Abstract
The aim of this study was to clarify the general status and historical transition of endovascular therapy (EVT) of acute stroke with major vessel occlusion before approval of mechanical thrombectomy devices in Japan from January 2005 to December 2009. We extracted 1,409 acute ischemic stroke patients receiving EVT (513 women, 69.8 ± 11.8 years) from two nationwide registry studies, the Japanese Registry of Neuroendovascular Therapy (JR-NET) and JR-NET 2. The median baseline National Institutes of Health Stroke Scale (NIHSS) score was 18, and 81.3% of the patients received EVT within 6 hours after symptom onset. The culprit occluded arteries were the internal carotid artery (ICA) in 21.2%, middle cerebral artery (MCA) in 53.0%, and basilar artery (BA) in 20.6%. Intravenous thrombolysis was administered to 6.7% of the patients, and EVT mainly consisted of intra-arterial thrombolysis and percutaneous balloon angioplasty/balloon clot disruption. The final recanalization rate was 82.5%, and the clinical outcome was favorable in 35.8% and fatal in 11.6% at 30 days after onset or at discharge. There was no significant change in neurological severity at baseline throughout the study period, but the onset-to-treatment time became longer and the proportion of ICA or BA occlusion increased annually. Although the final recanalization rate was similar throughout the study period, the incidence of a favorable outcome tended to decreased annually from 41.0% to 29.0%. These results could be considered as baseline data that can be used to validate the beneficial effects of novel EVT devices in Japan.
Keywords: acute ischemic stroke, intracranial major vessel occlusion, endovascular treatment, nationwide survey, recanalization
Introduction
Acute stroke with major vessel occlusion is generally recognized as a serious disease. Immediate recanalization by intravenous (IV) thrombolysis using recombinant tissue plasminogen activator (t-PA) is not sufficiently achieved in such stroke patients. In recent years, endovascular therapy (EVT) using mechanical thrombectomy devices has been evaluated in stroke patients with major vessel occlusion. The results of several studies have shown immediate recanalization with better clinical outcomes than were obtained with thrombolytic therapy.1–6)
The Japanese Registry of Neuroendovascular Therapy (JR-NET) 1 and JR-NET 2 were created to clarify the general status of neuroendovascular therapy performed by specialist physicians certified by the Japanese Society for Neuroendovascular Therapy (JSNET). Clinical and procedural data on a total of 32,000 neuroendovascular procedures were retrospectively collected from January 2005 to December 2006 (JR-NET 1) and from January 2007 to December 2009 (JR-NET 2). The two study periods encompassed the time form approval of IV t-PA (October 2005) in Japan until the approval of the Merci Retriever (Concentric Medical, Mountain View, California, USA, 2010) and Penumbra System (Penumbra Inc., Alameda, California, USA, 2011) thrombectomy devices.
The aim of this study was to clarify the general status of endovascular treatment of acute stroke with major vessel occlusion before approval of mechanical thrombectomy devices in Japan, and to determine if there were changes in stroke characteristics or treatment patterns throughout the periods encompassed by JR-NET 1 and 2.
Materials and Methods
Among all datasets of JR-NET 1 and 2, we extracted 1,409 patients (619 from JR-NET 1 and 790 from JR-NET 2; 513 women; mean age, 69.8 ± 11.9 years) who underwent endovascular revascularization therapy for acute stroke with major vessel occlusion. The Institutional Review Board at each center approved the use of retrospective data from the patients.
We compared the following baseline characteristics between JR-NET 1 and 2: age, sex, baseline National Institutes of Health Stroke Scale (NIHSS) score, stroke subtypes, and culprit occluded arteries. Furthermore, we compared the use of computed tomography (CT), magnetic resonance imaging (MRI), and the onset-to-treatment time (OTT) between the two studies. We also compared the two studies for the use of revascularization procedures such as endovascular treatment (EVT) with or without preceding IV t-PA, stand-alone intra-arterial thrombolysis (IAT), stand-alone percutaneous transluminal angioplasty (PTA)/balloon clot disruption (BCD), other single procedures including stenting, thromboaspiration or clot retrieval, the combination of IAT and PTA/BCD, and other procedural combinations. Moreover, we determined if there were differences between the two studies in the recanalization rate (complete, partial, or none), symptomatic procedural complications with any clinical deterioration within 24 hours of EVT (intracranial hemorrhage or ischemia, puncture site, and other systemic complications) and clinical outcomes. A favorable clinical outcome was defined as a modified Rankin scale (mRS) score of 0–2 and a poor outcome as a mRS score of 5–6 or death at 30 days after stroke onset. Finally, we analyzed the annual transition in baseline characteristics, the use of IV t-PA and EVT procedures, and the treatment results in the two studies. For the annual transition analysis, we divided the entire period encompassed by the two studies into six periods: the first period (pre-IV t-PA period, until September 2005), the second period (October 2005–September 2006), the third period (October 2006–September 2007), the fourth period (October 2007–September 2008), the fifth period (October 2008–September 2009), and the last period (October–December 2009).
