Abstract
Surgical intervention is expected to improve the quality of life in patients with intractable epilepsy by providing adequate seizure control. Although many previous studies showed various rates of seizure freedom, definite conclusions have not yet been made regarding outcomes. In order to clarify the long-term postoperative outcome for a period up to 10 years, a retrospective review of our patients was performed longitudinally by using the survival analysis method. The postoperative state of epilepsy in 76 patients who underwent resection surgery was assessed based on Engel’s criteria. In addition, Kaplan-Meier survival analysis was used to calculate the probability of seizure freedom. In this patient group, abnormal lesion were detected by MRI in 70 out of 76 cases, and the ictal onset zone was finally identified within temporal lobe in 51 cases. The most favorable outcome, defined as Engel Class Ia, was observed in 26 (37%), 24 (40%), and 18 (41%) cases at 2, 5, and 10 years after surgery, respectively. The Kaplan-Meier survival curve in the overall group estimated the probability of seizure freedom as 75% (95% confidence interval [CI] 70–80%), 67% (62–72%), and 51% (45–57%) at 2, 5, and 10 years follow up, respectively. Half of all seizure recurrences occurred within the first 2 postoperative years. In this study, we showed that long-term favorable outcome of seizure control following resection surgery can be achieved in more than half of the patients.
Keywords: epilepsy surgery, resection surgery, long-term outcome, longitudinal analysis, Kaplan-Meier analysis
Introduction
Epilepsy surgery, which usually consists of ablation of the epileptogenic area in an attempt to improve seizure control, can be classified into two broad categories: palliative surgery and curative surgery. While palliative surgery lessens seizure severity and/or frequency or prevents the occurrence of a certain seizure type, curative resection surgery aims to eradicate seizures, leading to an improvement in daily life and decreased mortality. Surgically remediable epilepsy syndrome is referred to as mesial and/or lateral temporal lobe epilepsy (TLE), lesional neocortical epilepsy, non-lesional neocortical epilepsy, diffuse hemispheric epilepsy, and symptomatic generalized epilepsy.9) As Wiebe et al. demonstrated the advantages of epilepsy surgery over medical treatment, the role of surgical resection in TLE has become well established within a relatively short period.44) With long-term adequate control of seizures, surgical intervention is also expected to set the stage for improved self-esteem, greater social opportunity, and career advancement, thereby improving the quality of life for a patient with seizure disorder.20) Surgical success relies upon complete resection of the ictal onset zone, especially in the case of lesional/nonlesional neocortical epilepsy.5,43) Accurate demarcation of both seizure foci and eloquent cortices is essential for this purpose.26) Previous reports showed that seizure freedom rates after resection surgery vary from 15% to 84%,4,8,12,19,22–24,39,40,46) but more consistent conclusions on outcome have not yet been made due to short-term follow-up periods41) or due to little knowledge about longitudinal outcomes. The aim of the current study is to examine both short- and long-term seizure outcomes by using the statistical methods of survival analysis while accounting for variation in the duration of follow-up among patients in a single institute.
Materials and Methods
Since 1992, more than 150 patients with medically intractable epilepsy have been treated surgically in Kyoto University Hospital. In order to clarify the long-term postoperative outcome for a period up to 10 years, a retrospective chart review of patients who underwent epilepsy surgery at our department between May 1992 and February 2003 was performed. Only patients who underwent resection surgery for curative purpose and had multiple seizure episodes with adequate usage of the appropriate antiepileptic drugs were included. Patients who underwent a hemispherectomy, a palliative surgery such as callosotomy, or tumor resection surgery were excluded from this study. Although hemispherectomy is generally considered as a curative surgery, the candidate of hemispherectomy may have widely damaged brain and/or severe developmental disorder, and will not be considered for further resective surgery. Eventually, 76 patients were included in this study with the aforementioned criteria.
I. Acquisition of perioperative data
Data collected from medical records included demographics, neuroimaging data, information on prior electrode implantation surgery, the location and extent of the epileptogenic area, the type of surgery, the language dominant hemisphere, and pathological findings.
All patients first underwent a detailed history and neurological estimation. Long-term video-electroencephalogram (EEG) monitoring was performed with scalp electrodes placed according to the international 10–20 system. Preoperative imaging included magnetic resonance imaging (MRI) using a standardized epilepsy protocol that always included T1-weighted, T2-weighted, and fluid-attenuated inversion recovery (FLAIR) sequences. MRI studies were classified as normal (“non-lesional”) or abnormal (“lesional”). Selected patients also underwent fluorodeoxyglucose-positron emission tomography (FDG-PET), interictal/ictal 99 m Tc HMPAO or 123I IMP single-photon emission computed tomography (SPECT), or magneto encephalography (MEG). Additional examinations, which focused on the preservation of normal brain function, included functional MRI, the Wada test (intracarotid amobarbital procedure),32) and neuropsychological testing.
