Abstract
The effect of irrigation with artificial cerebrospinal fluid (CSF) containing various magnesium ion (Mg2+) concentrations on vasospastic arteries was investigated in the dog. Cerebral vasospasm was induced by the experimental subarachnoid hemorrhage model in 15 beagle dogs. Cisternal irrigation was performed for 1 hour via a microcatheter placed in the cisterna magna with commercially available artificial CSF (ARTCEREB®) with physiological concentration of Mg2+ (2.2 mEq/l) (ACM group, n = 5), ARTCEREB solution without Mg2+ (ACR group, n = 5), and ARTCEREB solution with higher Mg2+ concentration (5 mEq/l) (ACMM group, n = 5). CSF electrolyte concentrations and the diameters of the basilar and vertebral arteries were measured. In the ACM group, no changes were detected in either CSF Mg2+ concentration or arterial diameters. In the ACR group, the CSF Mg2+ decreased significantly to 0.8 ± 0.07 mEq/l from the baseline value of 1.4 ± 0.03 mEq/l, and both basilar and vertebral artery diameters were significantly decreased to 0.61 ± 0.18 mm and 0.57 ± 0.23 mm from their baseline values of 0.74 ± 0.22 mm and 0.68 ± 0.17 mm, respectively. In the ACMM group, the CSF Mg2+ significantly increased to 2.4 ± 0.15 mEq/l from the baseline value of 1.4 ± 0.05 mEq/l, and both basilar and vertebral artery diameters were significantly increased to 0.84 ± 0.19 mm and 0.90 ± 0.22 mm from their baseline values of 0.71 ± 0.21 mm and 0.69 ± 0.24 mm, respectively. Irrigation with artificial CSF solution without Mg2+ causes vasoconstriction of the cerebral artery. Irrigation with artificial CSF with appropriate Mg2+ concentration is essential, especially in patients with subarachnoid hemorrhage.
Keywords: magnesiumion, artificial cerebrospinal fluid, vasoconstriction, vasospasm
Introduction
Vascular muscle tone is mainly regulated by extracellular calcium ion (Ca2+) and magnesium ion (Mg2+) concentrations. The extracellular Mg2+ acts as a vasodilator under both physiological and pathological conditions.7,15) Recently, the extracellular Mg2+ concentration was shown to regulate the cerebral arteriole diameter that controls cerebral blood flow (CBF).11) Cisternal infusion of magnesium sulfate solution increased the cerebrospinal fluid (CSF) Mg2+ concentration, resulting in dilated spastic cerebral arteries and amelioration of the reduced CBF after experimental subarachnoid hemorrhage (SAH).8–10) Therefore, maintenance of appropriate extracellular and cisternal Mg2+ concentrations is considered to be clinically important under pathophysiological conditions.
Commercially available artificial CSF solution (ARTCEREB®; Otsuka Pharmaceutical Co., Ltd., Tokushima) is widely used to irrigate and perfuse the brain during cerebrospinal surgery. This artificial CSF has similar pH (7.3) and electrolyte concentrations to the human CSF.1,2) ARTCEREB solution is characterized as containing the physiological level of Mg2+ (2.2 mEq/l). Recently, we reported that cisternal infusion of Ringer solution without magnesium worsens the cerebral arterial vasospasm induced in the canine SAH model, whereas infusion of Ringer solution containing magnesium resulting in CSF Mg2+ concentration of more than 3 mEq/l ameliorated such vasospasm.10) Ringer solution's electrolytes concentrations (130 mEq/l Na+, 4 mEq/l K+, 109 mEq/l Cl−, and 28 mEq/l lactate) are different from those of normal CSF. However, whether Mg2+ in the mock CSF solution acts as same as that in the Ringer solution has not been examined.
The present study investigated the effects of commercially available artificial CSF solutions with or without Mg2+ on the spastic cerebral arteries induced in the canine SAH model to assess the potential risks of using artificial CSF solution without Mg2+ for irrigation of the brain, and to provide the rationale for use of artificial CSF solution with appropriate Mg2+ concentrations for routine neuro-surgical procedures including cisternal irrigation therapy in patients with SAH.