The culprit occluded arteries were separated into the internal carotid artery [ICA, including simultaneous middle cerebral artery (MCA) and/or other vessel occlusion]; the MCA (isolated MCA occlusion); the basilar artery (BA), including simultaneous vertebral and/or posterior cerebral artery occlusion); and the other arteries.
IV t-PA was performed using 0.6 mg/kg of alteplase, and EVT procedures were done according to protocol at each center by a JSNET-certified specialist physician or operator.
The recanalization status was evaluated angiographically at the end of EVT by the attending physician at each center. Partial recanalization was defined as any degree of recanalization other than complete. The mRS score at hospital discharge was used as a substitute for outcome when 30-day outcome data were unavailable. Hemorrhagic events after severe stroke that were unrelated to any EVT procedure were not collected in this retrospective analysis.
Data were statistically analyzed with IBM SPSS Statistics 20 software (IBM SPSS, Chicago, Illinois, USA). We used a chi-square test, Fisher's exact test, Mann-Whitney U-test, or Kruskal-Wallis test, as appropriate. A p-value < 0.05 was considered significant.
Results
I. Patient characteristics
Table 1 shows the patients' baseline characteristics and diagnostic imaging modalities. The median baseline NIHSS score was 18 [interquartile range (IQR), 11–23], and 11.7% of the patients had a mild neurological deficit (NIHSS score < 8). Cardioembolic stroke was the most frequent stroke subtype (64.9%) and atherothrombotic stroke was the second most frequent subtype (22.6%). The culprit occluded arteries were ICA in 21.2%, MCA in 53.0%, and BA in 20.6%. MRI was the most frequently used imaging modality (86.5%), and CT was used in 55.9%. The OTT was ≤ 3 hours in 37.4%, 3–6 hours in 43.9%, 6–12 hours in 11.6%, and > 12 hours in 7.3%.
Table 1.
Patients' baseline characteristics and imaging modalities
| Total | JR-NET 1 | JR-NET 2 | p | |
|---|---|---|---|---|
| n | 1,409 | 619 | 790 | |
| Age, mean ± SD (range) | 69.8 ± 11.9 (6–96) | 69.5 ± 11.4 (16–96) | 70.0 ± 12.2 (6–93) | 0.237 |
| Male (%) | 896/1,391 (64.4) | 400/601 (66.6) | 496/790 (62.8) | 0.146 |
| NIHSS | (n = 875) | (n = 293) | (n = 582) | |
| Baseline NIHSS, median (IQR) | 18 (11–23) | 17 (11–24) | 18 (12–22) | 0.969 |
| NIHSS < 8 (%) | 102 (11.7) | 37 (12.6) | 65 (11.2) | 0.525 |
| Stroke subtypes (%) | (n = 1,249) | (n = 471) | (n = 778) | 0.660 |
| Cardioembolic | 811 (64.9) | 305 (64.8) | 506 (65.0) | |
| Atherothrombotic | 282 (22.6) | 103 (21.9) | 179 (23.0) | |
| Iatrogenic | 44 (3.5) | 21 (4.5) | 23 (3.0) | |
| Others | 44 (3.5) | 18 (3.8) | 26 (3.3) | |
| Unknown | 68 (5.4) | 24 (5.1) | 44(5.7) | |
| Culprit occluded artery (%) | (n = 1,248) | (n = 470) | (n = 778) | < 0.001 |
| ICA | 264 (21.2) | 84 (17.9) | 180 (23.1) | |
| MCA | 661 (53.0) | 269(57.2) | 391 (50.3) | |
| BA | 257 (20.6) | 82 (17.4) | 174 (22.4) | |
| Other arteries | 68 (5.4) | 35 (7.4) | 33 (4.2) | |
| Imaging modalities (%) | (n = 1,212) | (n = 455) | (n = 757) | |
| CT | 677 (55.9) | 299 (65.7) | 378 (49.9) | < 0.001 |
| MRI | 1,048 (86.5) | 363 (79.8) | 685 (90.5) | < 0.001 |
| Onset-to-treatment time (%) | (n = 1,184) | (n = 453) | (n = 731) | < 0.001 |
| < 3 h | 443 (37.4) | 221 (48.8) | 222 (30.4) | |
| 3–6 h | 520 (43.9) | 183 (40.4) | 337 (46.1) | |
| 6–12 h | 137 (11.6) | 34 (7.5) | 103 (14.1) | |
| ≥ 12 h | 84 (7.1) | 15 (3.3) | 69 (9.4) |
BA: basilar artery, CT: computed tomography, ICA: internal carotid artery, IQR: interquartile range, JR-NET: Japanese Registry of Neuroendovascular Therapy, MCA: middle cerebral artery, MRI: magnetic resonance imaging, NIHSS: National Institutes of Health Stroke Scale.