These clinical history, semiology, and results of the non-invasive evaluation were presented at a multidisciplinary patient management conference. The strategy of resection surgery was discussed, and the recommendation of the invasive evaluation prior to resection was determined.21)
Based on the preoperative protocol, only selected patients, in whom the localization of the ictal onset zone was not confirmed with non-invasive examinations because of lack of adequate information and/or discordance of them, underwent invasive monitoring with subdural grid electrodes, sometimes with depth or rarely epidural electrodes. Although intracranial EEG has greater sensitivity and spatial specificity than scalp EEG, it has limited spatial sampling.42) We used commercially available platinum subdural strips and grid electrodes provided by two different manufacturers (Ad-tech Medical Instrument, Racine, Wisconsin, USA, and/or Unique Medical, Tokyo, Japan) for chronic implantation. These techniques were also used to perform functional mapping if the ictal onset zone was thought to be close to the eloquent cortical area.
Surgical resection was classified based on the location of the surgical site: the frontal lobe (FLE), temporal lobe (TLE), parietal lobe (PLE), occipital lobe (OLE), and over multiple lobes (multilobular). The type of surgery was classified as anterior temporal lobectomy (ATL), selective amygdalo-hippocampal (SAH) resection, and lesionectomy only/and tailored resection of focus (focus resection). Following pathological examination, the specimens were divided into the following five categories: hippocampal sclerosis, gliosis, cortical malformation, tumor, and vascular anomaly.
II. Postoperative evaluation
All patients were seen at the time of their regular visits at the outpatient clinic, and all medical records were reviewed retrospectively up to 10 years after the surgery. The state of epilepsy after surgical treatment was assessed by the classification of Engel’s criteria as follows: Class Ia (seizure free), Class Ib–d (free of disabling seizures), Class II (rare disabling seizures, almost seizure-free), Class III (worthwhile improvement), and Class IV (no worthwhile improvement).10) Seizure outcome was evaluated at 2 years, 5 years, and 10 years after the resection surgery.
In addition to this conventional evaluation of seizure control, we adopted Kaplan-Meier survival analysis. In this analysis, patients were classified as seizure free if they achieved an Engel Class I rating postoperatively throughout the entire period of 10 years. The primary endpoint was decided at the first occurrence of disabling complex partial seizure and/or the occurrence of a secondarily generalized seizure. Because most of the medical records lacked a detailed description of each aura and minor simple partial seizure symptom, it was often difficult to differentiate these sporadic episodes. Therefore, isolated auras and simple partial seizures were not counted as seizure recurrence.
III. Statistical analysis
Descriptive statics were obtained with means and standard deviations for continuous variables and frequencies of categorical variables. Factors such as the interval in months between the onset of the seizure and the surgical procedure (duration of epilepsy), the presence of an abnormality detected by MRI (lesional), the side of resection along with the dominant language hemisphere (dominant), the extent of the surgical site, the type of surgery, and pathological findings were assumed to be potential predicting factors. Kaplan-Meier survival analysis was used to calculate the probability of seizure freedom in the group. Statistical significance was tested by using the log-rank test and comparison of 95% confidence intervals (CIs).2)
Results
Clinical data on 76 consecutive patients who underwent resection surgery during the study period were reviewed and analyzed. The subjects included 39 female patients and the mean (SD) age of all subjects was 30 (14) (range, 4–65) years. The mean duration of epilepsy was 14.8 ± 10.6 years at the time of resection. The clinical features of the entire group are summarized in Table 1 .
Table 1.
Clinical features of patients treated with resection surgery for medically intractable epilepsy
| Characteristics | Data |
|---|---|
| Number | 76 |
| Mean age, years | 30 ± 14 |
| Male | 37 |
| Mean duration of epilepsy, years | 14.8 ± 10.6 |
| MRI positive lesion | 70 |
| Dominance of surgical site | |
| Dominant | 23 |
| Non-dominant | 35 |
| Undetermined | 18 |
| Electrodes implantation cases | 28 |
| Location | |
| FLE | 15 |
| TLE | 51 |
| PLE | 3 |
| Multilobular | 7 |
| Type of surgery | |
| ATL | 30 |
| SAH | 10 |
| Focus resection | 36 |
| Pathological findings | |
| HS | 22 |
| Gliosis | 21 |
| Cortical dysplasia | 11 |
| Tumor | 15 |
| Vascular anomaly | 7 |
ATL: anterior temporal lobectomy, FLE: frontal lobe, HS: hippocampal sclerosis, MRI: magnetic resonance imaging, SAH: selective amygdalo-hippocampal, PLE: parietal lobe, TLE: temporal lobe.
