Abstract
Glioblastoma multiforme (GBM) harbors are not only rapidly dividing cells but also small populations of slowly dividing and dormant cells with tumorigenesity, self-renewal, and multi-lineage differentiation capabilities. Known as glioblastoma stem cells (GSCs), they are resistant to conventional chemo- and radiotherapy and may be a causative factor in recurrence. The treatment outcome in patients with GBM remains unsatisfactory and their mean survival time has not improved sufficiently. We studied clinical evidence and basic research findings to assess the possibility of new treatment strategies that target GSCs and their specific microenvironments (GBM niches) and raise the possibility of adding new treatments to eradicate GSCs and GBM niches.
Keywords: glioblastoma, stem cells, niches, treatment, cancer stem cells
Introduction
Glioblastoma multiforme (GBM) is one of the most malignant tumors in humans. Despite postoperative chemo- and radiotherapy the mean survival time of GBM patients is 12–14 months and only a few survive for more than 5 years.74,75) Cancers are comprised of heterogeneous populations of cancer cells and include specific subpopulations that possess stem cell-like characteristics. They are known as cancer stem cells (CSCs) and they can produce CSCs and differentiated non-CSCs.65) Singh et al.69,70) who proposed the “cancer stem cell hypothesis” in human brain tumors reported that they contain small populations of cells that can initiate brain tumors and that they are concentrated in the CD133+ fraction. Vescovi et al.78) defined brain tumor stem cells as cells with cancer initiation and extensive self-renewal ability, karyotypic or genetic alterations, aberrant differentiation properties, and the capacity to generate non-tumorigenic end cells.
It is now known that specific microenvironments (niches) play an important role in maintaining the stemness of normal somatic stem cells and CSCs, and that, changes in the niches lead to the differentiation of stem cells. Cell-cell- and cell extracellular matrix (ECM) interactions take place in niches and several secreting molecules are involved.25,61) Glioblastoma stem cells (GSCs) and GBMs niches play a pivotal role in the initiation, progression, resistance to therapy, and recurrence of GBM.
The standard treatment for GBM consists of a combination of surgical resection and chemo-radiotherapy. Attempts are made to remove the tumor mass as thoroughly as possible. Neuronavigation systems, intraoperative magnetic resonance (MR) imaging, neurological monitoring, and photodynamic diagnosis using 5-aminolevulinic acid may facilitate maximal tumor removal and avoid the induction of neurological deficits.19,46,77) However, GBM tumor cells migrate into the brain parenchyma far from the tumor mass80) and recurrence is commonly seen along the periphery of the tumor removal cavity even in cases with complete postoperative disappearance of the enhanced lesion (Fig. 1A–C).27) This suggests that migrated tumor cells far from the tumor mass are killed by conventional chemo-radiotherapy or that the growth of residual tumor cells is below the level of detection. Thus GSCs around the removal cavity may be able to escape the effects of current multimodal therapies and the recurrence of GBM may be attributable to the persistence of surviving dormant GSCs in GBM niches around the removal cavity (Fig. 1C, D).
Fig. 1.

Recurrence of GBM. A–C: Contrast-enhanced MRI of a patient with GBM in the right frontal lobe obtained before surgery (A), after treatment with TMZ and radiotherapy (B), and at recurrence (C). D: Schematic drawing of GBM cell invasion into the deep brain. Although the GBM tumor mass is removed, invaded cells remain in the brain. Clusters of GSCs and non-GSCs form specific environments (GBM niches) with some extracellular matrix and secreting molecules. GBM recurs in the periphery of the removal cavity (marginal area) where some GSCs in GBM niches are resistant to chemo-radiotherapy and survive. GSCs without GBM niches in the invasive area die, stop growing, or grow below the detection level on MRI. GBM: glioblastoma multiforme, GSCs: glioblastoma stem cells, MRI: magnetic resonance imaging, TMZ: temozolomide.
Despite extensive efforts to cure GBM patients, curative therapies remain elusive. Beier et al.8) who summarized accumulated information on the chemoresistance of GBMs concluded that the interactions of GSCs and chemotherapy are highly complex and that intrinsic and extrinsic factors are involved. Here we focus on GSCs and GBM niches as therapeutic targets and discuss the need for additive treatments.
GSC markers
According to Singh et al.,69,70) brain tumor initiating cells are concentrated in the CD133+ but not in the CD133− fraction. Clinically, CD133− GBMs are characterized by a lower proliferation index.6,7) However, the CD133 status alone is not sufficient as a GSC marker. Beier et al.7) reported that cells from primary GBM contained CD133+ subpopulations that formed spheres, and that cells from GBMs that harbored no CD133+ cells grew adherently, and that CD133− tumor cells could initiate tumors and fulfilled stem-cell criteria. Chen et al.15) had shown that some CD133− cells were more primitive than CD133+ cells and that CD133− cells could produce CD133+ and CD133− cells. Nishide et al.56) established induced GSCs (iGSCs) derived from mouse neural stem cells (NSCs). They deleted CD133-expressing cells by tamoxifen-dependent Cre activation and obtained cells that could form GBM. They concluded that CD133 expression was not required for the tumorigenesis of GSCs in nude mice.
