Abstract
The vascular type of Ehlers-Danlos syndrome (vEDS) is an autosomal dominant hereditary disease characterized by connective tissue fragility throughout the body, including the arteries, viscera, and gastrointestinal tract. We report a case in which we performed transvenous embolization (TVE) via direct superior ophthalmic vein (SOV) approach to treat a direct carotid-cavernous fistula (CCF) in a patient with Ehlers-Danlos syndrome (EDS). The patient was a 37-year-old woman who developed tinnitus in her left ear and a headache during examination in the outpatient clinic of another hospital in order to make a definitive diagnosis of vEDS, and she was referred to our hospital and examined. Based on the results of all of the studies she was diagnosed with a CCF. Conservative treatment was attempted, but was not very effective. Because of progressing aphasia, TVE was performed via the SOV direct cut. There were no intraoperative or postoperative complications. It has been reported that cerebral angiography is generally contraindicated in vEDS and that the morbimortality associated with endovascular treatment is very high. When performing treatment it is necessary to be sufficiently aware of the risks it entails.
Keywords: carotid-cavernous fistula, Ehlers-Danlos syndrome, vascular type, embolization
Introduction
Ehlers-Danlos syndrome (EDS) is known to be an autosomal dominant hereditary disease that is characterized by connective tissue fragility throughout the body, including the arteries, viscera, and gastrointestinal tract, and its manifestations are widely known to include thin, transparent skin, a bleeding diathesis, and hypermobile joints. The first report of EDS is thought to have been made by a Dutch surgeon in 1668,1) and the descriptions by Ehlers2) and Danlos3) were made in the early 1900s.
EDS has been classified into approximately 10 subtypes, and type IV is also called the vascular type of Ehlers-Danlos syndrome (vEDS). In 1967, the classification was modified by Barabas,4) and the vEDS was found to be attributable to a genetic abnormality of type III collagen (COL3A1) and to be the most pernicious and highly life-threatening type, with ruptures and dissection of blood vessels and arterial aneurysm formation being more common than in the other types.5–7)
While making the diagnosis, there is a strong likelihood of vEDS if any two of the following four criteria are met: (1) arterial rupture, (2) intestinal rupture, (3) uterine rupture during pregnancy, and (4) family history of vEDS. A definitive diagnosis can be made on the basis of biochemistry tests and genetic testing.
We performed transvenous embolization (TVE) via the superior ophthalmic vein (SOV) to treat a carotid-cavernous fistula (CCF) in patients with vEDS and were able to achieve a favorable therapeutic outcome. There have been occasional reports of TVE in the literature that we were able to collect in the past,8) but there have been no reports of treatment via the SOV, making this the first such case. We report this case based on the pitfalls and aspects that require caution when this treatment is selected.
Case Report
I. History of the present illness
The patient was a 37-year-old woman with a history of intestinal perforation, but no history of cranial or orbital trauma recently.
While undergoing genetic diagnosis of vEDS in the outpatient clinic of another hospital, the patient experienced a sudden onset of a vascular murmur in the vicinity of the left ear, tinnitus, and a headache. The tinnitus disappeared when the left carotid artery was compressed, and a bruit was heard over the left eyeball. No clear abnormal findings were detected by computed tomography (CT) of the head, but magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) of the head revealed a CCF, and the patient was referred to our hospital and examined.
The patient's consciousness level was clear on arrival at our hospital. Ophthalmological examination indicated corrected visual acuity of 1.0/1.0. Bilateral Amsler grid testing indicated no abnormalities at any point in either visual field. The left eye was markedly red with dilated irregular conjunctival and subconjunctival vessels. Hess chart testing indicated abducens nerve paralysis. Based on the patient's family history, past medical history, and the imaging findings, there was a strong possibility of a CCF complicating EDS, and because of the possibility that angiography would cause vascular injury, the preoperative evaluation was performed by 3D CTA.