Intracranial electrodes were implanted in 28 (37%) out of 76 patients prior to resection surgery. The monitoring period following the placement of the electrodes was 9.5 ± 3.8 (range, 3–15) days. Resection surgery was performed based on non-invasive and invasive data available within the limited period of monitoring for 3 patients who required emergent electrode removal before the scheduled surgery. MRI scans detected an abnormality in 70 cases out of 76, and these cases were classified as “lesional” cases. Out of these lesional cases, only 5 cases showed abnormal lesion over multiple lobes. The other 65 cases showed signal abnormality within the single lobe.
The cases were classified into 3 groups based on the language dominant side in accordance with the side of the resection area: 23 language dominant side, 35 language non-dominant side, and 18 undetermined cases. The ictal onset zones were identified for resection within a single lobe such as the frontal (FLE: 15 cases, 20%), temporal (TLE: 51 cases, 67%), and parietal (PLE: 3 cases, 4%) lobes; the occipital lobe was not identified for any of the cases. Surgical treatment over multiple lobes (multilobular) was required in 7 cases (9%). The most common etiology was hippocampal sclerosis in 22 cases (29%), followed by gliosis (28%), tumor (20%), cortical malformation (14%), and vascular anomaly (9%).
Postoperative course and outcome of seizure control
Out of 76 patients, 6, 17, and 32 patients did not turn for a follow-up at 2, 5, and 10 years after surgery, respectively. It is because of various reasons: referral to another hospital (25 cases), reoperation (6 cases), and death (1 case). In the cases of reoperation, outcome of seizure control was evaluated before the second operation. The cause of death in the latter patient was acute myocardial infarction, which was thought to have no relation to the surgical procedure.
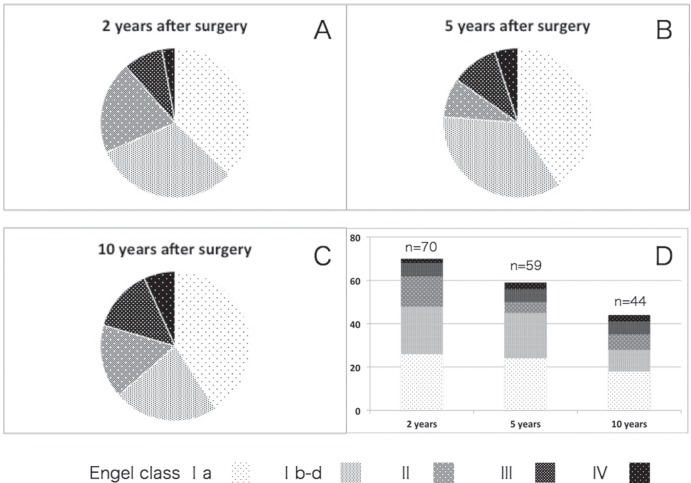
The comparison of postoperative seizure control of each time period over the 10 years of follow-up is illustrated in Fig. 1. The most favorable outcome, defined as an Engel Class Ia, was achieved in 26 (37%), 24 (40%), and 18 (41%) cases at 2, 5, and 10 years after surgery, respectively. The second favorable, outcome defined as an Engel Class Ib-d, was achieved in 22 (31%), 21 (36%), and 19 (22%) cases at 2, 5, and 10 years, respectively. The course of outcome values based on Engel’s criteria for 44 out of 76 (58%) patients who were followed continuously during the entire study period is shown in Fig. 2. These results did not reach significant differences regarding the favorable outcome group among the three different time periods of evaluation after surgery.
Fig. 1.
Circle graphs demonstrated proportion of each surgical outcome based on Engel’s criteria at 2 (A), 5 (B), 10 (C) years after resection surgery. Bar graph (D) showed comparison in number of patients for each Engel class amongthe three different times after surgery. Evaluation at 2 years contained 26, 22, 14, 6, 2 cases of Engel ClassIa, Ib-d, II, III, IV, respectively. Seizure control at 5 years ended as 24, 21, 5, 6, 3 cases for Engel Class Ia, Ib-d, II, III, IV, respectively. Seizure control at 10 years ended as 18, 10, 7, 6, 3 cases for Engel Class Ia, Ib-d, II, III, IV, respectively.
Fig. 2.
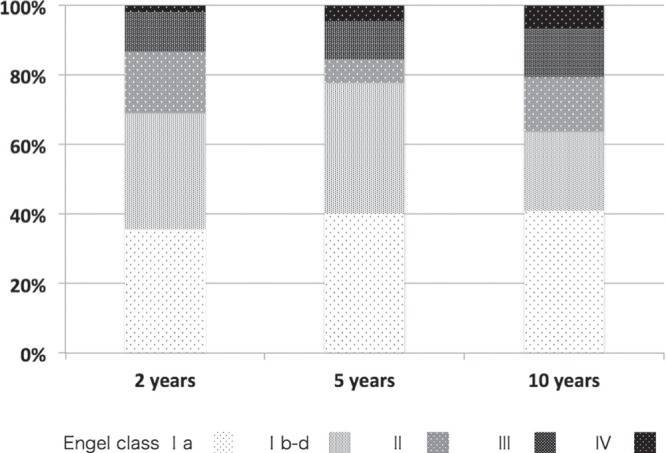
Bar graph demonstrated proportion of each surgical outcome of the patients in who have been followed in detail throughout the study period of 10 years. The proportion of Engel Class Ia, III, and IV increased a bit from 36% to 41%, from 11% to 14%, from 2% to 7%, respectively. As for Engel Class Ib-d, II, there was no constant tendency in proportional change.