While CD133 is one of the markers of GSCs, it is not sufficient for their purification. Other markers used for the detection of GSCs are CD15/SSEA-1/ LewisX, A2B5, L1CAM, integrin alpha 6, and CXCR4.1,47,57,72,83) Kijima et al.41) who reported that CD166/activated leukocyte cell adhesion molecule (ALCAM) was highly expressed in CD133+ GSCs showed that ALCAM and its soluble isoform are involved in the regulation of glioblastoma invasion and progression.
Another technique used to identify GSCs is utilization of their drug efflux ability through ATP-binding cassette (ABC) drug transporters. Hematopoietic stem cells (HSCs) express high levels of ABCG2, but the gene is turned off in committed progenitors and mature blood cells.68) These transporters protect HSCs from cytotoxic agents. Cells expressing ABCG2 excrete Hoechst 33342 fluorescent dye; they are detected by fluorescence-activated cell sorting (FACS) as fluorescent dye-negative cells. Stem cells are concentrated in this small unstained population and this cell fraction is referred to as the side population (SP). The fluorescence-excreting function is inherent in normal somatic stem cells and CSCs. GSCs are concentrated in the SP fraction34,44) and SP cells are different from non-SP cells in their ability for self-renewal, tumorigenesity, and resistance to therapy. The drug efflux ability is controlled by several genes of the ABC transporter family and protects CSCs from the effects of chemotherapeutic agents.20) The ABCG2 gene plays a major role in the control of this function. In the transgenic mouse model a nuclear form of GFP expression under the control of the ABCG2 promoter was detected in the ventricular zone of the developing forebrain and spinal cord where NSCs exist.58) Patrawala et al.60) reported that a subpopulation of ABCG2− cells produced ABCG2+ cells and that both ABCG2+ and ABCG2− cells are tumorigenic. They concluded that ABCG2 expression primarily identifies fast cycling tumor progenitors and that the ABCG2− population contains primitive stem-like cancer cells. On the other hand, Broadley et al.10) documented that doxsorubicin-exposed cells showed a transient increase in SP cells without being enriched for the stem cell phenotype.
Taken together, these findings suggest that GSCs can be enriched by using some cell surface markers and/or the drug efflux ability. However, these techniques are suboptimal if the goal is the purification of bona fide GSCs.
Cell origin of GBM
Core signaling pathways, e.g. receptor tyrosine kinase (RTK), p53, and Rb are crucial in clinical studies of glioblastoma.13) They are also significant for gliomagenesis in both genetically manipulated mouse models and several types of iGSCs transformed from neural lineage cells via the over- and down-regulation of these core pathway genes.14,26,50,52) To investigate the cell origin of GBMs, we established iGSCs derived from p53-/- NSCs, astrocytes, and oligodendrocyte precursor cells (OPCs). These were transformed by the over-expression of the active form of H-ras. While 10 injected iGSCs from NSCs and OPCs formed GBMs in the brains of nude mice, the injection of 104 iGSCs from astrocytes was required to form anaplastic astrocytomas, indicating that NSCs and OPCs have a higher potential for gliomagenesis than astrocytes.32,33) Liu et al.50) who used a mouse model of p53/Nf1 mutation showed that GBM originates from OPCs and Friedmann-Morvinski et al.26) who performed p53/Nfl knockdown in mouse brains demonstrated that even mature neurons and astrocytes can induce malignant gliomas. They proposed that upon defined genetic alterations, most differentiated cells in the central nervous system (CNS) undergo dedifferentiation to generate an NSC- or progenitor state, to maintain tumor progression, and to give rise to the heterogeneous populations observed in malignant gliomas. Thus, not only NSCs and OPCs but also mature neurons and astrocytes can be the target of gliomagenesis.14,26,32,33,50,52)
Characteristics of GSCs
Clinically, GSCs are resistant to conventional chemo- and radiotherapy.5,20) Residual tumor cells, especially GSCs in GBM niches, lead to recurrence even after primary intensive treatment consisting of surgery and chemo- and radiotherapy.
Bao et al.2) who studied the radioresistance of GSCs showed that CD133+ glioma cells recovered more quickly from deoxyribonucleic acid (DNA) damage than CD133− cells by expressing checkpoint kinase (Chk) 1 and 2. Ropolo et al.67) examined DNA repair in five stem and non-stem glioma cell lines. They found that the population-doubling time was significantly longer for stem- than non-stem glioma cell lines, and that the activation of Chk1 and Chk2 was enhanced in untreated CD133+ compared to untreated CD133− cells. After irradiation, DNA base excision repair, single-strand break repair, and the resolution of phospho-H2AX nuclear foci, an indicator of double-strand break repair, were not significantly greater in CD133+ than CD133− cells. They suggested that an elongated cell cycle and enhanced basal activation of checkpoint proteins contribute to the radio-resistance of GSCs and that enhanced DNA repair is not a common feature of these cells.
