Abstract
Traumatic cerebrovascular injury (TCVI) is a serious complication of severe head injury, with a high mortality rate. To establish a proper treatment strategy for TCVI, we investigated patients with a high risk of TCVI according to the Guidelines for the Management of Severe Head Injury (hereafter “the Guidelines”) to elucidate the validity of the criteria for TCVI in the Guidelines and the appropriate screening timing and methods. Of those transported to our facility between December 2008 and June 2012, 67 individuals with a high risk of TCVI were evaluated to reveal the proper timing and methods of vascular evaluation. Of the 67 patients, 21 had a diagnosis of TCVI based on cerebral angiography, three-dimensional computed tomography angiography (3DCTA), or magnetic resonance imaging (MRI), accounting for 6.4% of all patients with severe head injury and as high as 31.3% of patients with a high risk of TCVI according to the Guidelines. In addition, according to the Glasgow Outcome Scale (GOS), outcomes were three deaths due to primary brain injury, six cases of persistent vegetative state, five cases of severe disability, three cases of moderate disability, and four cases of good recovery. Although 3DCTA is a simple and convenient diagnostic method, cerebral angiography is necessary to evaluate dissecting lesions. If patients have any signs or symptoms of TCVI, as described in the Guidelines, cerebral angiography or 3DCTA should be performed as an initial screening method within 72 hours of admission, followed by cerebral angiography on postadmission Day 14 ± 2 to prevent failed diagnosis.
Keywords: traumatic cerebrovascular injury, severe head injury, cerebral angiography, three-dimensional computed tomography angiography, timetable
Introduction
Traumatic cerebrovascular injury (TCVI) is a complication of severe head injury that accounts for only 0.5–1% of all injury cases. However, if left untreated, TCVI can cause stroke in the carotid and vertebral arterial circulation in almost 50% and 25% of patients, respectively, with a high mortality rate. Thus, TCVI is a serious pathological condition that requires prompt diagnosis.2,3,6,18) On the other hand, due to the development of noninvasive and fast diagnostic imaging methods, such as computed tomography (CT) and magnetic resonance imaging (MRI), it is rare to perform cerebral angiography as an initial diagnostic measure in brain injury cases. This has inevitably complicated the early diagnosis of a vascular disorder in the head and neck region. It is also problematic to evaluate the temporal profiles of neurological symptoms or to decide on a proper timing for vascular evaluation in patients with disturbed consciousness. In Japan, evidence-based treatments have been provided for TCVI caused by head injury in accordance with the Guidelines for the Management of Severe Head Injury (hereafter, “the Guidelines”) issued in 2000 and revised in 2006.12) Even with a prompt diagnosis of TCVI, it is often difficult to establish a proper treatment strategy, especially during the acute phase, because craniotomy, for example for parent artery occlusion, may not be indicated due to severe brain swelling. It is also challenging to select an appropriate diagnostic method. Although we follow the Guidelines to treat TCVI patients at our facility, there are many severe cases with multiple injuries for which it is difficult to provide prompt vascular evaluation.
Purpose
We investigated the validity of the criteria for TCVI in the Guidelines, the proper timing of early diagnosis, and appropriate diagnostic methods that reflect the recent advances in imaging methods, such as three-dimensional computed tomography angiography (3DCTA). We also discuss the results of the present study as well as results reported in the literature with the aim of establishing an optimum treatment strategy for patients with suspected TCVI at our facility.
Materials and Methods
Between December 2008 and June 2012, 1,340 injured patients were transported to our facility for urgent care. After excluding cases of cardiac arrest, 568 patients had a head injury that was equivalent to 3+ on the Abbreviated Injury Scale (AIS3+), of which 330 had severe head injury. According to the Guidelines, 67 of the 330 patients had suspected TCVI and were subsequently enrolled in this study. Patients comprised 51 men and 16 women (mean age 51.0 ± 21.1 years; range 6–89 years), and the causes of injury were fall impact or traffic accident. 3DCTA, cerebral angiography, and MRI (MR angiography and venography [MRA/MRV]) were used to diagnose TCVI. Of the patients meeting the criteria for TCVI as per the Guidelines, hypovolemic shock patients with bleeding from the external carotid artery due to, for example, facial trauma were treated immediately for embolization in cerebral angiography. Multiple-trauma patients who required transcatheter arterial embolization (TAE) underwent concurrent cerebrovascular evaluation during cerebral angiography. Patients also underwent 3DCTA and/or cerebral angiography immediately after the appearance of neurological abnormalities or new lesions in CT imaging. In patients with stable conditions, 3DCTA or cerebral angiography was performed within 72 hours, followed by additional cerebral angiography on postadmission 14 ± 2 days. If patients were initially unable to receive vascular evaluation as scheduled due to severe complications, they underwent the above procedures when their conditions had been stabilized.