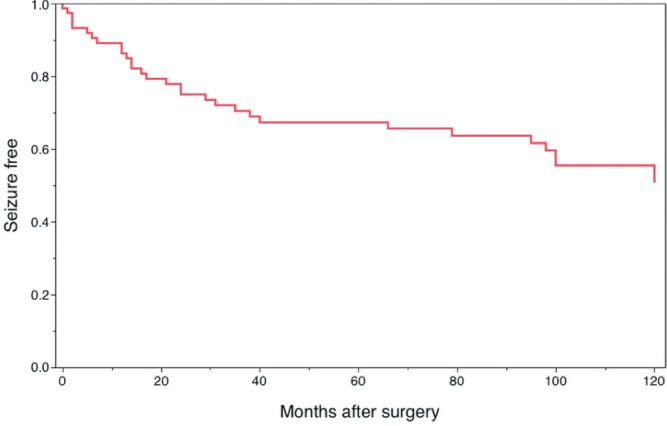
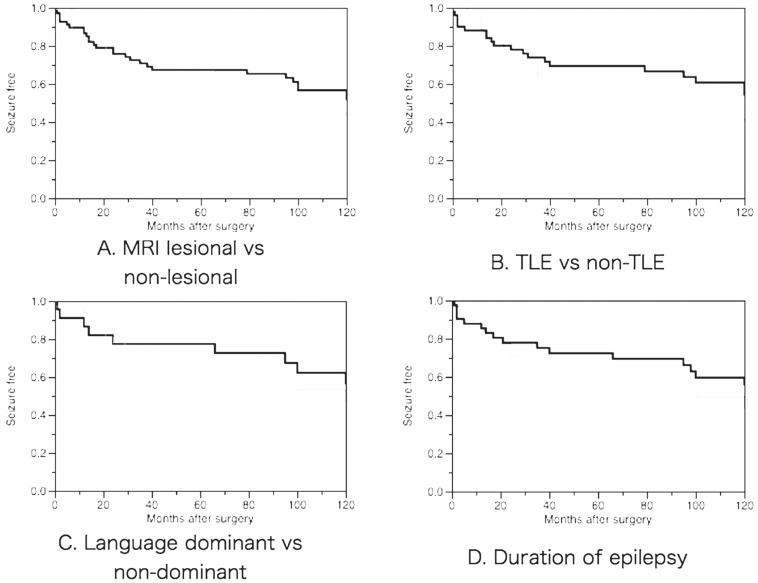
The Kaplan-Meier survival curve illustrating seizure recurrence in the overall group is shown in Fig. 3. The estimated probability of seizure freedom was 86% (95% CI, 82–90%) at 1 postoperative year, 75% (70–80%) at 2 years, 67% (62–72%) at 5 years, and 51% (45–57%) at 10 years. The variables of interest in this analysis were sex, duration of epilepsy (less than/not less than 10 years), presence of MRI abnormality (lesional/non-lesional), location of surgical site (TLE/other than TLE), type of surgery (ATL/SAH/focus resection), the side of surgery corresponding to language dominancy (dominant/non-dominant), and pathological findings (5 categories). The proportion of favorable surgical outcome assessed with Engel’s criteria did not show any differences according to these variables. The duration of epilepsy showed a tendency to correlate with long-term outcome of seizure control in the survival analysis (Fig. 4), but this failed to reach a significant level with any comparison.
Fig. 3.
Kaplan-Meier survival curve in the overall group. It estimated the probability of seizure freedom as 86% (95% confidence interval [CI] 82–90%), 75% (95% CI 70–80%), 67% (95% CI 62–72%), and 51% (95% CI 45–57%) at 1, 2, 5, and 10 years follow-up after surgery, respectively.
Fig. 4.
Kaplan-Meier survival curves in various interest groups. A: Comparison between groups of MRI lesional (thick line) and non-lesional (thin line). B: Comparison between groups of TLE (thick line) and non-TLE (thin line). C: Comparison between groups of language dominant side surgery (thick line) and non-dominant (thin line). D: Comparison between groups with duration of epilepsy more than 10 years (thick line) and not more than 10 years (thin line). MRI: magnetic resonance imaging, TLE: temporal lobe epilepsy.