II. Family history
Elder brother: Dissection of the thoracic aorta
Father's family: Rupture of the abdominal aorta, sudden death
III. Course after admission
Although the symptoms temporarily improved in response to intermittent manual compression of the carotid artery, they became more severe again (Fig. 1). Because of the development of higher function disorders, including aphasia and agnosia, associated with marked cortical reflux within the cranium, endovascular treatment (TVE) was performed under general anesthesia. A direct CCF from the vicinity of the left internal carotid artery (ICA) at the C3–4 level was confirmed by digital subtraction angiography (DSA). Marked retrograde flow into the intracranial veins was observed, and treatment was performed as described below (Fig. 2).
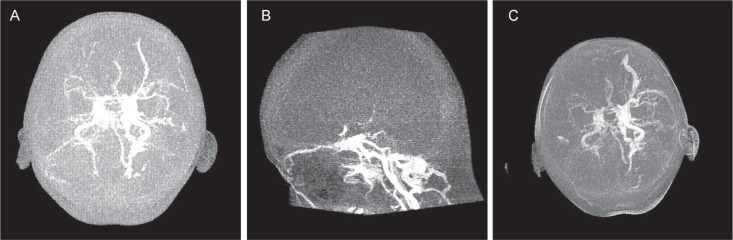
Fig. 1.
Dynamic CTA (preop: axial lateral). A: Dynamic CTA shows dilatation of the left SOV and insufficient intracranial circulation. B: Dynamic CTA shows aggravation of CCF. An increase in the dilatation of the left SOV can be observed in addition to intracranial cortical vein reflux. C: Dilatation of the left SOV and marked retrograde flow in the left intracranial veins can be seen. CCF: carotid-cavernous fistula, CTA: computed tomography angiography, SOV: superior ophthalmic vein.
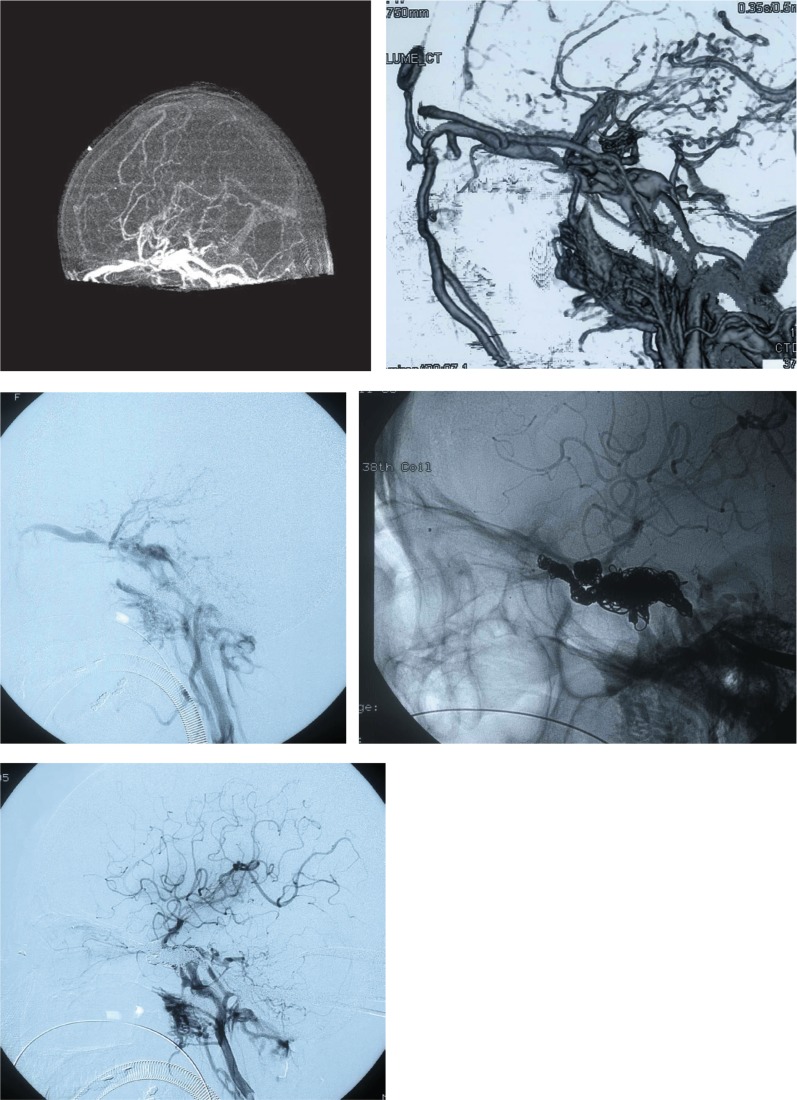
Fig. 2.