Results
In the present study, 49 patients received cerebrovascular evaluation 14 ± 2 days postadmission. Another 17 patients received the evaluation when needed. One patient did not receive vascular evaluation as scheduled due to acute respiratory distress syndrome and experienced seizure on postadmission Day 17 (Table 1). Head CT findings were remarkable acute subdural hematoma along the cerebral falx and 3DCTA findings were a traumatic distal anterior cerebral artery aneurysm. Twenty-one patients had TCVI, accounting for 31.3% of the 67 patients with a high risk of TCVI according to the Guidelines (Table 2). These 21 patients also accounted for 1.6% of all 1,340 injured patients, 3.7% of 568 patients with AIS3+ head injury, and 6.4% of 330 patients with severe head injury. The prevalence of TCVI was clearly high among the 67 patients who met the TCVI criteria in the Guidelines. When cases with abnormal findings were evaluated based solely on the Guidelines, 1 of 3 cases (33.3%) had neurological symptoms that could not be caused by traumatic brain injury alone, 4 of 4 cases (100%) had late-onset exacerbation of clinical symptoms or produced changes in CT imaging that could not be explained by primary injury alone, 3 of 5 cases (60%) had thick diffuse subarachnoid hemorrhage or severe localized subarachnoid hemorrhage, 6 of 46 cases (13.0%) had basal skull fracture (frontal skull base fracture extending over two-thirds of the interior of sphenoid bone, or fracture of the middle cranial fossa extending to the carotid artery canal), and 9 of 9 cases (100%) had severe untreatable bleeding from the mouth, nose, or ear. Abnormal findings were also observed in two of three patients (66.7%) with cervical vertebral fracture and cervical spinal cord injury (Table 3).
Table 1.
Baseline and clinical characteristics of patients admitted for major head trauma during the study period*
| Number of patients | n = 67 |
|---|---|
| Age, median (range) (yrs) | 51.0 (6–89) |
| Injury, % (n) | |
| Brain injury only | 50.7 (34) |
| Brain injury with major facial fractures | 9.0 (6) |
| Brain injury with cervical spine fractures | 4.5 (3) |
| Brain injury with thoracic lesions | 32.8 (22) |
| Brain injury with abdominal or pelvic lesions | 17.9 (12) |
| GCS, range (median) | 3–15 (11) |
| Injury severity score, range (median) | 9–66 (25) |
| Mortality, % (n) | 11.9 (8) |
Some patients had multiple lesions.
Table 2.
Characteristics of patients with traumatic cerebrovascular injury*
| Number of patients | n = 21 |
|---|---|
| Age, median (range) (yrs) | 43.8 (6–89) |
| Injury, % (n) | |
| Brain injury only | 28.6 (6) |
| Brain injury with major facial fractures | 19.0 (4) |
| Brain injury with cervical spine fractures | 9.5 (2) |
| Brain injury with thoracic lesions | 42.9 (9) |
| Brain injury with abdominal or pelvic lesions | 28.6 (6) |
| GCS, range (median) | 3–15 (10) |
| Injury severity score, range (median) | 16–66 (29) |
| Mortality, % (n) | 14.3 (3) |
Some patients had multiple lesions.
Table 3.