Discussion
Quality of life has been gradually recognized as an important component of epilepsy care, especially as the understanding of the complex impact on the patient’s daily life has increased.27) The elimination of seizures by surgical intervention would be expected to decrease mortality in addition to providing opportunities for improved self-esteem, greater social opportunity, and even career advancement.25) Spencer and Huh reviewed that 48–84% of patients were seizure-free after temporal lobe resection: 66–70% at short-term (less than 5 years) and 41–79% at long-term (more than 5 years) follow-up. On the other hand, 36–76% of patients were seizure-free after neocortical resection.36) In other studies, seizure-free outcomes at the last follow-up ranged from 15% to 55%.6,7,15,24) Direct comparison of seizure freedom rates derived across various surgical series may appear to be conflicting. In addition, the lack of longitudinal follow-up in many of the prior studies may lead to more optimistic conclusions about surgical outcomes because the results may not be sustained over a period of more than 5 years. Since epilepsy is considered a life-long disease, its treatment should aim to obtain satisfactory control over the condition for a substantially long period. From this point of view, a definite estimation of seizure control after resection surgery will be required over a relatively long period of time. Traditionally, epilepsy after surgical treatment was assessed by Engel’s criteria, which categorizes patients into four major classes according to the degree of seizure reduction. The individual’s classification differs according to the time of estimation, because the categorization is based only on the recent attack. In addition to this system, we used Kaplan-Meier survival analysis.1,2,31,46) Although several reports addressed long-term seizure outcomes,3,29) a clear understanding of the mechanism governing seizure recurrence at various postoperative periods has not been achieved.1)
Van Gompel et al. (2008) reported that 47% of patients were seizure-free or exhibited only auras in a follow-up period of at least 2 years.41) Moreover, it was reported that 66% experienced seizure remission within first 2 years, and these rates did not significantly differ between medial temporal (68%) and neocortical (50%) resections.37) Our longitudinal analyses demonstrated that seizure freedom varied with the postoperative time course, with approximately half of the recurrences occurring during the first 2 years following surgical resection and the other half occurring between 2 and 10 years. Thus, the period around 2 years after resection surgery might be one of the critical points, which is independent of the type of epilepsy and surgery.
This phenomenon and pattern of seizure recurrence is in concordance with previously published data.16–18,46) Although the reason for these differences between early and late seizure recurrence is not clear, the initial rapid recurrence may be explained by incorrect localization of the epileptic focus, leading to inappropriate/incomplete resection. On the other hand, it is suspected that the later recurrence with slow rate is more likely attributable to the progressive manifestation of potential epileptogenicity, de novo development of epileptogenicity, or the influence habit changes in the patient’s daily life like the usage of antiepileptic drugs, sleep cycle, alcohol consumption, and employment. This suggests that the major epileptic focus in medically intractable epilepsy patients is not stable over time because the initial success in seizure control will be followed by the delayed development of active epileptic foci. Moreover, the late seizure recurrence in this patient population raises the question of epilepsy progression.38) The pattern of relapsing seizure in the current study seems very close to the observational study ofrecurrent seizures after one or two unprovoked seizures.13,14) Such similarity may suggest that the resection surgery has already fulfilled the main purpose to change intractable partial epilepsy into controllable to a certain level. Recent advances in imaging technology allow the current seizure onset zone to be well defined and resected.28) However, no methods currently exist to delineate the “potential seizure onset zone,” which may be the source of this delayed recurrence.
Regarding the long-term evaluation of seizure control, another classification has been recently proposed that categorizes the state of epilepsy into four different types: inactive, delayed seizure, intermittent, and intensive. This novel classification makes use of empirical data on long-term frequency and duration of attacks and provides a clue to the quality of life. Eventually, it can be more informative for predicting long-term mortality than the Engel classification.34)
Determination of long-term relapse risk and prognosis is essential, but the available information is neither comprehensive nor conclusive because various factors may influence long-term seizure control. The presence of hippocampal sclerosis, the focal localization of interictal epilepti form discharges, the absence of preoperative generalized seizures, tumor etiology, complete resection of the lesion,36) and MRI with visible focal lesions were indicated as positive predictive factors for resection surgery of TLE.45) The presence of a discrete lesion on MRI, complete resection of the lesion, localized scalp EEG ictal onset, concordant hypometabolism on FDG-PET (with lesion or localized EEG), longer duration of epilepsy, lack of febrile seizures,36) milder semiology, location in temporal lobe, and positive findings in histology of dysplastic cortex29) were indicated as positive predictors for neocortical epilepsy. In contrast, normal pathology and earlier relapse were assumed to be negative predictive factors for good seizure outcome.46) In comparison of the factors such as MRI lesional/non-lesional, TLE/non-TLE, language dominant/non-dominant, and duration of epilepsy longer/shorter than 10 years, two estimated survival curves cross each other over the period.