In GBM, CD133+ cells highly express drug resistance genes and this result in chemoresistance.9,51) Despite treatment with temozolomide (TMZ), an important anti-GBM drug, some GBM cells survive, leading to tumor recurrence within a year. TMZ kills GBM cells but the ratio of SP cells among residual tumor cells increases.17) Consequently, although treatment with TMZ plus radiation has extended the mean survival time of GBM patients, this therapy fails to eradicate all GSCs.75)
Somatic stem cells and CSCs have been identified among slow-dividing and/or dormant cell populations but have not been shown among GSCs.73) Deleyrolle et al.21) reported that glioma cells were stained with carboxyfluorescein diacetate succinimidylester (CSFE) and that this fluorescent dye was diluted by cell division. Characteristically, CFSEhigh cells, i.e., slow-dividing cells, showed a higher expression of stem cell markers and stronger tumor forming more ability than CSFElow cells. This was the first evidence that label-retaining tumor-initiating cell populations within the human GBM-derived glioma sphere are highly tumorigenic GSCs and their findings may help to explain the resistance of GSCs to conventional therapies.
GSCs and hypoxia
According to Pistollato et al.,64) oxygen tension controls the expansion of precursors in the human CNS. Low physiological oxygen tension maintains stemness, while higher oxygen tension promotes the differentiation of normal human neural precursors into astrocytes and oligodendrocytes. Hypoxia has critical effects on CSCs.30,36,38,39,45) With respect to gliomas, it promotes the expression of GSC markers and expands the GSC pool.4,31,53,54,71,81) Natsume et al.55) reported that girdin maintains the stemness of GSCs; under hypoxic conditions its expression was up-regulated in parallel with the expression of CD133. Earlier, Pistollato et al.63) had documented that the intratumoral hypoxic gradient drives stem cell distribution and the expression of MGMT in glioblastoma.
An essential gene regulating the hypoxic condition is hypoxia-inducible factor (HIF). It regulates GBM recurrence and its poor response to treatment and is involved in the poor prognosis of GBM.37,38,81) Calabrese et al.11) reported that the stem cell pool in the brain tumor mass physically interacts with the tumor vasculature and endothelial cells. In particular, HIF-1 alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion.23) HIF regulates the tumorigenic capacity of GSCs and HIF-2 alpha is specifically expressed in GSCs.49) In addition, HIF-2 alpha expression correlates with the poor survival of glioma patients.49) Kolenda et al.43) showed that in addition to the expression of HIF-1 alpha and HIF-2 alpha, the expression of stem cell and chemoresistant markers was increased under hypoxic conditions while Ki-67 was reduced. Together, these findings indicate that hypoxia promotes not only chemoresistance but also stem cell marker expression and slowing of the cell cycle.
GSCs and GBM niches
With respect to HSCs, both perivascular and osteoblastic niches play an essential role in the existence of progenitor and stem cells.40,82) Doetsch et al.22) studied neurogenesis in the adult mouse brain. They showed that characteristic microenvironments help NSCs to maintain their ability for self-renewal, multi-lineage differentiation, and infinite proliferation. They designated stem- and proliferative progenitor cells as type B and C cells, respectively, and migrating neuroblasts as type A cells assembled in the subventricular zone where NSCs were in touch with vessels. NSCs reside in the perivascular niche and their self-renewal ability is regulated by this specific microenvironment.59) Cell-cell- and cell-ECM interactions and interactions among several secreting molecules are important in NSC and GBM niches.22,25,61)
Hypoxic- and perivascular niches are strongly involved in the initiation, progression, chemotherapy resistance, and recurrence of GBM (Fig. 2A, B).29) Hypoxia promotes angiogenesis and the migration and expression of stemness genes, resulting in the exacerbation of clinical symptoms due to tumor cell invasion, expansion of the tumor mass and perifocal edema, and it induces resistance to therapy. HIFs are key regulators of vascular endothelial growth factor (VEGF) expression and other hypoxia-responsive genes such as Oct4, Sox2, and Glut1.29,38,39,49,81) The number of capillaries in GBM tumors correlates with the patient prognosis48) and CD133+ GSCs promote tumor angiogenesis through VEGF.3)
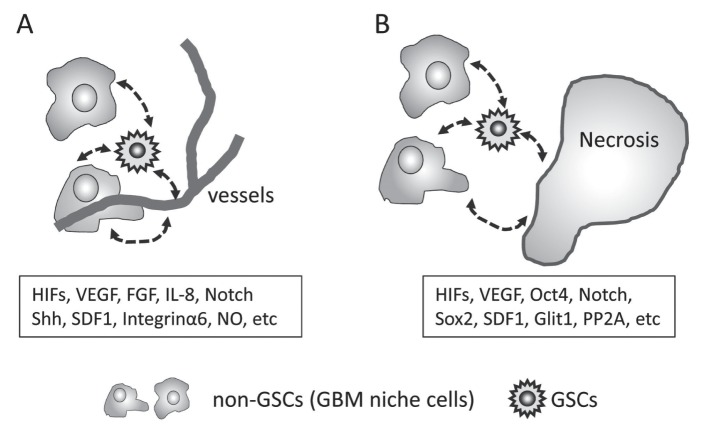
Fig. 2.