Preoperative, intraoperative, and postoperative DSA, DA (lateral view). Preoperative (top), intraoperative (middle), and postoperative (bottom). Digital subtraction angiograms (left common carotid arteriogram, lateral view). Preoperative angiogram: A large-volume direct carotid-cavernous fistula (CCF) shunt into the jugular vein is visible in the vicinity of the internal carotid artery at C3–4 in the early arterial phase. Outflow via the shunt into the left superior ophthalmic vein, left sylvian vein, left pterygoid sinus, and venous plexus is visible. More specifically, there was retrograde flow from the left sylvian vein to the cortical veins, and the veins had become dilated and tortuous. In addition, there was also retrograde flow to the right superior ophthalmic vein via the cavernous sinus. Hardly any normal circulation from the arteries into the cranium was observed. Postoperative angiogram: Retrograde flow from posterior to the cavernous sinus into the posterior cranial fossa and retrograde flow into the venous plexus were observed, and although a residual CCF was visible, because the retrograde flow into the right cavernous sinus had resolved and sufficient normal circulation had developed.
IV. Treatment
General anesthesia: No heparinilization
Micro catheter: Excelsior 45° (Boston Scientific, Natick, Massachusetts, USA)
SL-10 45° (Boston Scientific, Natick, Massachusetts, USA)
Micro guidewire: GT-0.016W.90° × 2 (Terumo, Tokyo)
Synchro14S (Boston Scientific, West Valley, Utah, USA)
Diagnostic catheter: An incision was made in the skin of the right inguinal area, and after directly puncturing the femoral artery with an 18G needle under direct vision, a 0.035 GW was inserted. A 4Fr diagnostic catheter (Berenstein type; Terumo Cinical Supply Co., Ltd., Gifu) was then directly inserted into the artery and gently advanced to the left ICA.
Treatment catheter: A transverse incision was made in the left superciliary area, and the SOV was exposed based on Doppler ultrasonography and the markers placed in advance when 3D CTA was performed. Since the tissue was fragile, with postoperative hemostatic maneuvers in mind, after lifting the SOV with 3-0 silk, we punctured it with a 20G needle, and inserted the Excelsior 45° inside to the cavernous sinus (CS), guiding the TERUMO GT0.016.
Coil embolization was started at the left superficial sylvian vein, where retrograde intracranial flow had been observed, and elimination of the intracranial retrograde flow was confirmed. We changed the working angle frequently to prevent the coil from migrating to the internal carotid artery. After embolizing the CS, we continued the embolization as we returned to the SOV. Although a residual CCF was observed, it had been possible to sufficiently reduce it, and since normal circulation had been adequately restored and the retrograde flow into the right CS had also stopped. After removing the femoral catheter, we manually compressed the puncture site with fibrin glue and polyglycolic acid (PGA). We observed the puncture site under direct vision and confirmed hemostasis, following which we concluded the operation.
V. Postoperative course
The patient's intraoperative and postoperative course was uneventful. The higher function disorders, including the aphasia, resolved and the tinnitus had completely disappeared by about 2 weeks postoperatively. The patient was discharged in a state of mRS 0. Because of the problems created by the coil artifacts, the postoperative evaluation was performed by MRDSA, but no deterioration was observed as of the 18-month follow-up examination (Fig. 3).