In case of insufficient postoperative seizure control, a second resection surgery in addition to further medical treatment and/or palliative neuromodulation surgery such as vagal nerve stimulation can be considered as treatment options. In this study, 6 patients (8%) underwent further resection surgery within 10 years. In a recent series of reports, 1–11% of patients underwent reoperation for surgical failure,11,30,35) which was usually performed in the same region as the previous surgery. After reoperation, 39–57% of patients became seizure–free,36) which is usually a less favorable outcome than the previous operation.33)
Our longitudinal analyses showed relatively good outcome in this study, but did not indicate any significant predicting factors for good outcome. This may be due to the predominant proportion of lesional cases, a mixed study group with various types of epilepsy, the limited number of patients, or multiple surgeons. And there remains another possibility that the subsequent medical treatment may be strongly biased depending on the etiology of epilepsy. For example, in case of non-lesional or non-TLE, reduction of antiepileptic drugs may be intentionally refrained for long time.
Conclusion
In this study, long-term favorable outcome following resection surgery was verified in more than half of patients with intractable partial epilepsy. In addition, our longitudinal analyses of seizure control during a postoperative period up to 10 years in a single institute raises questions to be addressed in future studies that will further our understanding of epilepsy progression.
Acknowledgments
Work by the authors in the present study was supported by Grant-in-Aid for Scientific Research C (24592159) from Japanese Society for the Promotion of Science.
References
- 1). Bulacio JC, Jehi L, Wong C, Gonzalez-Martinez J, Kotagal P, Nair D, Najm I, Bingaman W: Long-term seizure outcome after resective surgery in patients evaluated with intracranial electrodes. Epilepsia 53: 1722– 1730, 2012. [DOI] [PubMed] [Google Scholar]
- 2). Burneo JG, Villanueva V, Knowlton RC, Faught RE, Kuzniecky RI: Kaplan-Meier analysis on seizure outcome after epilepsy surgery: do gender and race influence it? Seizure 17: 314– 319, 2008. [DOI] [PubMed] [Google Scholar]
- 3). Carrette E, Vonck K, De Herdt V, Van Dycke A, El Tahry R, Meurs A, Raedt R, Goossens L, Van Zandijcke M, Van Maele G, Thadani V, Wadman W, Van Roost D, Boon P: Predictive factors for outcome of invasive video-EEG monitoring and subsequent resective surgery in patients with refractory epilepsy. Clin Neurol Neurosurg 112: 118– 126, 2010. [DOI] [PubMed] [Google Scholar]
- 4). Cohen-Gadol AA, Ozduman K, Bronen RA, Kim JH, Spencer DD: Long-term outcome after epilepsy surgery for focal cortical dysplasia. J Neurosurg 101: 55– 65, 2004. [DOI] [PubMed] [Google Scholar]
- 5). de Oliveira RS, Santos MV, Terra VC, Sakamoto AC, Machado HR: Tailored resections for intractable rolandic cortex epilepsy in children: a single-center experience with 48 consecutive cases. Childs Nerv Syst 27: 779– 785, 2011. [DOI] [PubMed] [Google Scholar]
- 6). Elsharkawy AE, Behne F, Oppel F, Pannek H, Schulz R, Hoppe M, Pahs G, Gyimesi C, Nayel M, Issa A, Ebner A: Long-term outcome of extratemporal epilepsy surgery among 154 adult patients. J Neurosurg 108: 676– 686, 2008. [DOI] [PubMed] [Google Scholar]
- 7). Elsharkawy AE, May T, Thorbecke R, Koch-Stoecker S, Villagran A, Urak L, Pfafflin M, Pannek H, Pietila TA, Ebner A: Long-term outcome and determinants of quality of life after temporal lobe epilepsy surgery in adults. Epilepsy Res 86: 191– 199, 2009. [DOI] [PubMed] [Google Scholar]
- 8). Engel J, Wiebe S, French J, Sperling M, Williamson P, Spencer D, Gumnit R, Zahn C, Westbrook E, Enos B: Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Epilepsia 44: 741– 751, 2003. [DOI] [PubMed] [Google Scholar]
- 9). Engel J, Jr, Cascino GD, Shields WD: Surgically remediable syndromes, in Engel J, Jr, Pedley TA, Aicardi J, Dichter MA, Moshe S, Perucca E, Trimble M. (eds): Epilepsy: A Comprehensive Textbook. Philadelphia, Lippincott Williams & Wilkins, 2007, pp 1761– 1769 [Google Scholar]