GBM niches. The stemness of GSCs is maintained by reciprocal signaling in the GBM niche. Perivascular and hypoxic (peri-necrotic) niches are important for GSCs. HIFs and VEGFs are key molecules for the establishment and maintenance of GBM niches. They harbor GSCs, non-GSCs, extracellular matrix, and secreting molecules. Representative genes and molecules are indicated in the box. A: Perivascular niche. Endothelial cells and pericytes in the perivascular area produce molecules for angiogenesis, and also interact with GSCs, non-GSCs, extracellular matrix, and secreting molecules that regulate the expression of genes involved in the maintenance of stemness. B: Hypoxic (peri-necrotic) niche. Hypoxia induces necrosis and regulates the expression of stemness genes. The population of GSCs increases and their proliferation slows. Some GSCs start to invade or enter into dormant state. FGF: fibroblast growth factor, GBM: glioblastoma multiforme, GSCs: glioblastoma stem cells, HIF: hypoxia-inducible factor, VEGF: vascular endothelial growth factor.
Zhu et al.84) reported that endothelial cells create a stem cell niche in GBMs by providing NOTCH ligands that nurture the self-renewal of GSCs. In addition, GSCs recruit endothelial cells and GSCs transdifferentiate into endothelial cells.3,11,66,79) According to Cheng et al.,16) GSCs generate vascular pericytes to support vessel function and tumor growth. Like endothelial cells, pericytes are important constituents of GBM niches. Specific microenvironments in hypoxic- and perivascular areas result in the formation of GBM niches. Thus, several genes and molecules in the GBM niches control the maintenance and expansion of GSCs (Fig. 2A, B).
GSCs and GBM niches as treatment targets
The usual targets of chemo- and radiotherapy are rapidly dividing cancer cells because expansion and invasion of the tumor mass into surrounding tissue results in organ dysfunction and local pain. GBM is comprised of heterogeneous cell populations that contain not only rapidly-, slowly- and non-dividing cells but also dormant cells. The fraction of dormant and slow-dividing cells appears to be able to resist chemo-radiotherapy due to drug reflux and DNA repair. Accumulated knowledge regarding GSCs and GBM niches has led to the realization that a paradigm shift is necessary with respect to the targets of GBM treatments. In efforts to eradicate GSCs, the blocking of several key pathways related to the maintenance of stemness has been found to effectively reduce their tumorigenic potential. In fact, inhibition of some pathways, e.g., Sonic hedgehog (Shh), Notch, and Wingless-type (Wnt) attenuated the characteristics of stemness and inhibited the formation of GBMs.18,24,42)
Differentiation therapy is an additional strategy that targets GSCs. Piccirillo et al.62) reported that bone morphogenetic protein inhibits the tumorigenic potential of human GSCs. All-trans-retinoic acid (ATRA), a standard drug for the treatment of acute promyelocytic leukemia, was effective against GSCs; it induced differentiation and therapy-sensitizing effects, impaired the secretion of angiogenic cytokines, and disrupted GSCs motility.12) Hofstetter et al.35) documented the relationship between hypoxia and the dormancy of GSCs. They showed that protein phosphatase 2A (PP2A) mediates the dormancy of GSCs under hypoxic conditions and that inhibition of PP2A activity results in increased cell proliferation, ATP exhaustion, and the acceleration of P53-independent cell death of hypoxic GSCs.
The perivascular niche is a potential target for GBM treatment. Blocking the SDF-1/CXCR4 pathway prevents or delays tumor recurrence after irradiation by inhibiting the recruitment of monocytes and macrophages that participate in tumor revascularization.76) In addition, the deletion of vascular pericytes generated from GSCs inhibits tumor growth16) and a reduction in pro-angiogenic gene expression interrupts perivascular niche formation and results in a decrease in the number of GSCs.28) Thus, not only specific cells, i.e., endothelial cells and vascular pericytes, but also important genes, i.e., stemness genes and pro-angiogenic genes, are candidate targets in efforts to eradicate GSCs.
Although current conventional GBM treatment strategies can decrease and/or minimize the number of GSCs and GBM niches, they are not curative. Post-treatment, some enhanced lesions indicative of residual tumor disappear on MR imaging scans. Theoretically, both therapy-resistant GSCs and GBM niche cells are minimized at that time, suggesting that nearly “naked” GSCs exist in incomplete GBM niches (Fig. 3). This presents an excellent opportunity for attacking GSCs directly. Besides conventional chemotherapeutic drugs, novel treatment strategies targeting GSCs, and GBM niches may help to cure patients with GBM. The further disruption of GBM niches evacuates GSCs, abolishes their stemness, and induces chemo-radio sensitivity and terminal differentiation. Additionally, due to the specific metabolism and immunoreactivity of GCS, the targeting of GSC-specific cell surface markers may render these cells dormant and/or prove eradicative (Fig. 3).