Materials and Methods
All animal experiments were performed in accordance with the Institutional Guidelines and Rules of Animal Experimentation and the Guidelines for the Care and Use of Laboratory Animals of Juntendo University Shizuoka Hospital. Fifteen female beagle dogs (weighing 9–11 kg) were anesthetized with an intravenous bolus injection of 20 mg/kg of pentobarbital and subsequent continuous intravenous infusion of 1 ml/kg/hr of propofol. The dogs were intubated using a 6 Fr endotracheal tube and mechanically ventilated. On Days 1 and 2, the experimental SAH model was conducted to induce vasospasm by puncturing the cisterna magna with a 20-gauge needle and injecting autologous blood (0.5 ml/kg) after the removal of 0.3 ml/kg CSF (double hemorrhage SAH model). On Day 7, the animals were anesthetized and intubated as on Days 1 and 2. A 4 Fr double-lumen sheath catheter was placed in the femoral artery for cerebral angiography and blood gas sampling. End-tidal CO2 was carefully monitored and the PaCO2 was maintained between 35 mmHg and 45 mmHg.
The left vertebral artery was cannulated using a 4 Fr catheter under fluoroscopic control, and digital subtraction angiography was performed after injecting 2.5 ml iopamidol (Bayer AG, Leverkusen, Germany). Vertebral angiograms were obtained to measure the diameters of the vertebral artery and basilar artery, performed by radiological technicians unaware of the experimental protocols. The diameter of the basilar artery was measured at three points, the vertebro-basilar artery junction (lower point), basilar-superior cerebellar artery junction (upper point), and the midpoint between them. The mean value of the diameters at these three points was taken as the basilar artery diameter. The vertebral artery diameters were measured at both sides of the distal point of the artery just proximal to the basilar artery, and the mean of the two diameters was taken as the vertebral artery diameter.
Immediately after the first angiography, the dogs were placed in the lateral position. Lumbar puncture was performed with a 17-gauge Tuohy needle. A microcatheter (RenegradeTM; Boston Scientific Corp., Natick, Massachusetts, USA) with a guide-wire (TransendTM EX; Boston Scientific Corp.) was introduced into the lumbar subarachnoid space under fluoroscopic observation, and the tip of the microcatheter was advanced and positioned in the cisterna magna. Three types of irrigation solution were prepared: commercially available artificial CSF solution with Mg2+ (2.2 mEq/l) (ARTCEREB, ACM), ARTCEREB without Mg2+ provided by Otsuka Pharmaceutical Co., Ltd. (ACR), and ARTCEREB with higher Mg2+ concentration (5 mEq/l) (ACMM). The compositions of these three artificial CSF solutions are listed in Table 1. The cisterna magna was punctured with a 20-gauge needle to obtain CSF samples for measurement of the CSF electrolyte concentrations. After CSF sampling, one of the three artificial CSF solutions was injected through the microcatheter as a bolus of 0.5 ml/kg and then infusion was continued at 5 ml/hr for 1 hour. The animals were divided into three groups (ACR group, ACM group, ACMM group) according to the artificial CSF solution, and each group contained five dogs.
Table 1.
Compositions of three kinds of artificial cerebro-spinal fluid
| ACR | ACM | ACMM | |
|---|---|---|---|
| pH | ≒ 7.3 | ≒ 7.3 | ≒ 7.3 |
| Na+ (mEq/l) | 145 | 145 | 145 |
| K+ (mEq/l) | 2.8 | 2.8 | 2.8 |
| Cl− (mEq/l) | 129 | 129 | 129 |
| Ca2+ (mEq/l) | 2.3 | 2.3 | 2.3 |
| Mg2+ (mEq/l) | 0 | 2.2 | 5.0 |
| HCO3− (mEq/l) | 23.1 | 23.1 | 23.1 |
| P (mEq/l) | 1.1 | 1.1 | 1.1 |
| Glucose (g/l) | 0.61 | 0.61 | 0.61 |
ACM: artificial cerebro-spinal fluid solution with normal Mg2+ concentration (2.2 mEq/l), ACMM: artificial cerebro-spinal fluid solution with increased Mg2+ concentration (5 mEq/l), ACR: artificial cerebro-spinal fluid solution without Mg2+.
After infusion was completed, vertebral angiography was repeated to measure the arterial diameters again, and CSF sampling was also repeated. The dogs were euthanized with overdose intravenous injection of pentobarbital. The CSF Na+, K+, Cl−, Ca2+, and Mg2+ ionized concentrations were measured with ion-selective electrodes (StatProfile CCX; NOVA Biomedical, Waltham, Massachusetts, USA).