- 10). Engel JJ, Van Ness P, Rasmussen T: Outcome with respect to epileptic seizures., in Surgical Treatment of the Epilepsies, Engel J, Jr , (ed). Raven Press, New York, pp 609– 621, 1993. [Google Scholar]
- 11). González-Martínez JA, Srikijvilaikul T, Nair D, Bingaman WE: Long-term seizure outcome in reoperation after failure of epilepsy surgery. Neurosurgery 60: 873– 880; discussion 873–880, 2007. [DOI] [PubMed] [Google Scholar]
- 12). Harkness W: Temporal lobe resections. Childs Nerv Syst 22: 936– 944, 2006. [DOI] [PubMed] [Google Scholar]
- 13). Hauser WA, Anderson VE, Loewenson RB, McRoberts SM: Seizure recurrence after a first unprovoked seizure. N Engl J Med 307: 522– 528, 1982. [DOI] [PubMed] [Google Scholar]
- 14). Hauser WA, Rich SS, Lee JR, Annegers JF, Anderson VE: Risk of recurrent seizures after two unprovoked seizures. N Engl J Med 338: 429– 434, 1998. [DOI] [PubMed] [Google Scholar]
- 15). Hemb M, Velasco TR, Parnes MS, Wu JY, Lerner JT, Matsumoto JH, Yudovin S, Shields WD, Sankar R, Salamon N, Vinters HV, Mathern GW: Improved outcomes in pediatric epilepsy surgery: the UCLA experience, 1986–2008. Neurology 74: 1768– 1775, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Jeha LE, Najm IM, Bingaman WE, Khandwala F, Widdess-Walsh P, Morris HH, Dinner DS, Nair D, Foldvary-Schaeffer N, Prayson RA, Comair Y, O'Brien R, Bulacio J, Gupta A, Lüders HO: Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology 66: 1938– 1940, 2006. [DOI] [PubMed] [Google Scholar]
- 17). Jeha LE, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Lüders H: Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain 130 ( Pt 2): 574– 584, 2007. [DOI] [PubMed] [Google Scholar]
- 18). Jehi LE, O'Dwyer R, Najm I, Alexopoulos A, Bingaman W: A longitudinal study of surgical outcome and its determinants following posterior cortex epilepsy surgery. Epilepsia 50: 2040– 2052, 2009. [DOI] [PubMed] [Google Scholar]
- 19). Jeong SW, Lee SK, Kim KK, Kim H, Kim JY, Chung CK: Prognostic factors in anterior temporal lobe resections for mesial temporal lobe epilepsy: multivariate analysis. Epilepsia 40: 1735– 1739, 1999. [DOI] [PubMed] [Google Scholar]
- 20). Lowe AJ, David E, Kilpatrick CJ, Matkovic Z, Cook MJ, Kaye A, O'Brien TJ: Epilepsy surgery for pathologically proven hippocampal sclerosis provides long-term seizure control and improved quality of life. Epilepsia 45: 237– 242, 2004. [DOI] [PubMed] [Google Scholar]
- 21). Luders HO: Protocols and outcome statistics from epilepsy surgery centers, in Luderrs HO. (ed): Epilepsy Surgery. Philadelphia, Lippincott Williams & Wilkins, 2001, pp 973– 977 [Google Scholar]
- 22). McIntosh AM, Wilson SJ, Berkovic SF: Seizure outcome after temporal lobectomy: current research practice and findings. Epilepsia 42: 1288– 1307, 2001. [DOI] [PubMed] [Google Scholar]
- 23). McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briellmann RS, Berkovic SF: Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain 127( Pt 9): 2018– 30, 2004. [DOI] [PubMed] [Google Scholar]
- 24). McIntosh AM, Averill CA, Kalnins RM, Mitchell LA, Fabinyi GC, Jackson GD, Berkovic SF: Long-term seizure outcome and risk factors for recurrence after extratemporal epilepsy surgery. Epilepsia 53: 970– 978, 2012. [DOI] [PubMed] [Google Scholar]
- 25). Moritake K, Mikuni N, Akiyama Y, Nagai H, Maruyama N, Takada D, Daisu M, Nagasako N, Hashimoto N: Postoperative quality of life outcome and employment in patients undergoing resection of epileptogenic lesions detected by magnetic resonance imaging. Neurol Med Chir (Tokyo) 49: 281– 286, 2009. [DOI] [PubMed] [Google Scholar]
- 26). Palmini A, Gambardella A, Andermann F, Dubeau F, da Costa JC, Olivier A, Tampieri D, Gloor P, Quesney F, Andermann E: Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann Neurol 37: 476– 487, 1995. [DOI] [PubMed] [Google Scholar]
- 27). Räty LK, Wilde Larsson BM: Quality of life in young adults with uncomplicated epilepsy. Epilepsy Behav 10: 142– 147, 2007. [DOI] [PubMed] [Google Scholar]