Fig. 3.
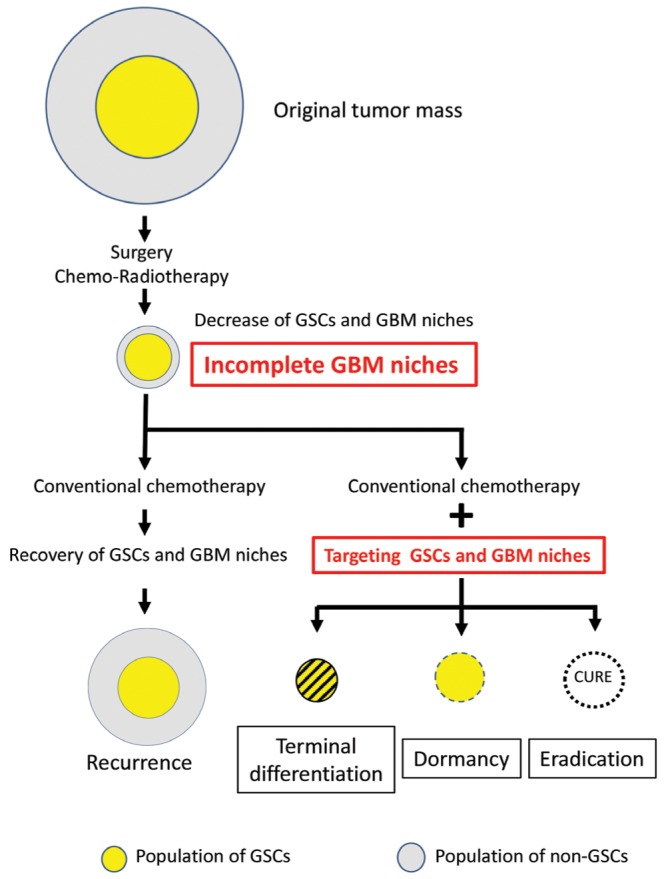
Additional treatment to eradicate GSCs. Postoperative chemo- and radiotherapy decreases the number of viable GSCs and GBM niche cells, although nearly “naked” GSCs remain in these incomplete GBM niches where suitable microenvironment for supporting GSCs is damaged. Direct attack on the GSCs and/or disruption of GBM niches may result in the terminal differentiation, dormancy, and eradication of GSCs. GBM: glioblastoma multiforme, GSCs: glioblastoma stem cells.
The development of multi-focal treatment strategies aimed at target cells and target functions and the optimal timing of treatments may improve the survival time and quality of life of GBM patients.
Concluding Remarks
Recently, leukemia has become a curable disease by the combination of chemotherapy, radiotherapy, and bone marrow transplantation, but GBM have not. The maximum removal of GBM tissue without eliciting neurological deficits is important for prolonging the survival of GBM patients and for retaining their good quality of life. Actually, the total resection of GBM tumor cells is extremely difficult because they invade into the deep brain.80) Occasionally, treatment may elicit pancytopenia, radiation necrosis, and the deterioration of cognitive functions in elderly patients. These issues make the radical treatment difficult.
The advent of CSC theory led to fine experiments on GSCs and GBM niches and then showed new insights. An advanced understanding of GSCs and GBM niches can be expected to lead to the development of new therapeutic strategies to cure GBM patients.
Acknowledgments
The authors research works focusing on GSCs and GBM niches were supported by JSPS KAKENHI Grant Number 25130710.
References
- 1). Bao S, Wu Q, Li Z, Sathornsumetee S, Wang H, McLendon RE, Hjelmeland AB, Rich JN: Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res 68: 6043– 6048, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN: Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444: 756– 760, 2006. [DOI] [PubMed] [Google Scholar]
- 3). Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN: Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res 66: 7843– 7848, 2006. [DOI] [PubMed] [Google Scholar]
- 4). Bar EE, Lin A, Mahairaki V, Matsui W, Eberhart CG: Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am J Pathol 177: 1491– 1502, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Baumann M, Krause M, Hill R: Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer 8: 545– 554, 2008. [DOI] [PubMed] [Google Scholar]
- 6). Beier CP, Beier D: CD133 negative cancer stem cells in glioblastoma. Front Biosci (Elite Ed) 3: 701– 710, 2011. [DOI] [PubMed] [Google Scholar]
- 7). Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP: CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res 67: 4010– 4015, 2007. [DOI] [PubMed] [Google Scholar]
- 8). Beier D, Schulz JB, Beier CP: Chemoresistance of glioblastoma cancer stem cells—much more complex than expected. Mol Cancer 10: 128, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Bleau AM, Huse JT, Holland EC: The ABCG2 resistance network of glioblastoma. Cell Cycle 8: 2936– 2944, 2009. [PubMed] [Google Scholar]
- 10). Broadley KW, Hunn MK, Farrand KJ, Price KM, Grasso C, Miller RJ, Hermans IF, McConnell MJ: Side population is not necessary or sufficient for a cancer stem cell phenotype in glioblastoma multiforme. Stem Cells 29: 452– 461, 2011. [DOI] [PubMed] [Google Scholar]
- 11). Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ: A perivascular niche for brain tumor stem cells. Cancer Cell 11: 69– 82, 2007. [DOI] [PubMed] [Google Scholar]
- 12). Campos B, Wan F, Farhadi M, Ernst A, Zeppernick F, Tagscherer KE, Ahmadi R, Lohr J, Dictus C, Gdynia G, Combs SE, Goidts V, Helmke BM, Eckstein V, Roth W, Beckhove P, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende C: Differentiation therapy exerts antitumor effects on stem-like glioma cells. Clin Cancer Res 16: 2715– 2728, 2010. [DOI] [PubMed] [Google Scholar]
- 13). Cancer Genome Atlas Research Network : Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455: 1061– 1068, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Chen J, McKay RM, Parada LF: Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell 149: 36– 47, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Chen R, Nishimura MC, Bumbaca SM, Kharbanda S, Forrest WF, Kasman IM, Greve JM, Soriano RH, Gilmour LL, Rivers CS, Modrusan Z, Nacu S, Guerrero S, Edgar KA, Wallin JJ, Lamszus K, Westphal M, Heim S, James CD, VandenBerg SR, Costello JF, Moorefield S, Cowdrey CJ, Prados M, Phillips HS: A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell 17: 362– 375, 2010. [DOI] [PubMed] [Google Scholar]