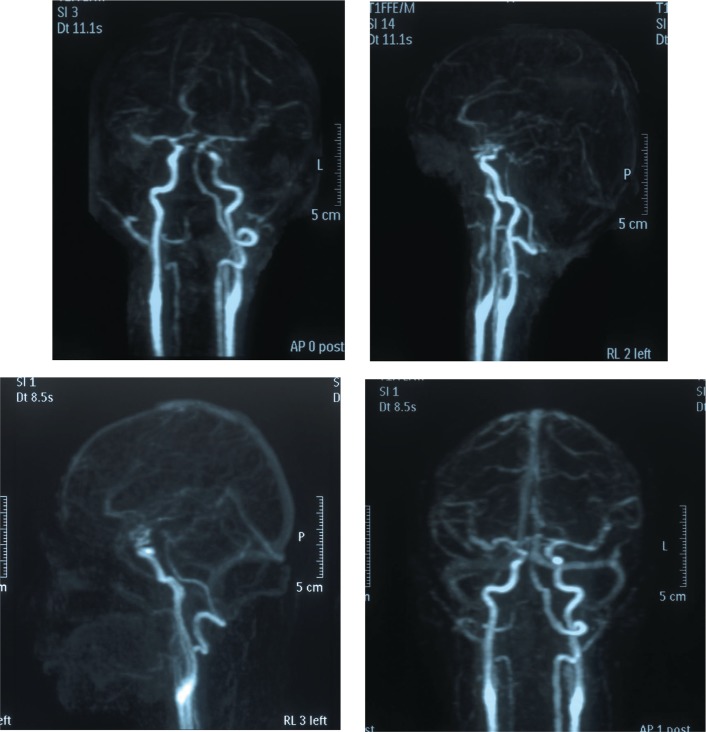
Fig. 3.
Postoperative follow-up MRDSA. Upper panel: One week postoperatively. Lower panel: 18 months postoperatively. No clear shunts were visible in either study, and no recurrence of the carotid-cavernous fistula was detected.
Discussion
I. Summary
EDS is an autosomal dominant hereditary disease that has been classified into approximately 10 subtypes. Type IV is also called the “vascular type” and accounts for approximately 4% of all cases. vEDS is a connective tissue disease with a very poor prognosis and is often the cause of sudden death in young people. It is characterized by a tendency for viscera and blood vessels to rupture idiopathically. The early mortality rate is high, and arterial and cardiac rupture, aortic dissection, and gastrointestinal rupture tend to occur, and recovery from the tissue fragility is difficult.9–11) Boutouyrie et al.12) conducted a study of the carotid arteries in vEDS cases and reported findings that wall stress was 22% to 43% higher and intima-media thickness 32% thinner than in a normal group. Because stress on vessel walls increases as a result of the reduced thickness of the intima and media, the risk of arterial dissection and vascular rupture is said to be higher, and fistulas, aneurysms, and dissections are cited as complications in the neuroendo vascular area.
II. Angiography
Freeman et al.13) reported a high major complication rate (22%) and mortality rate (5.6%) in 18 vEDS patients examined by cerebral angiography, and Schievink et al. reported a morbidity rate of 35% and mortality rate of 12% in a report on 25 cases,8) and thus both morbimortality rates were very high. Their causes have been reported to be not only bleeding at the puncture site and arterial dissection and retroperitoneal bleeding along the catheter access route, but vascular damage completely unrelated to the catheter maneuvers as well.14,15) Whenever possible, it is necessary to refrain from puncturing blood vessels, especially arteries, in vEDS, and we consider casual angiography to be contraindicated. If preoperative evaluation is possible, noninvasive CT and MRI (MRA) are useful, and in our patient we conducted the preoperative evaluation by three-dimensional computed tomography angiography (3D CTA) and the postoperative evaluation by magnetic resonance digital subtraction angiography (MR DSA).
III. Endovascular treatment (Table 1)
Table 1.