Guideline* diagnostic rates by category
| 1 | Neurological symptoms that could not be caused by traumatic brain injury alone | 33.3 (1) |
| 2 | Late-onset exacerbation of clinical symptoms or changes in CT imaging that could not be explained by primary injury alone | 100 (4) |
| 3 | Thick diffuse subarachnoid hemorrhage or severe localized subarachnoid hemorrhage | 60.0 (3) |
| 4 | Cervical injury (fracture proximal to C5, hyperextension/overrotation) | 13.0 (6) |
| 5 | Basal skull fracture (frontal skull base fracture that extended over two-thirds of the interior of sphenoid bone, or the fracture of the middle cranial fossa that extended to the carotid artery canal) | 66.7 (2) |
| 6 | Severe untreatable bleeding from the mouth, nose, or ear(s) | 100 (9) |
The Guidelines for the Management of Severe Head Injury Committee, second edition, Neurotrauma. Values are represented as percentages with numbers within parentheses.
Comparison by disease revealed three cases of internal carotid artery stenosis, for which conservative treatment was provided. This was followed by cerebral angiography 3 months later and follow-up observations with 3DCTA and MRA. The patients are currently on their way to recovery. There were four cases of posterior inferior cerebellar artery (PICA), vertebral artery dissection, or occlusion, for which we performed removal of hematoma and trapping or coil embolization to complete the treatment for TCVI. Although one patient had transverse sinus occlusion due to a fracture caused by minor injury, we confirmed recanalization of the sinus in routine follow-up cerebral angiography and MRV. We immediately operated on another patient with open depressed skull fracture to ligate the transverse sinus. In addition, we performed cerebral angiography and embolization for hemostasis in nine patients with hemorrhagic shock due to facial injury.
Cerebral angiography was performed on admission in patients requiring TAE because of multiple injuries or in patients with bleeding from the external carotid artery. Other patients received 3DCTA within 72 hours and cerebral angiography in 14 ± 2 days postadmission. Patients underwent 3DCTA or cerebral angiography immediately after the appearance of new symptoms or changes in imaging findings.
According to the Glasgow Outcome Scale (GOS), the outcomes of the present study were three deaths due to primary brain injury, six cases of persistent vegetative state, five cases of severe disability, three cases of moderate disability, and four cases of good recovery. No patients had additional diagnosis of TCVI during the follow-up period. In addition, no new episodes of TCVI in the other 223 cases were observed during the admission or follow-up periods.
Illustrative cases
Case 1:
A 21-year-old woman was transported to our facility after falling from height. On admission, her neurological state was 6 on the Glasgow Coma Scale (GCS6, E1V1M4), and the patient had traumatic subarachnoid hemorrh age and cerebral contusion accompanied by hemorrhagic shock due to unstable pelvic fracture and severe respiratory failure due to pulmonary contusion. Although we initially used the extracorporeal membrane oxygenation system to support respiratory function, it was removed on postadmission Day 6 when the patient's general condition stabilized. The patient's consciousness improved to the level of GCS10 (E3VTM6). On postadmission Day 8, however, the patient suddenly developed hemiplegia on the left side of the body. Because of severe cerebral vasospasm in 3DCTA, the patient underwent cerebral angiography and was diagnosed with the vasospasm of bilateral internal carotid arteries, bilateral middle cerebral artery, left anterior cerebral artery, and basilar artery (Fig. 1). Percutaneous transluminal angioplasty and intra-arterial infusion of nicardipine improved vasospasm and thus hemiplegia. After surgery for limb and pelvic fracture, the patient was transferred to a rehabilitation hospital.
Fig. 1.