Data are presented as the means ± SDs. The statistical significance of differences was analyzed with the paired t-test by using SPSS 7.5.1 J for Windows (SPSS Japan Inc., Tokyo). A p value < 0.05 was considered statistically significant.
Results
Table 2 shows the concentrations of arterial blood gases and CSF electrolytes before and after cisternal irrigation with the three types of artificial CSF solution. No changes in these data occurred except for the CSF Mg2+ concentration. In the ACR group, the Mg2+ concentration was 0.8 ± 0.07 mEq/l at 1 hour after irrigation, which was significantly (p < 0.01) decreased compared to 1.4 ± 0.03 mEq/l before the irrigation. In the ACM group, the Mg2+ concentration was 1.5 ± 0.08 mEq/l at 1 hour after irrigation, which was not significantly changed compared to 1.4 ± 0.10 mEq/l before irrigation. In the ACMM group, the Mg2+ concentration was 2.4 ± 0.15 mEq/l at 1 hour after irrigation, which was significantly (p < 0.01) increased compared to 1.4 ± 0.05 mEq/l before irrigation. In the ACMM group, the CSF Mg2+ significantly increased, but the vital signs (blood pressure, the heart rate, and the body temperature) were not significantly changed after irrigation (data were not shown).
Table 2.
Arterial blood gas and CSF electrolytes before and after cisternal irrigation
| ACR | ACM | ACMM | |
|---|---|---|---|
| Arterial blood gas | |||
| pH | |||
| before | 7.37 ± 0.02 | 7.39 ± 0.01 | 7.36 ± 0.02 |
| after | 7.39 ± 0.03 | 7.37 ± 0.02 | 7.37 ± 0.02 |
| pCO2 | |||
| before | 37 ± 2 | 38 ± 2 | 39 ± 4 |
| after | 38 ± 3 | 39 ± 2 | 39 ± 2 |
| pO2 | |||
| before | 343 ± 43 | 379 ± 40 | 396 ± 23 |
| after | 384 ± 44 | 384 ± 35 | 376 ± 13 |
| CSF electrolytes | |||
| Na+ | |||
| before | 142 ± 2 | 143 ± 1 | 144 ± 1 |
| after | 139 ± 1 | 141 ± 1 | 140 ± 1 |
| K+ | |||
| before | 2.7 ± 0.2 | 2.7 ± 0.1 | 2.7 ± 0.1 |
| after | 3.0 ± 0.2 | 2.9 ± 0.1 | 2.8 ± 0.1 |
| Cl− | |||
| before | 121 ± 4 | 123 ± 2 | 124 ± 1 |
| after | 121 ± 3 | 122 ± 2 | 125 ± 1 |
| Ca2+ | |||
| before | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.0 ± 0.1 |
| after | 2.2 ± 0.2 | 2.1 ± 0.1 | 2.0 ± 0.1 |
| Mg2+ | |||
| before | 1.4 ± 0.03 | 1.4 ± 0.10 | 1.4 ± 0.05 |
| after | 0.8 ± 0.07 * | 1.5 ± 0.08 | 2.4 ± 0.15 * |
ACM: mock cerebrospinal fluid with normal magnesium concentration, ACMM: mock cerebrospinal fluid with higher magnesium concentration, ACR: mock cerebrospinal fluid without magnesium, CSF: cerebrospinal fluid. Data are means ± standard deviation,
p < 0.05.
Table 3 shows the arterial diameters of the basilar and vertebral arteries before and after cisternal irrigation with the three types of artificial CSF solution. In the ACR group, the diameters of the basilar artery and vertebral artery were 0.61 ± 0.18 mm and 0.57 ± 0.23 mm, respectively, at 1 hour after irrigation, which were significantly (p < 0.05) decreased compared to the diameters of 0.74 ± 0.22 mm and 0.68 ± 0.17 mm, respectively, before irrigation. Representative vertebral angiograms before and after cisternal irrigation with artificial CSF solution without Mg2+ are presented in Fig. 1. In the ACM group, the diameters of the basilar artery and vertebral artery were 0.75 ± 0.10 mm and 0.72 ± 0.19 mm, respectively, at 1 hour after irrigation, which were not significantly changed compared with the diameters of 0.73 ± 0.10 mm and 0.66 ± 0.11 mm, respectively, before irrigation. In the ACMM group, the diameters of the basilar artery and vertebral artery were 0.84 ± 0.19 mm and 0.90 ± 0.22 mm, respectively, at 1 hour after irrigation, which were significantly (p < 0.05) increased compared with the diameters of 0.71 ± 0.21 mm and 0.69 ± 0.24 mm, respectively, before irrigation. Representative vertebral angiograms before and after cisternal irrigation with artificial CSF solution containing higher Mg2+ concentration than that of the physiological state are presented in Fig. 2.