Literature review table
| Author (yr) | Age | Sex | Fistula side/type | Complications of angiography | Treatment | Outcomes |
|---|---|---|---|---|---|---|
| Halbach et al. (1990)16) | 19 y/o | N/A | (–)/direct | (Cerebral angiography) Transfemoral→unsuccessful (excessive tortuous) Carotid puncture→massive hematoma |
Direct surgical ligation | Good (spontaneously closed) |
| 22 y/o | F | Lt/direct | (Cerebral angiography) Transfemoral→massive external hemorrhage (puncture site) |
1st TVE (silicon balloon) | Improve | |
| 2nd TVE (balloon) | Dead (POD4) fatal pontine hemorrhage | |||||
| 24 y/o | N/A | Rt/direct | (Cerebral angiography) Transfemoral→iliac artery dissection→graft repair |
Direct surgical repair | Visual loss, opthalmoplegia | |
| 39 y/o | F | Lt/direct | (Cerebral angiography) N/A | 1st TAE | Initial improvement (recurrence) | |
| 2nd TAE | Unsuccessful | |||||
| 3rd TAE (liquid) | Good | |||||
| 49 y/o | F | Rt/direct | (Cerebral angiography) N/A | TAE | Good (asymptomatic iliac dissection) | |
| Schievink et al. (1991)8) | 17 y/o | F | Lt/direct | (Cerebral angiography) Direct carotid puncture→large hematoma |
ICA ligation | Good |
| 20 y/o | N/A | Rt/direct | (Cerebral angiography) Transfemoral→large hematoma (puncture site) |
Rt ICA embolization | Fail (tortuous) | |
| Rt carotid puncture | Bleeding→CPA (common carotid clamp) | |||||
| Debrun et al. (1995)18) | 39 y/o | F | Rt/direct | (Cerebral angiography) N/A | TAE (balloon) | Recurrence (ICO) |
| 39 y/o | F | Lt/direct | (Cerebral angiography) N/A | TAE (balloon) | Good | |
| Bashir et al. (1999)23) | 53 y/o | F | Lt/direct | (Cerebral angiography) N/A | TVE (coil) | Dead (haemothorax, abdominal aortic rupture) |
| Chuman et al. (2002)24) | 57 y/o | M | Lt/direct | (Cerebral angiography) Multiple AN (abdomen-pelvis)→ dissection, groin hematoma |
TVE | Good (POD3 colon rupture→colostomy) |
| Mitsuhashi et al. (2004)14) | 30 y/o | F | Rt/direct | (Cerebral angiography) Rt femoral artery rupture→retroperitoneal bleeding |
Extracranial ligation | Good (watershed infarction) |
| Desal et al. (2005)17) | 48 y/o | F | Lt/direct | (Cerebral angiography) N/A | TAE (balloon×→ coil) | Dead (POD7) lt frontal hematoma |
CPA: cardio pulmonary arrest, F: female, ICA: internal carotid artery, ICO: internal carotid artery occlusion, M: male, N/A: not available, POD: postoperative day, TAE: transarterial embolization, TVE: transvenous embolization, y/o: years old.
The risk of angiography in relation to the treatment of direct CCF is even greater, and there is even a report in the literature of a 50% major morbidity rate and 25% mortality rate.16) Actually, there is also apprehension in the literature about postoperative intracranial hematomas,17) asphyxiation as a result of hematomas caused by direct punctures of the neck,5) the development of secondary arterial aneurysms as a result of hemodynamic changes associated with ligation, and recurrences of the CCF.18) The transvenous approach appears useful from the standpoint of avoiding invasion of the artery, but there is also a problem, for example postoperative massive intraperitoneal and retroperitoneal bleeding because of tissue fragility in relation to veins as well,19,20) we made the approach via direct SOV approach in our patient to avoid contact with the vessel as much as possible. We made a direct skin incision for intraoperative diagnostic cerebral angiography and punctured the femoral artery under direct vision. These enabled us to perform reliable hemostasis under direct vision. The reason we placed a direct catheter without placing a sheath is that we chose a method of treatment that avoided vascular stress as much as possible. In addition, the contrast injection must be performed manually and gently in order to avoid detachment of the vascular endothelium.