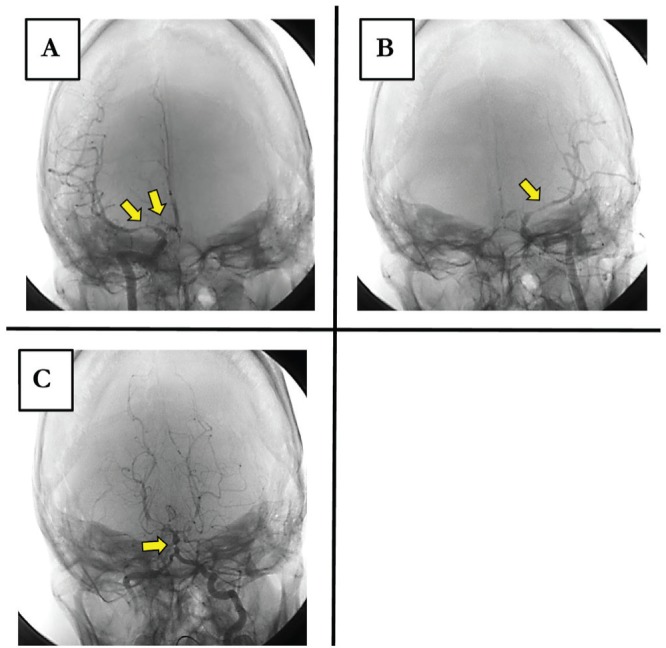
Case 1: A: Right internal carotid angiogram. B: Left internal carotid angiogram. C: Left vertebral angiogram, on postadmission Day 6. Vasospasm (arrows).
Case 2:
A 21-year-old man was involved in a traffic accident while riding a motorcycle. On admission, he had GCS12 (E3V4M5) with stable vital signs. Initial head CT findings were remarkable for thick subarachnoid hemorrhage; however, the patient was placed on observation because subsequent 3DCTA failed to pinpoint abnormalities. No injuries were present below the neck. During approximately 10 hours of observation, the patient's consciousness improved to the level of GCS15. In the morning following the injury, virtually no subarachnoid hemorrhage was observed on head CT. However, later that evening, the patient's neurological state rapidly declined to GCS3, with pupillary mydriasis and the loss of light reflex. Because of head CT findings of recurrent subarachnoid hemorrhage and hydrocephalus, the patient immediately received ventricular drainage, followed by cerebral angiography which revealed the dissection of the right PICA. Consequently, the patient underwent craniotomy and trap of PICA. Although the neurological state of the patient improved postoperatively, it again declined to the level of GCS3 on postadmission Day 14 due to cerebellar hematomas and subarachnoid hemorrhage. Because cerebral angiography revealed the dissection of the right vertebral artery proximal to the site trapped previously, the patient was operated for vertebral artery embolization (Fig. 2). The patient was subsequently discharged to start rehabilitation at another hospital. He currently walks with a cane and is followed up at our outpatient clinic.
Fig. 2.
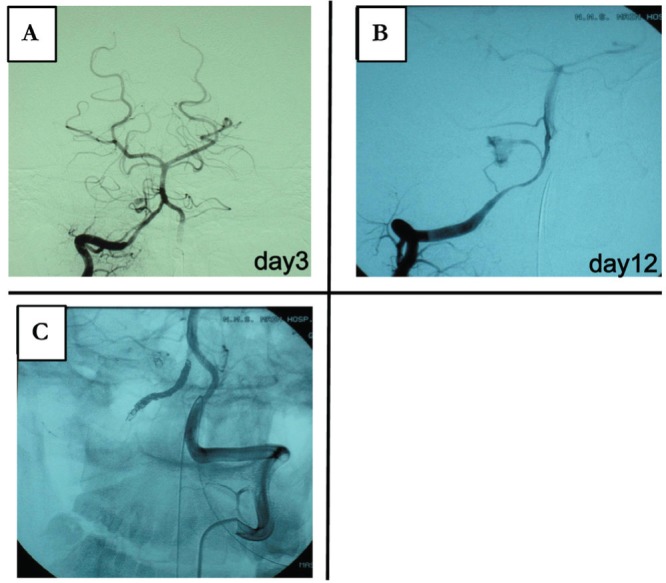
Case 2: Dissection is visible on: A: the right vertebral angiogram of the posterior inferior cerebellar artery (PICA). B: The right vertebral angiogram of the vertebral artery. C: Left vertebral angiogram after embolization of the right vertebral artery.
Case 3:
A 40-year-old man suffered acute subdural hematoma, epidural hematoma, and brain contusion in a motorcycle accident and immediately underwent craniotomy for removal of hematomas. On admission, he had already lost vision in his left eye and direct and indirect light reflexes. Cerebral angiography was performed on postadmission Day 8 because of basilar skull fracture and worsening of uncontrollable headache, and contrast findings were remarkable for left internal carotid-cavernous fistula accompanied by a steal phenomenon (Fig. 3). The general condition of the patient improved after transvenous embolization, and he was transferred to a rehabilitation hospital.