II. Revascularization procedures
Table 2 shows information on the revascularization procedures. IV t-PA was administered in 6.7% of the patients prior to EVT. EVT mainly consisted of IAT and PTA/BCD using a PTA balloon. Stand-alone IAT was performed in 37.4%: IA urokinase (UK) was administered in 34.4%, IA t-PA in 2.2%, and IA UK + t-PA in 0.7%; the combination of IAT and PTA/BCD was used in 27.4% and stand-alone PTA/BCD in 16.0%. The frequency of the combination including IAT was 73.7%.
Table 2.
Revascularization procedures
| Total | JR-NET 1 | JR-NET 2 | p | |
|---|---|---|---|---|
| n | 1,409 | 619 | 790 | |
| IV t-PA (%) | 81/1,202 (6.7) | 4/441 (0.9) | 77/761 (10.1) | < 0.001 |
| Endovascular procedures (%) | (n = 1,245) | (n = 471) | (n = 774) | < 0.001 |
| Stand-alone IAT | 466 (37.4) | 223 (47.3) | 243 (31.4) | |
| Stand-alone PTA/BCD | 199 (16.0) | 39 (8.3) | 160 (20.7) | |
| Other single procedures | 64 (5.1) | 24 (5.1) | 40 (5.2) | |
| IAT + PTA/BCD | 341 (27.4) | 135 (28.7) | 206 (26.6) | |
| Other procedural combinations | 175 (14.1) | 50 (10.6) | 125 (16.1) | |
| Any combination including IAT | 917 (73.7) | 388 (82.4) | 529 (68.3) | < 0.001 |
BCD: balloon clot disruption, IAT: intra-arterial thrombolysis, IV t-PA: intravenous thrombolysis using tissue plasminogen activator, JR-NET: Japanese Registry of Neuroendovascular Therapy, Other single procedures: includes stenting, thromboaspiration, clot retrieval and others, PTA: percutaneous transluminal angioplasty,.
III. Treatment results and procedural complications
Table 3 shows the treatment results and procedural complications. Final recanalization was obtained in 82.5% of the patients (partial, 50.0%; complete, 32.5%). The clinical outcome was favorable in 35.8%, poor in 27.3% and fatal in 11.6% at 30 days after onset.
Table 3.
Treatment results and procedural complications
| Total | JR-NET 1 | JR-NET 2 | p | |
|---|---|---|---|---|
| n | 1,409 | 619 | 790 | |
| Final recanalization (%) | 1,030/1,249 (82.5) | 384/471 (81.5) | 646/778 (83.0) | 0.299 |
| Partial | 624 (50) | 222 (47.1) | 402 (51.7) | |
| Complete | 406(32.5) | 162 (34.4) | 244 (31.4) | |
| Procedural complications (%) | 136/1,386 (9.8) | 68/599 (11.4) | 68/787 (8.6) | 0.093 |
| Intracranial hemorrhagic complication | 97 (7.0) | 50 (8.3) | 47 (6.0) | 0.086 |
| Intracranial ischemic complication | 23 (1.7) | 13 (2.2) | 10 (1.3) | 0.194 |
| Puncture site complication | 7 (0.5) | 2 (0.3) | 5 (0.6) | 0.706 |
| Other complications | 10 (0.7) | 4 (0.7) | 6 (0.8) | 1.00 |
| Clinical outcome at 30 days (%) | (n = 1,376) | (n = 600) | (n = 776) | |
| Favorable outcome | 492 (35.8) | 234 (39.0) | 258 (33.2) | 0.027 |
| Poor outcome | 375 (27.3) | 159 (26.5) | 216 (27.8) | 0.581 |
| Death | 159 (11.6) | 66 (11.0) | 93 (12.0) | 0.571 |
JR-NET: Japanese Registry of Neuroendovascular Therapy.
IV. Comparison of JR-NET 1 and 2
Tables 1, 2, and 3 present the clinical characteristics, treatment results, and statistical analysis of each registry study. There were no significant differences in age, sex, neurological severity, or stroke subtypes between JR-NET 1 and 2. However, there was a significantly higher rate of ICA occlusion and BA occlusion in JR-NET 2 than in JR-NET 1, whereas there was a significantly lower rate of MCA occlusion in JR-NET 2 than in JR-NET 1 (p < 0.001). CT was performed less frequently in JR-NET 2 than in JR-NET 1 (p < 0.001), whereas MRI was performed more frequently (p < 0.001). The OTT was significantly longer in JR-NET 2 than in JR-NET 1 (p < 0.001). IV t-PA and stand-alone PTA/BCD increased from JR-NET 1 to JR-NET 2, (both p-values < 0.001), whereas the rate of stand-alone IAT decreased from JR-NET 1 to JR-NET 2 (p < 0.001). Although the final recanalization and mortality rates were not significantly different, the proportion of patients with a favorable outcome significantly decreased from JR-NET 1 to JR-NET 2 (p = 0.027). The overall procedural complication rate, especially the rate of intracranial hemorrhage, tended to decrease from JR-NET 1 to JR-NET 2 (p = 0.093 and 0.086, respectively).