- 16). Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, Min W, McLendon RE, Rich JN, Bao S: Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 153: 139– 152, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Chua C, Zaiden N, Chong KH, See SJ, Wong MC, Ang BT, Tang C: Characterization of a side population of astrocytoma cells in response to temozolomide. J Neurosurg 109: 856– 866, 2008. [DOI] [PubMed] [Google Scholar]
- 18). Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A: HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol 17: 165– 172, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Colditz MJ, Jeffree RL: Aminolevulinic acid (ALA)-protoporphyrin IX fluorescence guided tumour resection. Part 1: Clinical, radiological and pathological studies. J Clin Neurosci 19: 1471– 1474, 2012. [DOI] [PubMed] [Google Scholar]
- 20). Dean M, Fojo T, Bates S: Tumour stem cells and drug resistance. Nat Rev Cancer 5: 275– 284, 2005. [DOI] [PubMed] [Google Scholar]
- 21). Deleyrolle LP, Harding A, Cato K, Siebzehnrubl FA, Rahman M, Azari H, Olson S, Gabrielli B, Osborne G, Vescovi A, Reynolds BA: Evidence for label-retaining tumour-initiating cells in human glioblastoma. Brain 134: 1331– 1343, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A: Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97: 703– 716, 1999. [DOI] [PubMed] [Google Scholar]
- 23). Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegué E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G: HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 13: 206– 220, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, Koh C, Zhang J, Li YM, Maciaczyk J, Nikkhah G, Dimeco F, Piccirillo S, Vescovi AL, Eberhart CG: NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells 28: 5– 16, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Filatova A, Acker T, Garvalov BK: The cancer stem cell niche(s): the crosstalk between glioma stem cells and their microenvironment. Biochim Biophys Acta 1830: 2496– 2508, 2013. [DOI] [PubMed] [Google Scholar]
- 26). Friedmann-Morvinski D, Bushong EA, Ke E, Soda Y, Marumoto T, Singer O, Ellisman MH, Verma IM: Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science 338: 1080– 1084, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Gaspar LE, Fisher BJ, Macdonald DR, LeBer DV, Halperin EC, Schold SC, Cairncross JG: Supratentorial malignant glioma: patterns of recurrence and implications for external beam local treatment. Int J Radiat Oncol Biol Phys 24: 55– 57, 1992. [DOI] [PubMed] [Google Scholar]
- 28). Gatson NN, Chiocca EA, Kaur B: Anti-angiogenic gene therapy in the treatment of malignant gliomas. Neurosci Lett 527: 62– 70, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Gilbertson RJ, Rich JN: Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer 7: 733– 736, 2007. [DOI] [PubMed] [Google Scholar]
- 30). Harris AL: Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2: 38– 47, 2002. [DOI] [PubMed] [Google Scholar]
- 31). Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN: The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle 8: 3274– 3284, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Hide T, Takezaki T, Nakatani Y, Nakamura H, Kuratsu J, Kondo T: Combination of a ptgs2 inhibitor and an epidermal growth factor receptor-signaling inhibitor prevents tumorigenesis of oligodendrocyte lineage-derived glioma-initiating cells. Stem Cells 29: 590– 599, 2011. [DOI] [PubMed] [Google Scholar]
- 33). Hide T, Takezaki T, Nakatani Y, Nakamura H, Kuratsu J, Kondo T: Sox11 prevents tumorigenesis of glioma-initiating cells by inducing neuronal differentiation. Cancer Res 69: 7953– 7959, 2009. [DOI] [PubMed] [Google Scholar]
- 34). Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK: A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA 101: 14228– 14233, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Hofstetter CP, Burkhardt JK, Shin BJ, Gürsel DB, Mubita L, Gorrepati R, Brennan C, Holland EC, Boockvar JA: Protein phosphatase 2A mediates dormancy of glioblastoma multiforme-derived tumor stem-like cells during hypoxia. PLoS ONE 7: e30059, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Jögi A, Øra I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, Axelson H, Påhlman S: Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci USA 99: 7021– 7026, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG: Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-oncology 7: 134– 153, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Keith B, Johnson RS, Simon MC: HIF1α and HIF2α: sibling rivalry in hypoxic tumor growth and progression. Nat Rev Cancer 12: 9– 22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Keith B, Simon MC: Hypoxia-inducible factors, stem cells, and cancer. Cell 129: 465– 472, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ: SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121: 1109– 1121, 2005. [DOI] [PubMed] [Google Scholar]