Fig. 3.
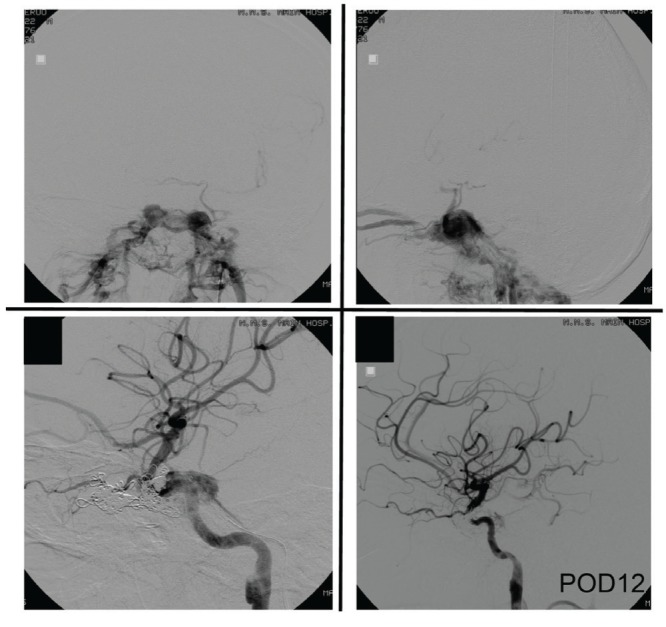
Case 3: Left internal carotid angiogram showing traumatic carotid-cavernous fistula with the steal phenomenon (upper panel). Venous reflux is visible in the left and right superior ophthalmic vein and basilar vein. Selective intravenous coil embolization of the fistula was performed. Blood flow in the left internal carotid artery improved after embolization (lower panel).
Case 4:
After falling from height, a 26-year-old man was admitted for brain contusion, traumatic subarachnoid hemorrhage, acute epidural hematoma, and the fracture of the skull and maxilla. He also had pelvic fracture with hemorrhagic shock, a burst fracture of the third lumbar vertebra, and fracture of the right tibiofibular and left calcaneus bones. His circulatory dynamics were stabilized by TAE of bilateral internal iliac artery. Concurrent cerebral angiography revealed no abnormalities (Fig. 4). On postadmission Day 5, the patient developed anisocoria (left pupil, 6 mm; right pupil, 3 mm), with no change in consciousness. Although no intracranial lesions or other abnormalities were detected on head CT and 3DCTA images, subsequent cerebral angiography revealed aneurysm formation in the left posterior communicating artery (Fig. 4). Although anisocoria disappeared spontaneously in a couple of hours, the patient underwent endovascular embolization of the aneurysm. He started rehabilitation and currently attends follow-up observation on foot.
Fig. 4.
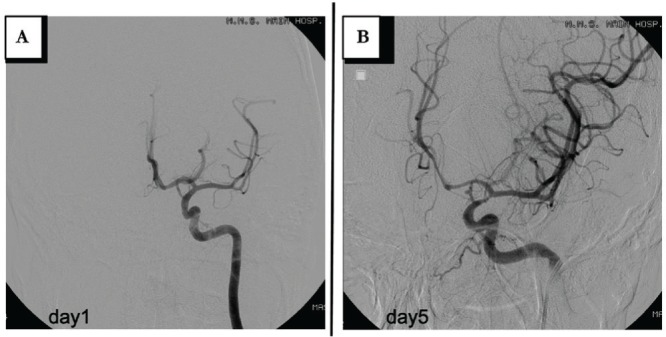
Case 4: A: Left internal carotid angiogram on admission. B: An aneurysm in the left posterior communicating artery.
Case 5:
A 26-year-old man fell from height and suffered severe bleeding from the mouth and nose due to Le Fort II and III fractures. After emergency puncture of the airway to treat life-threatening conditions caused by bleeding and the fracture of the mandible, we performed embolization to treat the extravasation of blood into the oral cavity from the bilateral maxillary artery (Fig. 5). The patient is currently stable.