- 28). Rosenow F, Luders H: Presurgical evaluation of epilepsy. Brain 124( Pt 9): 1683– 1700, 2001. [DOI] [PubMed] [Google Scholar]
- 29). Rowland NC, Englot DJ, Cage TA, Sughrue ME, Barbaro NM, Chang EF: A meta-analysis of predictors of seizure freedom in the surgical management of focal cortical dysplasia. J Neurosurg 116: 1035– 1041, 2012. [DOI] [PubMed] [Google Scholar]
- 30). Salanova V, Markand O, Worth R: Temporal lobe epilepsy: analysis of failures and the role of reoperation. Acta Neurol Scand 111: 126– 133, 2005. [DOI] [PubMed] [Google Scholar]
- 31). Sarkis RA, Jehi L, Najm IM, Kotagal P, Bingaman WE: Seizure outcomes following multilobar epilepsy surgery. Epilepsia 53: 44– 50, 2012. [DOI] [PubMed] [Google Scholar]
- 32). Schaller K: Propofol, amobarbital. . . is it the substance that matters, or the question about the role of the Wada test in brain tumor patients? World Neurosurg 75: 428– 430, 2011. [DOI] [PubMed] [Google Scholar]
- 33). Schulz R, Hoppe M, Boesebeck F, Gyimesi C, Pannek HW, Woermann FG, May T, Ebner A: Analysis of reoperation in mesial temporal lobe epilepsy with hippocampal sclerosis. Neurosurgery 68: 89– 97; discussion 97, 2011. [DOI] [PubMed] [Google Scholar]
- 34). Shih YH, Yen AM, Yen DJ, Hung LP, Chen HH, Liou HH: A novel postoperative seizure classification for long-term mortality of patients with intractable epilepsy: comparison with the Engel system. Neurosurgery 69: 64– 70; discussion 70–71, 2011. [DOI] [PubMed] [Google Scholar]
- 35). Siegel AM, Cascino GD, Meyer FB, McClelland RL, So EL, Marsh WR, Scheithauer BW, Sharbrough FW: Resective reoperation for failed epilepsy surgery: seizure outcome in 64 patients. Neurology 63: 2298– 2302, 2004. [DOI] [PubMed] [Google Scholar]
- 36). Spencer S, Huh L: Outcomes of epilepsy surgery in adults and children. Lancet Neurol 7: 525– 537, 2008. [DOI] [PubMed] [Google Scholar]
- 37). Spencer SS, Berg AT, Vickrey BG, Sperling MR, Bazil CW, Shinnar S, Langfitt JT, Walczak TS, Pacia SV, Multicenter Study of Epilepsy Surgery : Predicting long-term seizure outcome after resective epilepsy surgery: the multicenter study. Neurology 65: 912– 918, 2005. [DOI] [PubMed] [Google Scholar]
- 38). Sperling MR, Nei M, Zangaladze A, Sharan AD, Mintzer SE, Skidmore C, Evans JG, Schilling CA, Asadi-Pooya AA: Prognosis after late relapse following epilepsy surgery. Epilepsy Res 78: 77– 81, 2008. [DOI] [PubMed] [Google Scholar]
- 39). Téllez-Zenteno JF, Dhar R, Wiebe S: Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain 128( Pt 5): 1188– 1198, 2005. [DOI] [PubMed] [Google Scholar]
- 40). Téllez-Zenteno JF, Dhar R, Hernandez-Ronquillo L, Wiebe S: Long-term outcomes in epilepsy surgery: antiepileptic drugs, mortality, cognitive and psychosocial aspects. Brain 130( Pt 2): 334– 345, 2007. [DOI] [PubMed] [Google Scholar]
- 41). V Van Gompel JJ, Worrell GA, Bell ML, Patrick TA, Cascino GD, Raffel C, Marsh WR, Meyer FB: Intracranial electroencephalography with subdural grid electrodes: techniques, complications, and outcomes. Neurosurgery 63: 498– 505; discussion 505–506, 2008. [DOI] [PubMed] [Google Scholar]
- 42). Vulliemoz S, Carmichael DW, Rosenkranz K, Diehl B, Rodionov R, Walker MC, McEvoy AW, Lemieux L: Simultaneous intracranial EEG and fMRI of interictal epileptic discharges in humans. Neuroimage 54: 182– 190, 2011. [DOI] [PubMed] [Google Scholar]
- 43). Wagner J, Urbach H, Niehusmann P, von Lehe M, Elger CE, Wellmer J: Focal cortical dysplasia type IIb: completeness of cortical, not subcortical, resection is necessary for seizure freedom. Epilepsia 52: 1418– 1424, 2011. [DOI] [PubMed] [Google Scholar]
- 44). Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group : A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 345: 311– 318, 2001. [DOI] [PubMed] [Google Scholar]
- 45). Yang XL, Lu QC, Xu JW, Wang GS, Liu Q: Predictors of outcome in the surgical treatment for epilepsy. Chin Med J 124: 4166– 4171, 2011. [PubMed] [Google Scholar]
- 46). Yoon HH, Kwon HL, Mattson RH, Spencer DD, Spencer SS: Long-term seizure outcome in patients initially seizure-free after resective epilepsy surgery. Neurology 61: 445– 450, 2003. [DOI] [PubMed] [Google Scholar]