V. Annual transition analyses
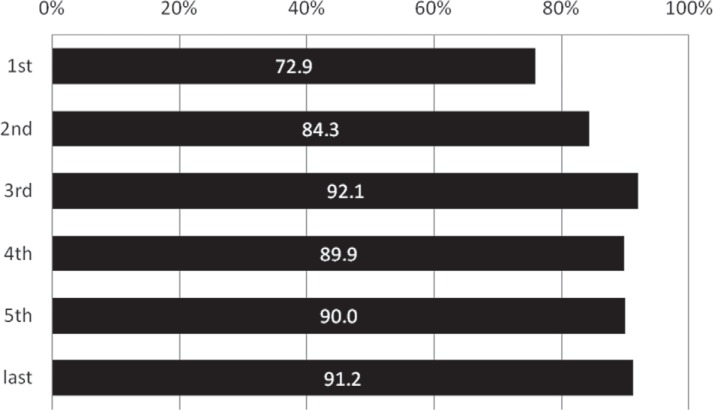
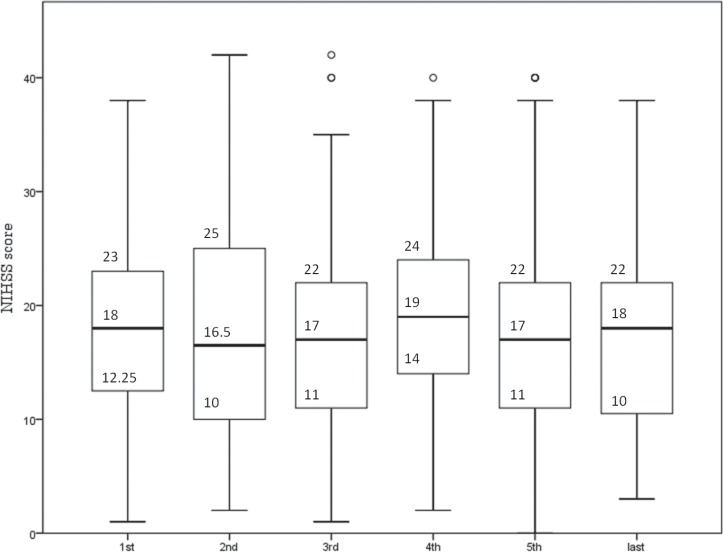
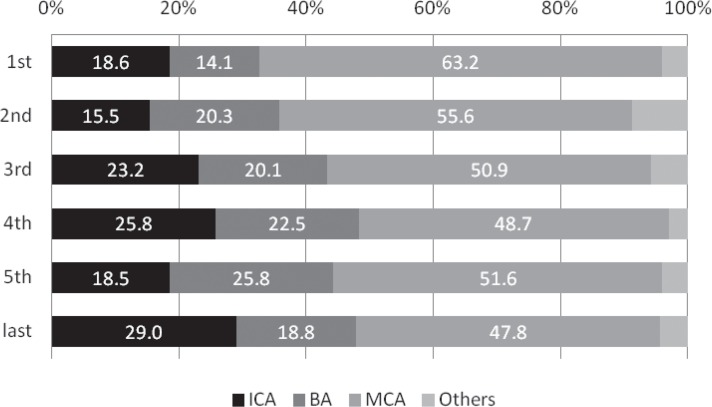
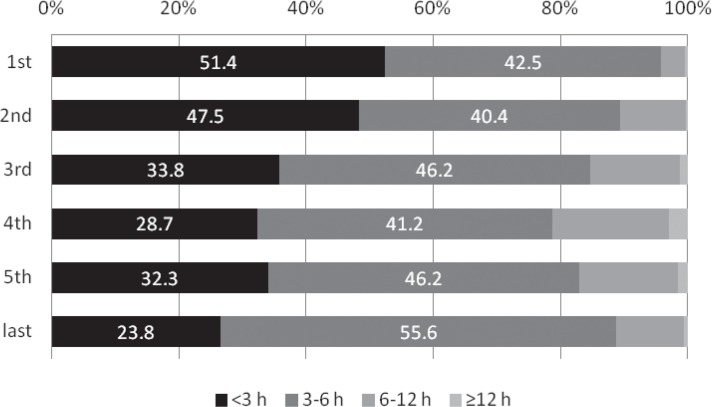
The rate that MRI was performed significantly increased from 72.9% in the first period to 92.1% in the third period (p = 0.005 comparing the first and second periods; p = 0.013 comparing the second and third periods), and was subsequently as high as around 90% (Fig. 1). Although stroke severity did not change annually throughout the study period (p = 0.352, Fig. 2), the incidence of ICA or BA occlusion tended to increase after approval of IV t-PA in contrast with MCA occlusion (ICA, p = 0.031; BA, p = 0.053; MCA, p = 0.026; Fig. 3). The proportion of patients with OTT within 3 hours significantly decreased from 51.4% in the first period to 23.8% in the last period (p < 0.001, Fig. 4).
Fig. 1.