- 41). Kijima N, Hosen N, Kagawa N, Hashimoto N, Nakano A, Fujimoto Y, Kinoshita M, Sugiyama H, Yoshimine T: CD166/activated leukocyte cell adhesion molecule is expressed on glioblastoma progenitor cells and involved in the regulation of tumor cell invasion. Neuro-oncology 14: 1254– 1264, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Kim KH, Seol HJ, Kim EH, Rheey J, Jin HJ, Lee Y, Joo KM, Lee J, Nam DH: Wnt/β-catenin signaling is a key downstream mediator of MET signaling in glioblastoma stem cells. Neuro Oncol 15: 161– 171, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Kolenda J, Jensen SS, Aaberg-Jessen C, Christensen K, Andersen C, Brünner N, Kristensen BW: Effects of hypoxia on expression of a panel of stem cell and chemoresistance markers in glioblastoma-derived spheroids. J Neurooncol 103: 43– 58, 2011. [DOI] [PubMed] [Google Scholar]
- 44). Kondo T, Setoguchi T, Taga T: Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA 101: 781– 786, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Koritzinsky M, Wouters BG: Hypoxia and regulation of messenger RNA translation. Meth Enzymol 435: 247– 273, 2007. [DOI] [PubMed] [Google Scholar]
- 46). Kubben PL, ter Meulen KJ, Schijns OE, ter Laak-Poort MP, van Overbeeke JJ, van Santbrink H: Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol 12: 1062– 1070, 2011. [DOI] [PubMed] [Google Scholar]
- 47). Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, Rich JN: Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 6: 421– 432, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Leon SP, Folkerth RD, Black PM: Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer 77: 362– 372, 1996. [DOI] [PubMed] [Google Scholar]
- 49). Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, Hjelmeland AB, Rich JN: Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15: 501– 513, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Liu C, Sage JC, Miller MR, Verhaak RG, Hippenmeyer S, Vogel H, Foreman O, Bronson RT, Nishiyama A, Luo L, Zong H: Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell 146: 209– 221, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS: Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 5: 67, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Marumoto T, Tashiro A, Friedmann-Morvinski D, Scadeng M, Soda Y, Gage FH, Verma IM: Development of a novel mouse glioma model using lentiviral vectors. Nat Med 15: 110– 116, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53). McCord AM, Jamal M, Shankavaram UT, Shankavarum UT, Lang FF, Camphausen K, Tofilon PJ: Physiologic oxygen concentration enhances the stem-like properties of CD133+ human glioblastoma cells in vitro. Mol Cancer Res 7: 489– 497, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54). Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E, Maclennan S, Borea PA: Adenosine modulates vascular endothelial growth factor expression via hypoxia-inducible factor-1 in human glioblastoma cells. Biochem Pharmacol 72: 19– 31, 2006. [DOI] [PubMed] [Google Scholar]
- 55). Natsume A, Kato T, Kinjo S, Enomoto A, Toda H, Shimato S, Ohka F, Motomura K, Kondo Y, Miyata T, Takahashi M, Wakabayashi T: Girdin maintains the stemness of glioblastoma stem cells. Oncogene 31: 2715– 2724, 2012. [DOI] [PubMed] [Google Scholar]
- 56). Nishide K, Nakatani Y, Kiyonari H, Kondo T: Glioblastoma formation from cell population depleted of Prominin1-expressing cells. PLoS ONE 4: e6869, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57). Ogden AT, Waziri AE, Lochhead RA, Fusco D, Lopez K, Ellis JA, Kang J, Assanah M, McKhann GM, Sisti MB, McCormick PC, Canoll P, Bruce JN: Identification of A2B5+CD133- tumor-initiating cells in adult human gliomas. Neurosurgery 62: 505– 514; discussion 514–515, 2008. [DOI] [PubMed] [Google Scholar]
- 58). Orford M, Mean R, Lapathitis G, Genethliou N, Panayiotou E, Panayi H, Malas S: Generation of an ABCG2(GFPn-puro) transgenic line—a tool to study ABCG2 expression in mice. Biochem Biophys Res Commun 384: 199– 203, 2009. [DOI] [PubMed] [Google Scholar]
- 59). Palmer TD, Willhoite AR, Gage FH: Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425: 479– 494, 2000. [DOI] [PubMed] [Google Scholar]
- 60). Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG: Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res 65: 6207– 6219, 2005. [DOI] [PubMed] [Google Scholar]
