Abstract
A 43-year-old woman was diagnosed with moyamoya disease (MMD) and underwent right-side bypass surgery. After surgery, previous symptoms disappeared. One month later, transient right hemiparetic attacks and motor dysphasia developed. Angiography revealed progressive severe stenosis of left supraclinoid segment of internal carotid artery. Angioplasty using a drug-eluting stent (DES) was performed. For 18 months, she presented no ischemic symptom and no in-stent stenosis was observed in follow-up angiography. This is the first case report about effect of DES use for MMD. Considering that intimal hyperplasia is a pathophysiology of stenosis, DES may have a role in reducing progression of stenosis in selected moyamoya patients.
Keywords: drug-eluting stent, moyamoya, stenting
Introduction
Moyamoya disease (MMD) is characterized by the spontaneous stenosis or occlusion of the distal internal carotid arteries (ICAs).1) The basic pathology of the progressive stenosis is intimal hyperplasia.2,3) There is no known effective medical therapy to prevent progressive stenosis of MMD. Surgical revascularization has been performed to reduce ischemic stroke.3) However, a direct approach to the stenotic segments has rarely been reported.4–8) These approaches were based on angioplasty technique with bare metal stent. But long-term outcomes were not favorable because of in-stent restenosis mainly. In this article, we describe the clinical and radiological outcomes of a patient with MMD who was treated with a drug-eluting stent (DES).
Case
A 43-year-old woman presented with repeated left-side transient ischemic attacks (TIAs). The magnetic resonance imaging revealed acute infarction at the right precentral gyrus. Right ICA angiography demonstrated stenosis of M1 and distal ICA with prominent collateral arteries. Left ICA angiography also showed middle cerebral artery (MCA) stenosis (Fig. 1). The resting hypoperfusion and decreased vascular reserve in the right MCA territory were observed in single photon emission computed tomography (SPECT). The patient was diagnosed with MMD. Right-side superficial temporal artery MCA anastomosis and encephaloduroarteriosynangiosis were performed. No more TIAs occurred after surgery. However, 1 month later, the patient experienced repeated contralateral side TIAs (right-hand weakness). The frequency of symptoms increased rapidly and transient motor dysphasia occurred for several months. Angiography revealed progression of the left supraclinoid ICA stenosis to 90%. The left ICA stenosis had been only 30% at the angiograms several months before (Fig. 2a, b). The SPECT showed decreased vascular reserve in the left MCA territory. The patient was reluctant to undergo bypass surgery. We decided to perform endovascular treatment using a DES considering the pathogenesis of progressive stenosis in MMD, which is intimal hyperplasia.
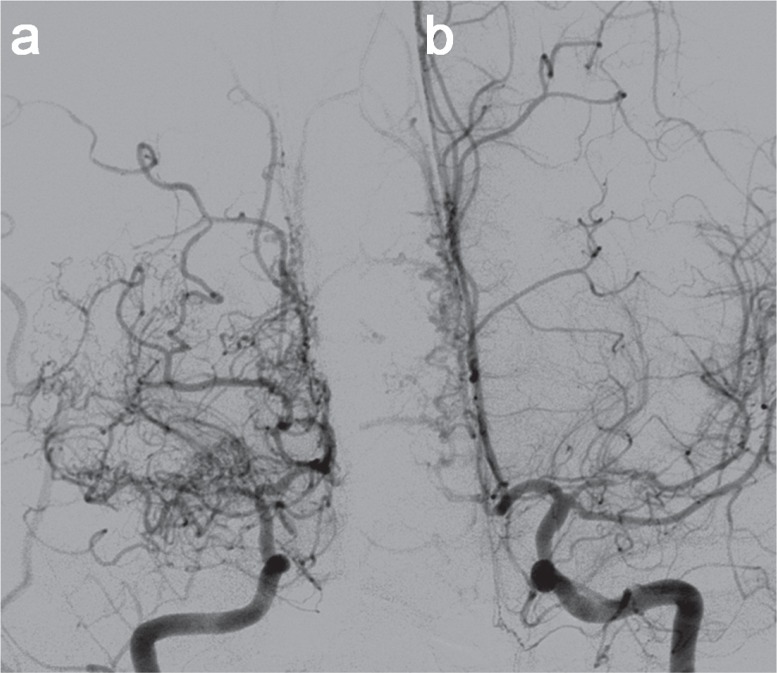
Fig. 1.
a: The right ICA angiography revealed stenosis of M1 and distal ICA with prominent collateral arteries. b: The left ICA angiography exhibited stenosis of the left middle cerebral artery and the ophthalmic segment of the left ICA. ICA: internal carotid artery.