Annual transition of the rate that magnetic resonance imaging (MRI) was performed (%). The rate that MRI was performed significantly increased from 72.9% in the first period to 92.1% in the third period (p = 0.005 comparing the first and second periods; p = 0.013 comparing the second and third periods), and was subsequently as high as around 90%.
Fig. 2.
Annual transition of stroke severity. Stroke severity did not change annually throughout the study period (p = 0.352). NIHSS: National Institutes of Health Stroke Scale.
Fig. 3.
Annual transition of the proportion of culprit occluded arteries (%). The incidence of ICA or BA occlusion tended to increase after approval of IV t-PA in contrast with MCA occlusion (ICA, p = 0.031; BA, p = 0.053; MCA, p = 0.026). BA: basilar artery, ICA: internal carotid artery, IV t-PA: intravenous thrombolysis using tissue plasminogen activator, MCA: middle cerebral artery.
Fig. 4.
Annual transition of the onset-to-treatment time (%). The proportion of patients with onset-to-treatment time within 3 hours significantly decreased from 51.4% in the first period to 23.8% in the last period (p < 0.001).
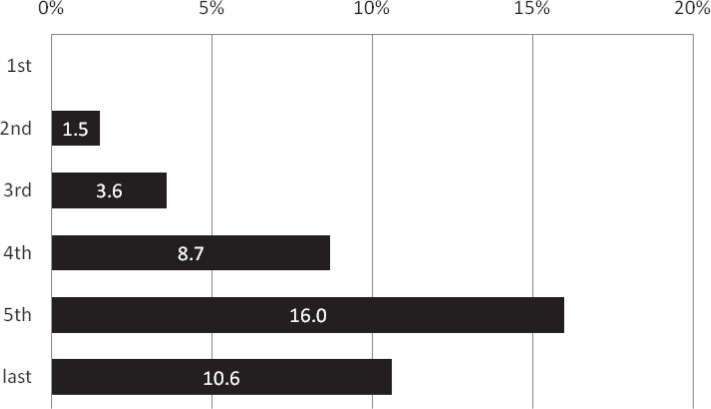
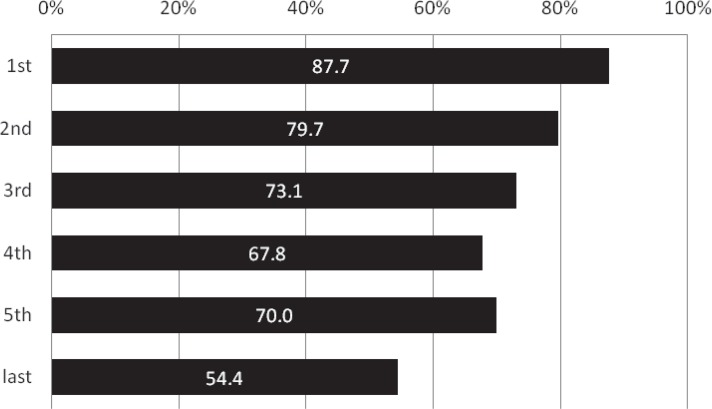
The proportion of patients receiving IV t-PA showed an annual increase from 1.5% in the second period just after the approval of IV t-PA to 16.0% in the fifth period (p = 0.015 comparing the third and fourth periods; p = 0.015 comparing the fourth and fifth periods; Fig. 5). In contrast, the frequency of EVT procedures including IAT abruptly dropped from 87.7% in the first period to 54.4% in the last period (p < 0.001, Fig. 6).
Fig. 5.
Annual transition of the proportion of the patients receiving IV t-PA (%). The proportion of patients receiving IV t-PA showed an annual increase from 1.5% in the second period just after the approval of IV t-PA to 16.0% in the fifth period (p = 0.015 comparing the third and fourth periods; p = 0.015 comparing the fourth and fifth periods). IV t-PA: intravenous thrombolysis using tissue plasminogen activator.
Fig. 6.
Annual transition of the proportion of any endovascular procedural combinations including intraarterial thrombolysis (%). The frequency of EVT procedures including IAT abruptly dropped from 87.7% in the first period to 54.4% in the last period (p < 0.001). EVT: endovascular procedure, IAT: intra-arterial thrombolysis.