Because the diagnosis of EDS had already been made before treatment in this case, we conducted the perioperative management cautiously, and favorable results of treatment were achieved. However, the diagnosis of EDS often may not have been made before the diagnostic studies or when the treatment is performed. While performing treatment in cases in which the diagnosis has already been made, it is important to determine in advance, before surgery, whether an aneurysm or dissection of the thoracoabdominal aorta or its major branches is present by multi detector-row computed tomography (MD-CT), etc.21,22) Moreover, quite a few complications of angiography and endovascular treatment are often recognized after treatment, for example arterial bleeding, abdominal organ rupture, vessel rupture, remote and delayed arterial rupture etc. So a careful postoperative monitoring of the patient's course is also necessary.14) It is also necessary to take the possibility of EDS into consideration as a cause when complications develop that cannot be explained by routine clinical tests or treatment. Cerebral angiography is generally considered to be contraindicated in vEDS, and very high morbimortality has been reported in association with interventional radiology. The risk must be sufficiently borne in mind when performing treatment.
References
- 1). van Meckeren J: Heel-en geneeskonstige aanmerkingen: Amsterdam. Casparus Commelijin 20: 495– 497, 1668. (Dutch) [Google Scholar]
- 2). Ehlers EL: Cutis laxa. Neigung zu Haemorrhagien in der Haut, Lockering mehrerer Artikulationen. Dermatol Zeitschr (Berlin) 8: 173– 174, 1901. (German) [Google Scholar]
- 3). Danlos M: Un cas de cutis lax a avec tumeurs par contusion chronique des coudes et des genoux. Bull Soc Fr Dermatol Syphiligr 19: 70– 72, 1908. (French) [Google Scholar]
- 4). Barabas AP: Heterogeneity of the Ehlers-Danlos syndrome: description of three clinical types and a hypothesis to explain the basic defect(s). Br Med J 2: 612– 613, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). North KN, Whiteman DA, Pepin MG, Byers PH: Cerebrovascular complications in Ehlers-Danlos syndrome type IV. Ann Neurol 38: 960– 964, 1995. [DOI] [PubMed] [Google Scholar]
- 6). Schievink WI, Michels VV, Piepgras DG: Neurovascular manifestations of heritable connective tissue disorders. A review. Stroke 25: 889– 903, 1994. [DOI] [PubMed] [Google Scholar]
- 7). Watanabe A, Kosho T, Wada T, Sakai N, Fujimoto M, Fukushima Y, Shimada T: Genetic aspects of the vascular type of Ehlers-Danlos syndrome (vEDS, EDSIV) in Japan. Circ J 71: 261– 265, 2007. [DOI] [PubMed] [Google Scholar]
- 8). Schievink WI, Piepgras DG, Earnest F, Gordon H: Spontaneous carotid-cavernous fistulae in Ehlers-Danlos syndrome Type IV. Case report. J Neurosurg 74: 991– 998, 1991. [DOI] [PubMed] [Google Scholar]
- 9). Habib K, Memon MA, Reid DA, Fairbrother BJ: Spontaneous common iliac arteries rupture in Ehlers-Danlos syndrome type IV: report of two cases and review of the literature. Ann R Coll Surg Engl 83: 96– 104, 2001. [PMC free article] [PubMed] [Google Scholar]
- 10). Imamura A, Nakamoto H, Inoue T, Yamada H, Okuno M, Takai S, Komada H, Kwon AH, Kamiyama Y: Ruptured dissecting aneurysm in bilateral iliac arteries caused by Ehlers-Danlos syndrome type IV: report of a case. Surg Today 31: 85– 89, 2001. [DOI] [PubMed] [Google Scholar]