Table 3.
Cerebral arterial diameters before and after cisternal irrigation
| ACR (n = 5) | ACM (n = 5) | ACMM (n = 5) | |
|---|---|---|---|
| BA diameter (mm) | |||
| before | 0.74 ± 0.22 | 0.73 ± 0.10 | 0.71 ± 0.21 |
| after | 0.61 ± 0.18* | 0.75 ± 0.10 | 0.84 ± 0.19* |
| VA diameter (mm) | |||
| before | 0.68 ± 0.17 | 0.66 ± 0.11 | 0.69 ± 0.24 |
| after | 0.57 ± 0.23 * | 0.72 ± 0.19 | 0.90 ± 0.22* |
ACM: mock cerebrospinal fluid with normal magnesium concentration, ACMM: mock cerebrospinal fluid with higher magnesium concentration, ACR: mock cerebrospinal fluid without magnesium, CSF: cerebrospinal fluid. Data are means ± standard deviation,
p < 0.05.
Fig. 1.
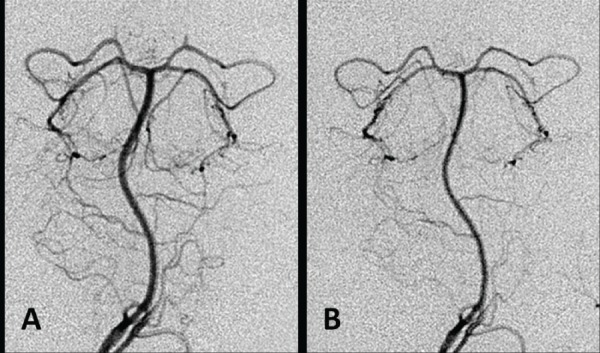
Representative angiograms obtained before (A) and 1 hour after (B) cisternal irrigation with artificial cerebrospinal fluid (CSF) solution without Mg2+. Note the vasoconstriction after irrigation.
Fig. 2.
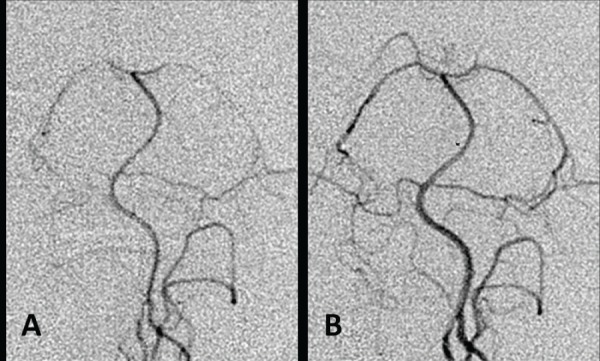
Representative angiograms obtained before (A) and 1 hour after (B) cisternal irrigation with artificial cerebrospinal fluid (CSF) solution containing higher than physiological Mg2+ concentration (5 mEq/l). Note the vasodilation after irrigation.
Discussion
ARTCEREB solution is an artificial CSF with similar characteristics to physiological CSF.2) The composition, pH, and concentrations of electrolytes including Mg2+ and glucose were determined based on the previously reported values for human physiological CSF.1) Generally, normal saline and lactated Ringer's solution have been used for irrigation and perfusion during neurosurgical procedures and post-operative continuous cisternal irrigation therapy to washout subarachnoid blood and prevent delayed vasospasm after SAH.4,5,12,14) The main advantage of ARTCEREB solution is the physiological concentration of Mg2+ provided by neither normal saline nor lactated Ringer's solution. Cerebral irrigation with normal saline has been reported to cause adverse neurotoxicity effects such as neuronal cell death, seizure, high fever, and headache in both experimental animals and humans.3,13) Moreover, irrigation by normal saline and lactated Ringer's solution are known to cause worsening of brain edema surrounding the injured brain, whereas this adverse effect is less prominent using ARTCEREB.2,6) Therefore, it is quite reasonable to irrigate and perfuse the brain by artificial CSF solution with the same composition of electrolytes and solutes as human CSF. However, the importance of appropriate Mg2+ concentration in the artificial CSF solution for the cerebral vasculature remains unclear.