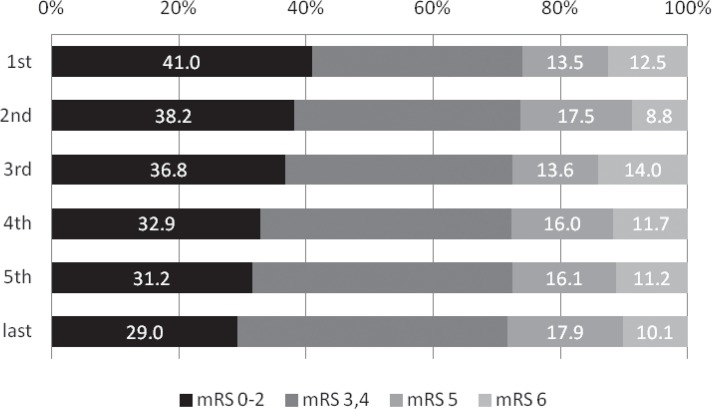
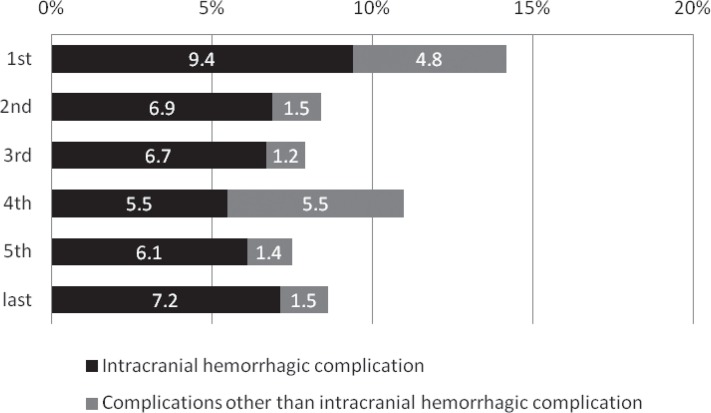
Despite the lack of a significant annual change in mortality or the proportion of patients with poor outcome, the proportion of patients with a favorable outcome decreased from 41.0% to 29.0% throughout the study period (p = 0.025 comparing the first and fifth periods; Fig. 7). The procedural complication rate showed no significant change throughout the study period, but there was a significant decrease in rate of all complications (p = 0.033); there was an insignificant trend for a decrease in the rate of intracranial hemorrhage between the first and second periods (p = 0.290, Fig. 8).
Fig. 7.
Annual transition of the proportion clinical outcomes at 30 days after onset (%). The proportion of patients with a favorable outcome decreased from 41.0% to 29.0% throughout the study period (p = 0.025 comparing the first and fifth periods) despite the lack of a significant annual change in mortality or the proportion of patients with poor outcome. mRS: modified Rankin scale.
Fig. 8.
Annual transition of the occurrence rate of procedural complications (%). The procedural complication rate showed no significant change throughout the study period, but there was a significant decrease in rate of all complications (p = 0.033); there was an insignificant trend for a decrease in the rate of intracranial hemorrhage between the first and second periods (p = 0.290).
Discussion
This study is the first nationwide survey of endovascular revascularization treatment for acute stroke with major vessel occlusion, and provides important information about EVT status after the introduction of IV t-PA in Japan before the use of thrombectomy devices. We found that EVT for acute stroke with major vessel occlusion was mainly performed for MRI-evaluated severe stroke patients (median NIHSS score, 18) within 6 hours after onset, using mainly IAT and PTA/BCD.
The final recanalization rate of EVT in JR-NET 1 and 2 combined was 82.5%, and this is higher than that obtained by intra-arterial urokinase therapy for MCA occlusion in the MCA Embolism Local Fibrinolytic Intervention Trial (MELT) in Japan (73.7%).7) The 82.5% recanalization rate that we observed is also higher than that reported for IV t-PA for MCA occlusion in the Japan Alteplase Clinical Trial (J-ACT) II (51.7%)8) and that obtained in the Japanese initial experience of 15 centers using the Merci Retriever (74.6%).9) However, the interpretation of the results of these different studies requires caution, as complete recanalization was limited to 32.5% in our study and the recanalization grade that we used is not congruent with the generally accepted ordinal recanalization scale [Thrombolysis in Myocardial Infarction (TIMI)10) or Thrombolysis in Cerebral Infarction (TICI)11) grade evaluated by angiography, or the modified Mori grade by MRA8)]. It has been reported that recanalization after PTA/BCD is better than stand-alone IAT,12,13) and PTA/BCD enhances the thrombolytic effect of IAT.14) The recanalization rate in our study reflects the final result of an EVT strategy that mainly consisted of IAT and PTA/BCD.
The proportion of patients with a favorable outcome was 35.8% in our study. This result is similar to or even better than the outcome results of some studies using thrombectomy devices (MERCI trial, 27.7%5); MultiMERCI trial, 36.0%4); Japanese initial experience using Merci Retriever, 28.2%9); Penumbra Pivotal Stroke trial, 25%2); The POST trial, 41.0%6)). Since there were some differences in baseline characteristics, especially neurological severity (our study included patients with an NIHSS score < 8 in 11.7%), a simple comparison among studies is difficult. A subanalysis limited to patients with a significant neurological deficit (NIHSS score ≥ 8) is needed. The procedural complication rate in our study was 9.8%, including intracranial hemorrhagic complications in 7%. These complication rates are thought to be acceptable compared with other studies (MERCI trial, 7.1%5); Multi MERCI trial, 5.5%4); Penumbra Pivotal Stroke trial, 12.8%2); The POST trial, 5.7%6)). However, we cannot draw any final conclusions about the safety of EVT in this study because the definition of procedural complications and the performance rate of IV t-PA were quite different among studies, and we lacked information on intracranial hemorrhage that was unrelated to EVT. Further study is needed to resolve this issue.