- 11). Watanabe A, Shimada T: Vascular type of Ehlers-Danlos syndrome. J Nippon Med Sch 75: 254– 261, 2008. [DOI] [PubMed] [Google Scholar]
- 12). Boutouyrie P, Germain DP, Fiessinger JN, Laloux B, Perdu J, Laurent S: Increased carotid wall stress in vascular Ehlers-Danlos syndrome. Circulation 109: 1530– 1535, 2004. [DOI] [PubMed] [Google Scholar]
- 13). Freeman RK, Swegle J, Sise MJ: The surgical complications of Ehlers-Danlos syndrome. Am Surg 62: 869– 873, 1996. [PubMed] [Google Scholar]
- 14). Mitsuhashi T, Miyajima M, Saitoh R, Nakao Y, Hishii M, Arai H: Spontaneous carotid-cavernous fistula in a patient with Ehlers-Danlos syndrome type IV—case report. Neurol Med Chir (Tokyo) 44: 548– 553, 2004. [DOI] [PubMed] [Google Scholar]
- 15). Horowitz MB, Purdy PD, Valentine RJ, Morrill K: Remote vascular catastrophes after neurovascular interventional therapy for type 4 Ehlers-Danlos Syndrome. AJNR Am J Neuroradiol 21: 974– 976, 2000. [PMC free article] [PubMed] [Google Scholar]
- 16). Halbach VV, Higashida RT, Dowd CF, Barnwell SL, Hieshima GB: Treatment of carotid-cavernous fistulas associated with Ehlers-Danlos syndrome. Neurosurgery 26: 1021– 1027, 1990. [DOI] [PubMed] [Google Scholar]
- 17). Desal HA, Toulgoat F, Raoul S, Guillon B, Bommard S, Naudou-Giron E, Auffray-Calvier E, de Kersaint-Gilly A: Ehlers-Danlos syndrome type IV and recurrent carotid-cavernous fistula: review of the literature, endovascular approach, technique and difficulties. Neuroradiology 47: 300– 304, 2005. [DOI] [PubMed] [Google Scholar]
- 18). Debrun GM, Aletich VA, Miller NR, DeKeiser RJ: Three cases of spontaneous direct carotid cavernous fistulas associated with Ehlers-Danlos syndrome type IV. Surg Neurol 46: 247– 252, 1996. [DOI] [PubMed] [Google Scholar]
- 19). Van Overmeire O, De Keukeleire K, Van Langenhove P, Defreyne L: Carotid-cavernous fistula in ehlers-danlos syndrome by pure transvenous approach. Interventional Neuroradiology 12: 45– 51, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Usinskiene J, Mazighi M, Bisdorff A, Houdart E: Fatal peritoneal bleeding following embolization of a carotid-cavernous fistula in Ehlers-Danlos syndrome type IV. Cardio vasc Intervent Radiol 29: 1104– 1106, 2006. [DOI] [PubMed] [Google Scholar]
- 21). Alkadhi H, Wildermuth S, Desbiolles L, Schertler T, Crook D, Marincek B, Boehm T: Vascular emergencies of the thorax after blunt and iatrogenic trauma: multi-detector row CT and three-dimensional imaging. Radiographics 24: 1239– 1255, 2004. [DOI] [PubMed] [Google Scholar]
- 22). Zilocchi M, Macedo TA, Oderich GS, Vrtiska TJ, Biondetti PR, Stanson AW: Vascular Ehlers-Danlos syndrome: imaging findings. AJR Am J Roentgenol 189: 712– 719, 2007. [DOI] [PubMed] [Google Scholar]
- 23). Bashir Q, Thornton J, Alp S, Debrun GM, Aletich VA, Charbel F, Ausman JI, Polet H: Carotid-cavernous fistula associated with Ehlers-Danlos syndrome type IV. A case report and review of literature. Interv Neuroradiol 5: 313– 320, 1999. [DOI] [PubMed] [Google Scholar]
- 24). Chuman H, Trobe JD, Petty EM, Schwarze U, Pepin M, Byers PH, Deveikis JP: Spontaneous direct carotid-cavernous fistula in Ehlers-Danlos syndrome type IV: two case reports and a review of the literature. J Neuro Ophthalmol 22: 75– 81, 2002. [DOI] [PubMed] [Google Scholar]