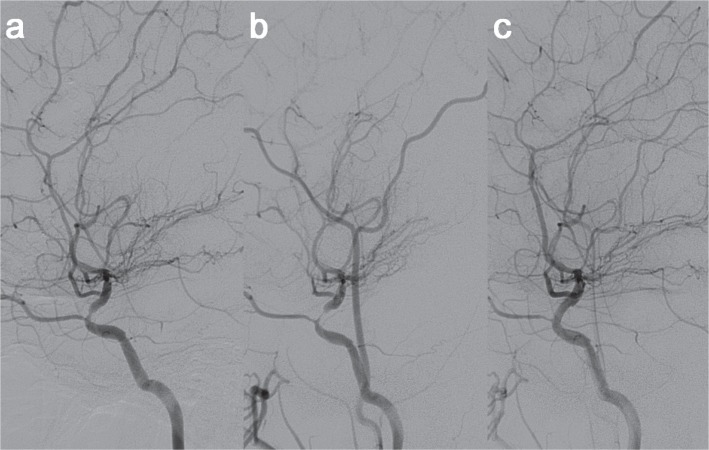
Fig. 2.
a, b: Progressive stenosis of the left ICA in the cerebral angiography performed at an interval of 7 weeks. c: Cerebral angiography performed after stent placement showed no residual stenosis in the ophthalmic segment of the left ICA. ICA: internal carotid artery.
Aspirin 100 mg and clopidogrel 75 mg were given daily from 5 days before stenting. Under general anesthesia, a 6F guide sheath was placed in the left cervical ICA. Using a coronary guide wire 190 cm (Whisper, Abbott, Illinois, USA), a DES (Xience 2.75 × 15 mm; Abbott) was deployed successfully by balloon inflation (Fig. 2c). All procedures were performed without any problem. TIAs completely disappeared after stenting. Dual antiplatelets were prescribed for a year. The poststenting SPECT demonstrated improved resting perfusion and vascular reserve in the left MCA territory. For 18 months, no recurrent symptom developed. Computed tomography angiography at 6 and 12 months revealed no in-stent stenosis.
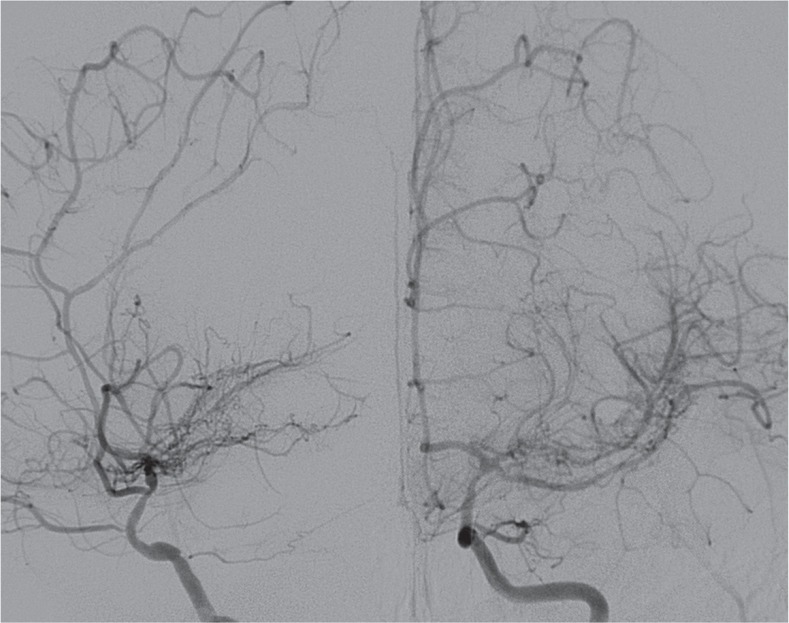
Eighteen months after stenting, the patient complained of a single episode of right-side TIA. Angiography demonstrated no restenosis at the stenting site but stenosis of the ICA more distal to the stenting segment and collateral vessels (Fig. 3). This study was exempted from deliberation by institutional review board of Seoul National University Bundang Hospital (IRB No. X-1205-153-902).
Fig. 3.
The 18-month follow-up cerebral angiography demonstrated progressive stenosis just distal to the previous stenting lesion. However, there was no in-stent stenosis.
Discussion
Endovascular dilatation was performed for selected MMD patients by previous authors.4–8) However, reported results were not encouraging. Six Wingspan stent (Boston Scientific, Natick, Massachusetts, USA) cases showed restenosis within 8 months after stenting. Five of them required surgical revascularization because of recurred ischemic symptoms.6) In one case in which the bare metal coronary stent was used, no in-stent stenosis was reported for 46 months. However, the disease of this case was not true MMD but moyamoya syndrome with essential thrombocythemia in which the pathophysiology of cerebral artery stenosis is known as different from the primary one.7)
Histopathological findings have shown that intimal hyperplasia is the main cause of progressive intracranial arterial narrowing.3,9) This respect may explain why just simple dilating with bare metal stents failed in stopping progression of stenosis. Reducing the intimal hyperplasia should be one therapeutic target for progressive stenosis of MMD. In cases of proven progression, inhibiting intimal hyperplasia would be a good therapeutic option. The stent we used for this patient contains Everolimus embedded in polymer which acts as an immunosuppressant leading to arrest of cell cycle at G1. By inhibiting smooth muscle cell mitosis in arterial wall, it reduces intimal hyperplasia. Efficacy of DES on inhibiting intimal hyperplasia is well known in the coronary artery stenosis as well as in intracranial stenosis.10,11)
The reasons we chose endovascular treatment with using a DES for our case were (1) the cause of the symptoms was short segment stenosis of supraclinoid ICA which had enough diameter for stenting; (2) it was rapidly progressive; and (3) we thought stenting would not interfere with bypass surgery, if necessary in the future, even though restenosis or complete occlusion of the stenting site could occur. After stenting, symptoms completely disappeared. Radiologically, no in-stent stenosis was found in the 18-month follow-up.