The present study demonstrated that cisternal irrigation with artificial CSF without Mg2+ caused significant reduction in the diameters of spastic cerebral arteries after experimental SAH, artificial CSF with normal concentration of Mg2+ did not cause any change in the arterial diameter, and artificial CSF with increased concentration of Mg2+ caused vasodilation of the spastic cerebral arteries. Continuous cisternal irrigation therapy for washout of the blood clot in the subarachnoid space can prevent the vasospasm and subsequent delayed neurological deficits after aneurysmal SAH.4,5,12,14) Such cisternal irrigation therapies use lactated Ringer's solution or normal saline as the vehicle for fibrinolytic agents such as urokinase and tissue typed plasminogen activator. The present findings suggest that use of irrigation solution without Mg2+ after SAH might potentially worsen vasospasm, and that artificial CSF solution with appropriate Mg2+ concentration would be better. Recently, investigation of the appropriate CSF Mg2+ concentration to dilate the spastic cerebral arteries in the experimental SAH models found that CSF Mg2+ concentration of more than 3 mEq/l caused dilation of all examined arterial diameters.10) However, reanalysis of the same data using the χ2 test showed that CSF Mg2+ concentration of more than 2.5 mEq/l significantly increased the spastic cerebral arterial diameter (data was not shown). The present study used artificial CSF solution with higher Mg2+ concentration (5 mEq/l) which resulted in CSF Mg2+ concentration of 2.4 ± 0.15 mEq/l after irrigation. Arterial diameter was slightly but significantly increased, and was well compatible with the previously reported data. CSF Mg2+ concentration of more than 2.5 mEq/l is supposed to be necessary for vasodilation therapy to treat and prevent vasospasm after SAH.
Adequate levels of Mg2+ in the irrigation solution during neurosurgical procedures are also important to maintain cerebral arterial and arteriolar diameters. Extracellular Mg2+ concentration controls the diameters of both cerebral arteries and the penetrating arterioles that act as resistance vessels to regulate CBF.11,15) Mechanical spasm of cerebral blood vessels caused by direct touch, the spasmogenic property of irrigation fluid without Mg2+, and temporary occlusion of the vasculature may all obstruct correct surgical manipulation of the blood vessels, and may cause CBF and metabolic disturbance.16) Therefore, brain irrigation with artificial CSF solution with appropriate Mg2+ concentration is highly recommended in neurosurgical procedures. The use of commercially available artificial CSF (ARTCEREB) is the ideal solution for irrigation and perfusion of brain in routine neurosurgical practice.
Acknowledgments
This study was supported by Grand-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 21591857).