Although there were no significant differences in neurological severity, sex, age, or stroke subtypes between JR-NET 1 and 2, the incidence of a culprit lesion in the ICA or BA was higher in JR-NET 2, whereas the incidence of a culprit lesion in the MCA was lower, with a similar tendency in the annual transition. Furthermore, the OTT became significantly longer throughout the study period. Considering revascularization procedures, the rate that IV t-PA and PTA/BCD were performed increased throughout the study period, whereas the rate that IAT was performed decreased from JR-NET 1 to JR-NET 2, with a similar tendency in the annual transition.
The proportion of patients who received IV t-PA in the fifth period was only 16%, and lower than that of the Japanese initial experience with the Merci Retriever (42.0%).9) This might suggest that interventionists had less enthusiasm for subsequent EVT after IV t-PA at that time for fear of hemorrhagic complications. Similarly, the changes in rate that EVT procedures were performed over time might reflect the fact that interventionists became more concerned about hemorrhagic complications due to proximal vessel occlusion such as ICA or BA with a prolonged OTT often causing a large infarct. In fact, the rate of procedural intracranial hemorrhagic complications in the first period was higher (9.4%) than those in the second to last periods after the approval of IV t-PA (5.5–7.2%).
Despite of lack of difference in the final recanalization rate between JR-NET 1 and 2, the proportion of patients who achieved a favorable outcome significantly decreased from 39% in JR-NET 1 to 32.2% in JR-NET 2 (p = 0.027), with a similar tendency in the annual transition. This provides additional evidence for shifting of the EVT indication to patients with higher risk factors such as ineligibility for IV t-PA, proximal vessel occlusion, and longer OTT.
This study has some limitations. This study was retrospective, and data were missing in some patients. The recanalization status and clinical outcomes were not evaluated by investigators who were blinded to the treatment strategy. In addition, the actual rate of TICI grade 2B + 3 as an index of substantial recanalization was not clear because of the ambiguous definition of recanalization status. Furthermore, the actual safety of EVT procedures could not be determined since we were not certain of the number of intracranial hemorrhages that were unrelated to EVT. Finally, no data on pre-EVT cerebral parenchymal imaging findings were collected.
The ongoing JR-NET 3 study is collecting recanalization status defined by TICI grade and information on the extent of the infarcted area defined by the Alberta Stroke Program Early CT Score (ASPECTS). This ongoing study is expected to give precise information on the general EVT status of acute stroke with major vessel occlusion in the post-thrombectomy device era in Japan.
Conclusion
EVT for acute stroke with major vessel occlusion was mainly performed with IAT and/or PTA/BCD and resulted in recanalization rate of up to 82.5%, and 35.8% of the patients had a favorable outcome at 30 days after onset before approval of mechanical thrombectomy devices in Japan. The results of this study may be used as baseline data for validation of the Merci Retriever (Concentric Medical, Mountain View, California, USA), Penumbra System (Penumbra Inc., Alameda, California, USA) or future novel EVT devices in Japan.
Acknowledgments
The authors would like to express heartfelt thanks to doctors who devoted their time to this investigation. This study was supported by research grants for cardiovascular diseases (17C-1, 20C-2) from the Ministry of Health, Labor, and Welfare of Japan.
The JR-NET Study Group: Principle Investigator; Nobuyuki Sakai, Kobe City Medical Center General Hospital, Kobe, Japan: Investigators; Akio Hyodo, Dokkyo Medical University Koshigaya Hospital, Koshigaya, Japan (17C-1, 20C-2), Shigeru Miyachi, Nagoya University, Nagoya, Japan (17C-1, 20C-2), Yoji Nagai, Translational Research Informatics Center, Kobe, Japan (17C-1, 20C-2), Chiaki Sakai, Institute of Biomedical Research and Innovation, Kobe, Japan (17C-1, 20C-2), Tetsu Satow, National Cerebral and Cardiovascular Center, Suita, Japan (17C-1, 20C-2), Waro Taki, Mie University, Tsu, Japan (17C-1, 20C-2), Tomoaki Terada, Wakayama Rosai Hospital, Wakayama, Japan (17C-1, 20C-2), Masayuki Ezura, Sendai Medical Center, Sendai, Japan (17C-1), Toshio Hyogo, Nakamura Memorial Hospital, Sapporo, Japan (17C-1), Shunji Matsubara, Tokushima University, Tokushima, Japan (17C-1), Kentaro Hayashi, Nagasaki University, Nagasaki Japan (20C-2); Co-Investigators; Toshiyuki Fujinaka, Osaka University, Suita, Japan, Yasushi Ito, Niigata University, Niigata, Japan, Shigeki Kobayashi, Chiba Emergency Medical Center, Chiba, Japan, Masaki Komiyama, Osaka City General Hospital, Osaka, Japan, Naoya Kuwayama, Toyama University, Toyama, Japan, Yuji Matsumaru, Toranomon Hospital, Japan, Yasushi Matsumoto, Konan Hospital, Sendai, Japan, Yuichi Murayama, Jikei Medical University, Tokyo, Japan, Ichiro Nakahara, Kokura Memorial Hospital, Kokura, Japan, Shigeru Nemoto, Jichi Medical University, Shimotsuke, Japan, Koichi Sato, Tokushima Red Cross Hospital, Tokushima, Japan, Kenji Sugiu, Okayama University, Okayama, Japan, Shinichi Yoshimura, Gifu University, Gifu, Japan, and certified specialist of Japanese Society for Neuroendovascular Therapy.