Fig. 5.
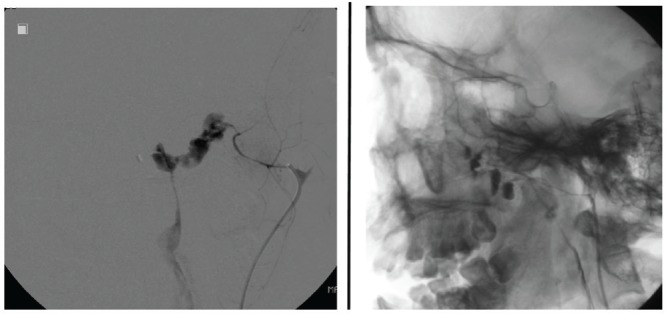
Case 5: Extravasation in the oral cavity is visible on a left external carotid angiogram.
Discussion
TCVI occurs in 0.03–0.1% of all head injury cases, but increases to 27–37% if the symptoms are more specific. The mortality rate of TCVI is reportedly > 50%.2,3,6,8,13,14,18,20,22) In this study, the incidence of TCVI was 1.6% of all injured patients brought to our facility during the study period, and 31.3% of the patients met the criteria specified by the Guidelines, demonstrating that the incidence at our facility was higher than previously reported. This is presumably because our facility is an urgent care center with a high number of severe trauma cases compared with general hospitals. Because TCVI has a high rate of complications and mortality, and because complications greatly affect the prognosis of TCVI patients, cerebrovascular evaluation should be considered a key diagnostic and treatment measure. In other cases, we did not observe the emergence of a new vascular injury during the follow-up periods.
With regard to the timing of cerebrovascular evaluation, if bleeding is observed in the mouth, nose, and ear, it is reasonable to provide testing and treatment immediately. With regard to other symptoms, approximately 70–80% of traumatic cerebral aneurysms form 1–2 weeks after injury and normally rupture 2–3 weeks after injury.1,4,11,22) There are conflicting reports on vasospasm at 5–41% after severe head injury:23) Vasospasm was previously reported to develop 2–3 days after injury,23) as in Case 1 of this study, whereas vasospasm was reported to develop within 2 weeks after injury,17) as with typical brain aneurysm ruptures. Impacts that cause diffuse brain injury tend to cause a vascular injury in the neck region, and the symptoms of ischemia generally appear a few hours to a few days after injury.5,10) The period between the development of a dissecting lesion and subsequent stroke ranges from immediately after injury to several years after injury; however, TCVI-related stroke reportedly occurs within 2 weeks after injury.15,19) Taken together, the present study suggests that it is important to perform cerebral angiography immediately when changes in clinical findings are noted; otherwise, cerebral angiography can be performed 14 ± 2 days postinjury.
To proceed with the potential development of TCVI in mind, simple and convenient 3DCTA as one of the initial screening methods or cerebral angiography at the time of angiography, such as TAE, can be performed for early TCVI evaluation and to compare the findings with subsequent imaging findings. As in Case 2 in the present study, imaging findings sometimes change over time, indicating that some patients need repeated cerebral angiography. Patients with severe head injury and signs of hernia require immediate surgery and subsequent multidisciplinary medical treatment. Therefore, it is necessary to pay special attention to these patients because they may not receive proper cerebrovascular evaluation.21)
Owing to recent advances in testing equipment, many studies have reported successful evaluation of TCVI by 3DCTA and MRA. In particular, the sensitivity and specificity of multidetector (16) row CTA (MDCTA) and CTA for the screening of TCVI in one study was as high as 97.7% and 100%, respectively.9) On the other hand, another study reported sensitivity and specificity of 74% and 86%, respectively,16) whereas yet another reported sensitivity of 51%. In the latter study, the authors even warned on the use of MDCTA as a sole screening tool for CVI.7) As stated earlier, to proceed with the potential development of TCVI in mind, the use of simple and convenient 3DCTA as a part of the initial treatment strategy can provide early diagnostic findings that can be compared with subsequent imaging findings. However, it is important to consider the precision of the individual instruments used at each institution. All the present cases received 3DCTA as the initial screening, except for those that required TAE for traumatic hemorrhagic shock, in which cerebral angiography was performed as the initial screening. Case 4, however, received cerebral angiography immediately after 3DCTA which failed to reveal abnormalities. Furthermore, when changes in CT imaging and/or clinical findings were noted, immediate 3DCTA was followed ultimately by cerebral angiography for a definitive diagnosis, as in Cases 1 and 4 of the present study. This means that cerebral angiography may be considered the gold standard because endovascular treatment can be initiated during cerebral angiography and because dissecting lesions and minor vascular abnormalities are difficult to visualize with 3DCTA.