- 61). Persano L, Rampazzo E, Basso G, Viola G: Glioblastoma cancer stem cells: role of the microenvironment and therapeutic targeting. Biochem Pharmacol 85: 612– 622, 2013. [DOI] [PubMed] [Google Scholar]
- 62). Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL: Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 444: 761– 765, 2006. [DOI] [PubMed] [Google Scholar]
- 63). Pistollato F, Abbadi S, Rampazzo E, Persano L, Della Puppa A, Frasson C, Sarto E, Scienza R, D'avella D, Basso G: Intratumoral hypoxic gradient drives stem cells distribution and MGMT expression in glioblastoma. Stem Cells 28: 851– 862, 2010. [DOI] [PubMed] [Google Scholar]
- 64). Pistollato F, Chen HL, Schwartz PH, Basso G, Panchision DM: Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Mol Cell Neurosci 35: 424– 435, 2007. [DOI] [PubMed] [Google Scholar]
- 65). Reya T, Morrison SJ, Clarke MF, Weissman IL: Stem cells, cancer, and cancer stem cells. Nature 414: 105– 111, 2001. [DOI] [PubMed] [Google Scholar]
- 66). Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, De Maria R: Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 468: 824– 828, 2010. [DOI] [PubMed] [Google Scholar]
- 67). Ropolo M, Daga A, Griffero F, Foresta M, Casartelli G, Zunino A, Poggi A, Cappelli E, Zona G, Spaziante R, Corte G, Frosina G: Comparative analysis of DNA repair in stem and nonstem glioma cell cultures. Mol Cancer Res 7: 383– 392, 2009. [DOI] [PubMed] [Google Scholar]
- 68). Scharenberg CW, Harkey MA, Torok-Storb B: The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood 99: 507– 512, 2002. [DOI] [PubMed] [Google Scholar]
- 69). Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB: Identification of a cancer stem cell in human brain tumors. Cancer Res 63: 5821– 5828, 2003. [PubMed] [Google Scholar]
- 70). Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB: Identification of human brain tumour initiating cells. Nature 432: 396– 401, 2004. [DOI] [PubMed] [Google Scholar]
- 71). Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, Engh J, Iwama T, Kunisada T, Kassam AB, Pollack IF, Park DM: Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene 28: 3949– 3959, 2009. [DOI] [PubMed] [Google Scholar]
- 72). Son MJ, Woolard K, Nam DH, Lee J, Fine HA: SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell 4: 440– 452, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73). Sottocornola R, Lo Celso C: Dormancy in the stem cell niche. Stem Cell Res Ther 3: 10, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74). Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups. National Cancer Institute of Canada Clinical Trials Group : Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10: 459– 466, 2009. [DOI] [PubMed] [Google Scholar]
- 75). Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987– 996, 2005. [DOI] [PubMed] [Google Scholar]
- 76). Tseng D, Vasquez-Medrano DA, Brown JM: Targeting SDF-1/CXCR4 to inhibit tumour vasculature for treatment of glioblastomas. Br J Cancer 104: 1805– 1809, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77). Tsugu A, Ishizaka H, Mizokami Y, Osada T, Baba T, Yoshiyama M, Nishiyama J, Matsumae M: Impact of the combination of 5-aminolevulinic acid-induced fluorescence with intraoperative magnetic resonance imaging-guided surgery for glioma. World Neurosurg 76: 120– 127, 2011. [DOI] [PubMed] [Google Scholar]
- 78). Vescovi AL, Galli R, Reynolds BA: Brain tumour stem cells. Nat Rev Cancer 6: 425– 436, 2006. [DOI] [PubMed] [Google Scholar]
- 79). Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V: Glioblastoma stem-like cells give rise to tumour endothelium. Nature 468: 829– 833, 2010. [DOI] [PubMed] [Google Scholar]
- 80). Wilson CB: Glioblastoma: the past, the present, and the future. Clin Neurosurg 38: 32– 48, 1992. [PubMed] [Google Scholar]
- 81). Yang L, Lin C, Wang L, Guo H, Wang X: Hypoxia and hypoxia-inducible factors in glioblastoma multi-forme progression and therapeutic implications. Exp Cell Res 318: 2417– 2426, 2012. [DOI] [PubMed] [Google Scholar]
- 82). Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L: Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425: 836– 841, 2003. [DOI] [PubMed] [Google Scholar]
- 83). Zheng X, Xie Q, Li S, Zhang W: CXCR4-positive subset of glioma is enriched for cancer stem cells. Oncol Res 19: 555– 561, 2011. [DOI] [PubMed] [Google Scholar]
- 84). Zhu TS, Costello MA, Talsma CE, Flack CG, Crowley JG, Hamm LL, He X, Hervey-Jumper SL, Heth JA, Muraszko KM, DiMeco F, Vescovi AL, Fan X: Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res 71: 6061– 6072, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]