References
- 1). Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, Liebeskind DS, Smith WS, TREVO 2 Trialists : Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet 380: 1231– 1240, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Penumbra Pivotal Stroke Trial Investigators : The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 40: 2761– 2768, 2009. [DOI] [PubMed] [Google Scholar]
- 3). Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, Clark W, Budzik R, Zaidat OO, SWIFT Trialists : Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet 380: 1241– 1249, 2012. [DOI] [PubMed] [Google Scholar]
- 4). Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, Lutsep HL, Rymer MM, Higashida RT, Starkman S, Gobin YP, Multi MERCI Investigators. Frei D, Grobelny T, Hellinger F, Huddle D, Kidwell C, Koroshetz W, Marks M, Nesbit G, Silverman IE: Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 39: 1205– 1212, 2008. [DOI] [PubMed] [Google Scholar]
- 5). Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, Lutsep HL, Nesbit GM, Grobelny T, Rymer MM, Silverman IE, Higashida RT, Budzik RF, Marks MP, MERCI Trial Investigators : Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 36: 1432– 1438, 2005. [DOI] [PubMed] [Google Scholar]
- 6). Tarr R, Hsu D, Alfke K, Stingele R, Jansen O, Frei D, Bellon R, Madison M, Struffert T, Dorfler A, Grunwald IQ, Reith W, Haass A: The POST trial: initial post-market experience of the Penumbra system: revascularization of the large vessel occlusion in acute ischemic stroke in the United States and Europe. J Neurointervent Surg 2: 341– 344, 2010. [DOI] [PubMed] [Google Scholar]
- 7). Ogawa A, Mori E, Minematsu K, Taki W, Takahashi A, Nemoto S, Miyamoto S, Sasaki M, Inoue T, MELT Japan Study Group : Randomized trial of intraarterial infusion of urokinase within 6 hours of middle cerebral artery stroke: the middle cerebral artery embolism local fibrinolytic intervention trial (MELT) Japan. Stroke 38: 2633– 2639, 2007. [DOI] [PubMed] [Google Scholar]
- 8). Mori E, Minematsu K, Nakagawara J, Yamaguchi T, Sasaki M, Hirano T, Japan Alteplase Clinical Trial II Group : Effects of 0.6 mg/kg intravenous alteplase on vascular and clinical outcomes in middle cerebral artery occlusion: Japan Alteplase Clinical Trial II (J-ACT II). Stroke 41: 461– 465, 2010. [DOI] [PubMed] [Google Scholar]
- 9). Sakai N, Ueda T, Hayakawa M, Nagahata M, Ota S, Nakahara I, Kimura K, Yoshimura S, Ezura M, Yamazaki S, Matsumoto Y, Nishino K, Toyota S, Yamasaki H, Onda T, Yamagami H, Imamura H: Periprocedural results of mechanical thrombectomy using Merci Retriever: Initial experience at Japanese top 15 centers. JNET 5: 23– 31, 2011. (Japanese) [Google Scholar]
- 10). The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med 312: 932– 936, 1985. [DOI] [PubMed] [Google Scholar]
- 11). Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, Sacks D, Technology Assessment Committee of the American Society of Interventional and Therapeutic Neuroradiology. Technology Assessment Committee of the Society of Interventional Radiology : Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 34: e109– 137, 2003. [DOI] [PubMed] [Google Scholar]
- 12). Nakano S, Iseda T, Yoneyama T, Kawano H, Wakisaka S: Direct percutaneous transluminal angioplasty for acute middle cerebral artery trunk occlusion: an alternative option to intra-arterial thrombolysis. Stroke 33: 2872– 2876, 2002. [DOI] [PubMed] [Google Scholar]
- 13). Nakano S, Wakisaka S, Yoneyama T, Kawano H: Reperfusion therapy for acute middle cerebral artery trunk occlusion. Direct percutaneous transluminal angioplasty versus intra-arterial thrombolysis. Interv Neuroradiol 10( Suppl 1): 71– 75, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Yoneyama T, Nakano S, Kawano H, Iseda T, Ikeda T, Goya T, Wakisaka S: Combined direct percutaneous transluminal angioplasty and low-dose native tissue plasminogen activator therapy for acute embolic middle cerebral artery trunk occlusion. AJNR Am J Neuroradiol 23: 277– 281, 2002. [PMC free article] [PubMed] [Google Scholar]