In practice, applying stents for MMD has many limitations. First of all, for stenting, target arteries should have enough size. In most typical MMD, supraclinoid ICA is not the only affected segment of the disease process. Smaller cerebral arteries are more often involved. Moreover, many MMD patients with ischemic presentation are children.3) Their cerebral arteries are often too small to adopt stents. In adult MMD patients, even supraclinoid ICA size may not be adequate. Angioplasty using drug-eluting balloons instead of stents may be an option for this situation if endovascular method should be chosen.
Another limitation is that pathology of MMD does not affect only one segment of cerebral arteries. Stenosis frequently occurs in multiple sites. DES inhibits progression of stenosis in stented segment only. Finally, efficacy of the ‘drugs’ (DES) on inhibiting intimal hyperplasia is time-limited for several months to years. Unlike the stenting-induced intimal hyperplasia, MMD activity (progressive stenosis) may not be transient. Disease activity may persist longer than drugs on stents. Considering these limitations, DES would be an option only for very limited patients with MMD (e.g. progressive stenosis of relatively large intracranial arteries).
Conclusion
In our case of MMD with rapid progressive supraclinoid ICA stenosis, stenting with a DES demonstrated clinical and radiological improvement. No restenosis was found at the 18-month follow-up. Longer-term follow-up and more experience will be necessary for clarifying efficacy and safety of DES for MMD involving supraclinoid ICA.
Acknowledgments
This article is supported by grant no. 03-2012-010 from the SNUBH Research Fund.
References
- 1). Suzuki J, Kodama N: Moyamoya disease—a review. Stroke 14: 104– 109, 1983. [DOI] [PubMed] [Google Scholar]
- 2). Hosoda Y, Ikeda E: Pathology of spontaneous occlusion of the circle of Willis (cerebrovascular Moyamoya disease). Neuropathology 19: 137– 138, 1999. [DOI] [PubMed] [Google Scholar]
- 3). Kuroda S, Houkin K: Moyamoya disease: current concepts and future perspectives. Lancet Neurol 7: 1056– 1066, 2008. [DOI] [PubMed] [Google Scholar]
- 4). Drazin D, Calayag M, Gifford E, Dalfino J, Yamamoto J, Boulos AS: Endovascular treatment for moyamoya disease in a Caucasian twin with angioplasty and Wingspan stent. Clin Neurol Neurosurg 111: 913– 917, 2009. [DOI] [PubMed] [Google Scholar]
- 5). El-Hakam LM, Volpi J, Mawad M, Clark G: Angioplasty for acute stroke with pediatric moyamoya syndrome. J Child Neurol 25: 1278– 1283, 2010. [DOI] [PubMed] [Google Scholar]
- 6). Khan N, Dodd R, Marks MP, Bell-Stephens T, Vavao J, Steinberg GK: Failure of primary percutaneous angioplasty and stenting in the prevention of ischemia in Moyamoya angiopathy. Cerebrovasc Dis 31: 147– 153, 2011. [DOI] [PubMed] [Google Scholar]
- 7). Kornblihtt LI, Cocorullo S, Miranda C, Lylyk P, Heller PG, Molinas FC: Moyamoya syndrome in an adolescent with essential thrombocythemia: successful intracranial carotid stent placement. Stroke 36: E71– 73, 2005. [DOI] [PubMed] [Google Scholar]
- 8). Rodriguez GJ, Kirmani JF, Ezzeddine MA, Qureshi AI: Primary percutaneous transluminal angioplasty for early moyamoya disease. J Neuroimaging 17: 48– 53, 2007. [DOI] [PubMed] [Google Scholar]
- 9). Houkin K, Ito M, Sugiyama T, Shichinohe H, Nakayama N, Kazumata K, Kuroda S: Review of past research and current concepts on the etiology of moyamoya disease. Neurol Med Chir (Tokyo) 52: 267– 277, 2012. [DOI] [PubMed] [Google Scholar]
- 10). Abou-Chebl A, Bashir Q, Yadav JS: Drug-eluting stents for the treatment of intracranial atherosclerosis: initial experience and midterm angiographic follow-up. Stroke 36: e165– 168, 2005. [DOI] [PubMed] [Google Scholar]
- 11). Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE, SIRIUS Investigators : Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 349: 1315– 1323, 2003. [DOI] [PubMed] [Google Scholar]