At present, when changes in CT or clinical findings are noted, cerebral angiography, but not 3DCTA, is the imaging modality of choice, even though the procedure is highly invasive. Because 3DCTA can be used to follow-up vascular stenosis accompanying basal skull fracture, we plan to increase the number of cases to elucidate evaluatory methods specific to different lesions. At present, we perform 3DCTA or cerebral angiography as an initial examination in patients with suspected TCVI, followed always by cerebral angiography on postadmission Day 14 ± 2. By performing vascular evaluation at least twice, we try to prevent missed diagnosis of TCVI. If any changes are noted in clinical or CT findings, cerebral angiography should be provided and repeated frequently.
This study has limitations. It was conducted at a single center. One of the limitations of this study which evaluated the validity of the TCVI criteria is that we failed to perform vascular evaluation in all 568 patients with head injury. Evaluating TCVI by means of cerebral angiography twice is not only invasive, but also economically impractical. The validity of the representative cases in the Guidelines is supported by the fact that no additional TCVI cases, besides the 67 cases, were observed during the study period or during follow-up periods at our facility or other affiliated facilities.
Conclusion
We examined patients with a high risk of TCVI according to the Guidelines to elucidate the validity of the criteria for TCVI, the proper timing of early diagnosis, and the appropriate diagnostic measures. The indication criteria in the Guidelines needs to be followed and cerebral angiography should be performed as a vascular diagnostic measure. In addition, repeated vascular evaluation may be necessary in some cases. If imaging and neurological findings indicate the exacerbation of symptoms, cerebral angiography needs to be performed immediately. Some cases may require individualized treatment strategies that combine a direct surgical approach or intravascular surgery for TCVI. We plan to increase the number of cases to further investigate appropriate examination timetables and methods by lesion.
References
- 1). Benoit BG, Wortzman G: Traumatic cerebral aneurysms: clinical features and natural history. J Neurol Neurosurg Psychiatry 36: 127– 138, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Berne JD, Norwood SH, McAuley CE, Vallina VL, Creath RG, McLarty J: The high morbidity of blunt cerebrovascular injury in an unscreened population: more evidence of the need for mandatory screening protocols. J Am Coll Surg 192: 314– 321, 2001. [DOI] [PubMed] [Google Scholar]
- 3). Biffl WL, Moore EE, Ryu RK, Offner PJ, Novak Z, Coldwell DM, Franciose RJ, Burch JM: The unrecognized epidemic of blunt carotid arterial injuries: early diagnosis improves neurologic outcome. Ann Surg 228: 462– 470, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Buckingham MJ, Crone KR, Ball WS, Tomsick TA, Berger TS, Tew JM, Jr: Traumatic intracranial aneurysms in childhood: two cases and a review of the literature. Neurosurgery 22: 398– 408, 1988. [DOI] [PubMed] [Google Scholar]
- 5). Cook A, Osler T, Gaudet M, Berne J, Norwood S: Blunt cerebrovascular injury is poorly predicted by modeling with other injuries: analysis of NTDB data. J Trauma 71: 114– 119, 2011. [DOI] [PubMed] [Google Scholar]
