oncol 31, 1–11 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell 39, 133–144 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang L. et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res 22, 107–126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson B. et al. Lack of detectable oral bioavailability of plant microRNAs after feeding in mice. Nat Biotechnol 31, 965–967 (2013). [DOI] [PubMed] [Google Scholar]
- Hirschi K. D. New foods for thought. Trends Plant Sci 17, 123–125 (2012). [DOI] [PubMed] [Google Scholar]
- Witwer K. W., McAlexander M. A., Queen S. E. & Adams R. J. Real-time quantitative PCR and droplet digital PCR for plant miRNAs in mammalian blood provide little evidence for general uptake of dietary miRNAs: limited evidence for general uptake of dietary plant xenomiRs. RNA Biol 10, 1080–1086 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. et al. Analysis of plant-derived miRNAs in animal small RNA datasets. BMC Genomics 13, 381 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N. et al. Draft genome sequence of the mulberry tree Morus notabilis. Nat Commun 4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L. et al. Identification of the Conserved and Novel miRNAs in Mulberry by High-Throughput Sequencing. PloS One 9, e104409 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. Small RNAs and their roles in plant development. Annu Rev Cell Dev Bi 25, 21–44 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z. et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res 25, 39–49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullokandov G. et al. High-throughput assessment of microRNA activity and function using microRNA sensor and decoy libraries. Nat Methods 9, 840–846 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. et al. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics 25, 1966–1967 (2009). [DOI] [PubMed] [Google Scholar]
- Mortazavi A., Williams B. A., McCue K., Schaeffer L. & Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5, 621–628 (2008). [DOI] [PubMed] [Google Scholar]
- Zhang Q. & Denlinger D. L. Molecular characterization of heat shock protein 90, 70 and 70 cognate cDNAs and their expression patterns during thermal stress and pupal diapause in the corn earworm. J Insect physiol 56, 138–150 (2010). [DOI] [PubMed] [Google Scholar]
- Gandhe A. S., Arunkumar K., John S. H. & Nagaraju J. Analysis of bacteria-challenged wild silkmoth, Antheraea mylitta (lepidoptera) transcriptome reveals potential immune genes. BMC Genomics 7, 184 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaoka S. et al. Functional analysis of four Gloverin - like genes in the silkworm, Bombyx mori. Arch Insect Biochem 67, 87–96 (2008). [DOI] [PubMed] [Google Scholar]
- Cox-Foster D. L. & Stehr J. E. Induction and localization of FAD-glucose dehydrogenase (GLD) during encapsulation of abiotic implants in Manduca sexta larvae. J Insect Physiol 40, 235–249 (1994). [Google Scholar]
- Sugiyama M. et al. Characterization of a Bombyx mori cDNA encoding a novel member of the attacin family of insect antibacterial proteins. Insect Biochem Molec 25, 385–392 (1995). [DOI] [PubMed] [Google Scholar]
- Carlsson A., Engström P., Palva E. T. & Bennich H. Attacin, an antibacterial protein from Hyalophora cecropia, inhibits synthesis of outer membrane proteins in Escherichia coli by interfering with omp gene transcription. Infect Immun 59, 3040–3045 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. et al. cDNA cloning and gene expression of cecropin D, an antibacterial protein in the silkworm, Bombyx mori. Comp Biochem Phys B 122, 409–414 (1999). [DOI] [PubMed] [Google Scholar]
- Zhu F. & Zhang X. The Wnt signaling pathway is involved in the regulation of phagocytosis of virus in Drosophila. Sci Rep 3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. & Granados R. R. An intestinal mucin is the target substrate for a baculovirus enhancin. P Natl Acad Sci USA 94, 6977–6982 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C., Ragan E. J. & Kanost M. R. Serpin-1 splicing isoform J inhibits the proSpätzle-activating proteinase HP8 to regulate expression of antimicrobial hemolymph proteins in Manduca sexta. Dev Comp Immunol 35, 135–141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H. et al. Cloning and Expression of a Novel Gene Encoding a New Antibacterial Peptide from Silkworm, Bombyx mori. Biochem Bioph Res Co 246, 388–392 (1998). [DOI] [PubMed] [Google Scholar]
- Kaneko Y., Furukawa S., Tanaka H. & Yamakawa M. Expression of antimicrobial peptide genes encoding Enbocin and Gloverin isoforms in the silkworm, Bombyx mori. Biosci Biotech Bioch 71, 2233–2241 (2007). [DOI] [PubMed] [Google Scholar]
- Panda D. et al. RNAi screening reveals requirement for host cell secretory pathway in infection by diverse families of negative-strand RNA viruses. P Natl Acad Sci USA 108, 19036–19041 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Whang L. H., Kumar V. & Kafatos F. C. Two genes encoding midgut-specific maltase-like polypeptides from Anopheles gambiae. Exp Parasitol 81, 272–283 (1995). [DOI] [PubMed] [Google Scholar]
- James A. A., Blackmer K. & Racioppi J. V. A salivary gland-specific, maltase-like gene of the vector mosquito, Aedes aegypti. Gene 75, 73–83 (1989). [DOI] [PubMed] [Google Scholar]
- Hanzlik T. N., Abdel-Aal Y., Harshman L. G. & Hammock B. D. Isolation and sequencing of cDNA clones coding for juvenile hormone esterase from Heliothis virescens. Evidence for a catalytic mechanism for the serine carboxylesterases different from that of the serine proteases. J Biol Chem 264, 12419–12425 (1989). [PubMed] [Google Scholar]
- Gao H., Wu X. & Fossett N. Odd-skipped maintains prohemocyte potency and blocks blood cell development in Drosophila. Genesis 49, 105–116 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgomanolis T., Iatrou K. & Swevers L. BmCAP, a silkmoth gene encoding multiple protein isoforms characterized by SoHo and SH3 domains: Expression analysis during ovarian follicular development. Insect Biochem Molec 39, 892–902 (2009). [DOI] [PubMed] [Google Scholar]
- Dason J. S. et al. Frequenin/NCS-1 and the Ca2+ -channel α1-subunit co-regulate synaptic transmission and nerve-terminal growth. J Cell Sci 122, 4109–4121 (2009). [DOI] [PubMed] [Google Scholar]
- Yoshihara M. & Littleton J. T. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron 36, 897–908 (2002). [DOI] [PubMed] [Google Scholar]
- Kern J. V., Zhang Y. V., Kramer S., Brenman J. E. & Rasse T. M. The Kinesin-3, Unc-104 regulates dendrite morphogenesis and synaptic development in Drosophila. Genetics 195, 59–72 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voegtli W. C., Madrona A. Y. & Wilson D. K. The structure of Aip1p, a WD repeat protein that regulates Cofilin-mediated actin depolymerization. J Biol Chem 278, 34373–34379 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.