References
- 1). Davson H: Chemical composition and secretory nature of the fluids, in Davson H, Landers J, Churchill A. (eds): Physiology of the Cerebrospinal Fluid. London, J & A Churchill, 1967, pp 33– 54 [Google Scholar]
- 2). Doi K, Kawano T, Morioka Y, Fujita Y, Nishimura M: Various irrigation fluids affect postoperative brain edema and cellular damage during experimental neurosurgery in rats. Surg Neurol 66: 565– 571; discussion 571–572, 2006. [DOI] [PubMed] [Google Scholar]
- 3). Doi K, Morioka Y, Nishimura M, Kawano T, Harada D, Naito S, Yamauchi A: Perfusion fluids used in neurosurgery affect cerebrospinal fluid and surrounding brain parenchyma in the rat ventriculocisternal perfusion model. J Toxicol Sci 34: 511– 518, 2009. [DOI] [PubMed] [Google Scholar]
- 4). Kinouchi H, Ogasawara K, Shimizu H, Mizoi K, Yoshimoto T: Prevention of symptomatic vasospasm after aneurysmal subarachnoid hemorrhage by intra-operative cisternal fibrinolysis using tissue-type plasminogen activator combined with continuous cisternal drainage. Neurol Med Chir (Tokyo) 44: 569– 575; discussion 576–577, 2004. [DOI] [PubMed] [Google Scholar]
- 5). Kodama N, Sasaki T, Kawakami M, Sato M, Asari J: Cisternal irrigation therapy with urokinase and ascorbic acid for prevention of vasospasm after aneurysmal subarachnoid hemorrhage. Outcome in 217 patients. Surg Neurol 53: 110– 117; discussion 117–118, 2000. [DOI] [PubMed] [Google Scholar]
- 6). Koizumi S, Hayasaka T, Goto-Inoue N, Doi K, Setou M, Namba H: Imaging mass spectrometry evaluation of the effects of various irrigation fluids in a rat model of postoperative cerebral edema. World Neurosurg 77: 153– 159, 2012. [DOI] [PubMed] [Google Scholar]
- 7). Miura K: [Changes in Mg++ concentration of CSF after subarachnoid hemorrhage and Mg++—effects on the contractions of bovine cerebral artery]. No Shinkei Geka 16: 1251– 1259, 1988. (Japanese) [PubMed] [Google Scholar]
- 8). Mori K, Miyazaki M, Hara Y, Aiko Y, Yamamoto T, Nakao Y: Novel vasodilatory effect of intracisternal injection of magnesium sulfate solution on spastic cerebral arteries in the canine two-hemorrhage model of subarachnoid hemorrhage. J Neurosurg 110: 73– 78, 2009. [DOI] [PubMed] [Google Scholar]
- 9). Mori K, Miyazaki M, Iwata J, Yamamoto T, Nakao Y: Intracisternal infusion of magnesium sulfate solution improved reduced cerebral blood flow induced by experimental subarachnoid hemorrhage in the rat. Neurosurg Rev 31: 197– 203; discussion 203, 2008. [DOI] [PubMed] [Google Scholar]
- 10). Mori K, Yamamoto T, Miyazaki M, Hara Y, Aiko Y, Koike N, Sakamoto S, Nakao Y, Esaki T: Optimal cerebrospinal fluid magnesium ion concentration for vasodilatory effect and duration after intracisternal injection of magnesium sulfate solution in a canine subarachnoid hemorrhage model. J Neurosurg 114: 1168– 1175, 2011. [DOI] [PubMed] [Google Scholar]
- 11). Murata T, Horiuchi T, Goto T, Li Y, Hongo K: Vasomotor response induced by change of extracellular potassium and magnesium in cerebral penetrating arterioles. Neurosci Res 70: 30– 34, 2011. [DOI] [PubMed] [Google Scholar]
- 12). Nakagomi T, Furuya K, Nagashima H, Tanaka J, Ishii T, Takanashi S, Shinohara T, Watanabe F, Ogawa A, Fujii N, Tamura A: Surgical procedure and results of cisternal washing therapy for the prevention of cerebral vasospasm following SAH. Acta Neurochir Suppl 110( Pt 2): 105– 109, 2011. [DOI] [PubMed] [Google Scholar]
- 13). Oka K, Yamamoto M, Nonaka T, Tomonaga M: The significance of artificial cerebrospinal fluid as perfusate and endoneurosurgery. Neurosurgery 38: 733– 736, 1996. [PubMed] [Google Scholar]
- 14). Sasaki T, Kodama N, Kawakami M, Sato M, Asari J, Sakurai Y, Watanabe K, Onuma T, Matsuda T: Urokinase cisternal irrigation therapy for prevention of symptomatic vasospasm after aneurysmal subarachnoid hemorrhage: a study of urokinase concentration and the fibrinolytic system. Stroke 31: 1256– 1262, 2000. [DOI] [PubMed] [Google Scholar]
- 15). Seelig JM, Wei EP, Kontos HA, Choi SC, Becker DP: Effect of changes in magnesium ion concentration on cat cerebral arterioles. Am J Physiol 245: H22– 26, 1983. [DOI] [PubMed] [Google Scholar]
- 16). Shimizu H, Inoue T, Fujimura M, Saito A, Tominaga T: Cerebral blood flow after surgery for unruptured cerebral aneurysms: effects of surgical manipulation and irrigation fluid. Neurosurgery 69: 677– 688; discussion 688, 2011. [DOI] [PubMed] [Google Scholar]