- 6). Cothren CC, Moore EE: Blunt cerevrovascular injury. Importance of early diagnosis and anticoagulant therapy. Ann Surg 223: 513– 525, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). DiCocco JM, Emmett KP, Fabian TC, Zarzaur BL, Williams JS, Croce MA: Blunt cerebrovascular injury screening with 32-channel multidetector computed tomography: more slices still don't cut it. Ann Surg 253: 444– 450, 2011. [DOI] [PubMed] [Google Scholar]
- 8). Dubey A, Sung WS, Chen YY, Amato D, Mujic A, Waites P, Erasmus A, Hunn A: Traumatic intracranial aneurysm: a brief review. J Clin Neurosci 15: 609– 612, 2008. [DOI] [PubMed] [Google Scholar]
- 9). Eastman AL, Chason DP, Perez CL, McAnulty AL, Minei JP: Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: is it ready for primetime? J Trauma 60: 925– 929, 2006. [DOI] [PubMed] [Google Scholar]
- 10). Fabian TC, George SM, Jr, Croce MA, Mangiante EC, Voeller GR, Kudsk KA: Carotid artery trauma: management based on mechanism of injury. J Trauma 30: 953– 961; discussion 961–963, 1990. [DOI] [PubMed] [Google Scholar]
- 11). Fleischer AS, Patton JM, Tindall GT: Cerebral aneurysms of traumatic origin. Surg Neurol 4: 233– 239, 1975. [PubMed] [Google Scholar]
- 12). The Guidelines Committee on the Management of Severe Head Injury : The Guidelines for the Management of Severe Head Injury, ed 2. JSNT Jpn Soc Neurotraumatol, 2007. [Google Scholar]
- 13). Holmes B, Harbaugh RE: Traumatic intracranial aneurysms: a contemporary review. J Trauma 35: 855– 860, 1993. [PubMed] [Google Scholar]
- 14). Larson PS, Reisner A, Morassutti DJ, Abdulhadi B, Harpring JE: Traumatic intracranial aneurysms. Neurosurg Focus 8: e4, 2000. [DOI] [PubMed] [Google Scholar]
- 15). Leys D, Debette S: Long-term outcome in patients with cervical-artery dissections. There is still a lot to know. Cerebrovasc Dis 22: 215, 2006. [DOI] [PubMed] [Google Scholar]
- 16). Malhotra AK, Camacho M, Ivatury RR, Davis IC, Komorowski DJ, Leung DA, Grizzard JD, Aboutanos MB, Duane TM, Cockrell C, Wolfe LG, Borchers CT, Martin NR: Computed tomographic angiography for the diagnosis of blunt carotid/vertebral artery injury: a note of caution. Ann Surg 246: 632– 642; discussion 642–643, 2007. [DOI] [PubMed] [Google Scholar]
- 17). Martin NA, Doberstein C, Zane C, Caron MJ, Thomas K, Becker DP: Posttraumatic cerebral arterial spasm: transcranial Doppler ultrasound, cerebral blood flow, and angiographic findings. J Neurosurg 77: 575– 583, 1992. [DOI] [PubMed] [Google Scholar]
- 18). Miller PR, Fabian TC, Croce MA, Cagiannos C, Williams JS, Vang M, Qaisi WG, Felker RE, Timmons SD: Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg 236: 386– 393; discussion 393–395, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Mokri B, Piepqras DG, Houser OW: Traumatic dissections of the extracranial internal carotid artery. J Neurosurg 68: 189– 197, 1988. [DOI] [PubMed] [Google Scholar]
- 20). Nakstad P, Nornes H, Hauge HN: Traumatic aneurysms of the pericallosal arteries. Neuroradiology 28: 335– 338, 1986. [DOI] [PubMed] [Google Scholar]
- 21). Onda H, Fuse A, Igarashi Y, Suzuki G, Matsumoto G, Kin S, Yokota H: [A case of severe head injury and complicated traumatic cerebral artery aneurysm rupture treated twice under therapeutic hypothermia and come back to normal life]. J Jpn Assoc Acute Med 23: 265– 272, 2012. (Japanese) [Google Scholar]
- 22). Parkinson D, West M: Traumatic intracranial aneurysms. J Neurosurg 52: 11– 20, 1980. [DOI] [PubMed] [Google Scholar]
- 23). Weber M, Grolimund P, Seiler RW: Evaluation of posttraumatic cerebral blood flow velocities by transcranial Doppler ultrasonography. Neurosurgery 27: 106– 112, 1990. [DOI] [PubMed] [Google Scholar]


