Abstract
In surgery for subthalamic nucleus (STN) deep brain stimulation (DBS), precise implantation of the lead into the STN is essential. Physiological refinement with microelectrode recording (MER) is the gold standard for identifying STN. We studied single tract MER findings and surgical outcomes and verified our surgical method using single tract MER. The number of trajectories in MER and the final position of lead placement were retrospectively analyzed in 440 sides of STN DBS in 221 patients. Bilateral STN DBS yielded marked improvement in the motor score, dyskinesia/fluctuation score, and reduced requirement of dopaminergic medication in this series. The number of trajectories required to obtain sufficient activity of the STN was one in 79.0%, two in 18.2%, and three or more in 2.5% of 440 sides. In 92 sides requiring altered trajectory, the final direction of trajectory movement was posterior in 73.9%, anterior in 13.0%, lateral in 5.4%, and medial in 4.3%. In 18 patients, posterior moves were required due to significant brain shift with intracranial air caused by outflow of CSF during the second side procedure. Sufficient STN activity is obtained with minimum trajectories by proper targeting and precise interpretation of MER findings even in the single tract method. Anterior–posterior moves rather than medial–lateral moves should be attempted first in cases with insufficient recording of STN activity.
Keywords: Parkinson’s disease, deep brain stimulation, subthalamic nucleus, microelectrode recording
Introduction
Subthalamic nucleus (STN) deep brain stimulation (DBS) has been widely performed for medically refractory Parkinson’s disease (PD). The clinical efficacy of DBS depends largely on the lead localization.16,18,21) Therefore, precise implantation of the DBS lead into the STN is a goal of the DBS surgery. Magnetic resonance imaging (MRI) is widely used for initial targeting3,5) and physiological refinement with microelectrode recording (MER) is the gold standard to identify the borders of the STN.6,8,10,12,19) When the length of the STN activity in MER is long enough, the trajectory is considered to have passed through the center of the STN. Sufficient length of the STN activity is easily obtained with proper targeting in most patients. However, several trajectories are required to obtain sufficient length of the STN activity in some patients due to individual anatomical variations.11,17) If possible, minimum trajectories of MER are preferable to avoid hemorrhagic complications. We studied single tract MER findings and its interpretation to optimize the use of MER.
Materials and Methods
Between November 2003 and December 2011, we performed bilateral STN DBS in 221 PD patients (86 men and 135 women, age 35–82 years, mean 63.9 years, duration of disease at surgery 3–40 years, mean 11.7 years). All patients underwent simultaneous bilateral implantation of DBS systems by the same surgeon (A.U.). STN DBS was indicated in most patients for significant motor complications from levodopa such as fluctuanation and dyskinesia. We retrospectively analyzed the number of trajectories in MER and the final position of lead placement in 440 sides of STN DBS in 221 patients with medically refractory PD.
I. Surgical procedure and targeting
Our goal for all cases was to implant quadripolar DBS electrodes (lead 3389; Medtronic) into the center of the STN as the most distal contact (contact 0) of the DBS lead placed at the bottom of the STN. Our routine procedure was as follows. The Leksell stereotactic G frame was used. Under sedation with intravenous administration of propofol, the spontaneously breathing patient was placed in a sitting position. After temporary fixation with ear bars, the frame was fixed tightly with four sharp pins. Then, the localizer for the MRI was attached on the frame and the patient was taken to the radiology suite for MR imaging.
MR imaging was performed for targeting with a 1.5-tesla MR unit (Intera, Phillips). Standard gadolinium-enhanced T1-weighted and T2-weighted images were used. The target localization was based on the Schaltenbrand–Wahren atlas and direct visualization on the MRI using surgical planning computer software (Frame Link; Medtronic). According to the atlas, the target of the bottom of the STN was 12 mm lateral from the midsagittal plane, 2–3 mm posterior to the midcommisural point, and 5 mm inferior to the anterior commissure to posterior commissure (AC-PC) line. In direct visualization with T2-weighted image, the red nucleus was employed for target localization.3,5) The y coordinate of the target was determined as on the line of the anterior margin of the red nucleus in the axial plane. Practically, the y coordinate of the tentative target determined by the atlas was adjusted by direct visualization of the red nucleus. The coordinates obtained from these two methods were used for the initial microelectrode track. Frame Link software was used not only for target localization but also for simulation of the trajectory from the entry point to the target. The entry point was also determined near the coronal suture 10–20 degrees from the sagittal plane as the trajectory to avoid the cortical vein, the cortical sulci, and the lateral ventricle. Gadolinium-enhanced MR image clearly identified the cortical veins or intracerebral abnormal vessels. The initial coordinates of the second side were revised according to the final position of the lead on the first side. Namely, the coordinates of the second side were determined as symmetric to the final lead position of the first side.
The patient returns to the operating room and was placed on the table in the supine position with slight head elevation. The patient was awake or minimally sedated to facilitate cooperation during the operation. The stereotactic arc was set according to the coordinates of the target. A marker for fluoroscopic visualization of the target was attached to the arc. A 4-cm sagittal linear skin incision was made on the planned entry point under local anesthesia with lidocaine. A 14-mm-diameter burr hole was made with a perforator and the dura was opened. The pial surface was cauterized to allow passage of the electrode cannula. Then, the guiding cannula through which the recording microelectrodes and DBS leads were passed was inserted into the brain slowly and gently until it was 25 mm above the target.
II. MER and evaluation
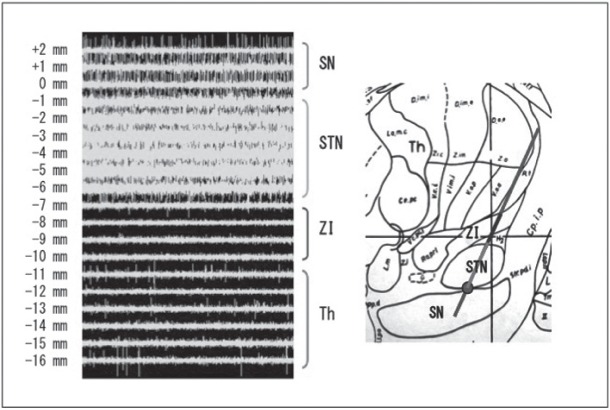
The target was refined physiologically by single tract MER. MER was performed to characterize the physiology of the neuronal environment and determine proper target localization by confirming the location of the dorsal and ventral borders of the STN. We used microelectrodes with a tungsten tip (FHC). The neuronal signal was amplified using the LeadPoint system (Medtronic), band-pass filtered between 500 Hz and 5 kHz. Recording began from approximately 15 mm above the target. The impedance of the electrodes was checked at first. If the impedance was not between 0.2 MOhm and 1.5 MOhm, the electrode was replaced. Then the electrode was advanced slowly using manual microdrive to permit recording of neuronal activity throughout the target area.6,8,10,12,19) The usual trajectories passed through the anterior thalamic nuclei (reticular nucleus), zona incerta, STN, and substantianigra (SN) (Fig. 1). The STN was characterized by increase of background activity and irregular firing pattern with large amplitude spikes. Passive joint movement was also used to confirm the entrance of the STN.1) MER was usually ended when cells of the SN were encountered.
Fig. 1.
Example of typical microelectrode recording pattern with LeadPoint system in subthalamic nucleus (STN) surgery. In this case, recording started from 16 mm above target (bottom of STN) and 5.7 mm length (from −6.1 mm to −0.4 mm) of STN activity was obtained.
Then we evaluated the results of MER. When a sufficient length of STN activity was obtained (length more than 4.0 mm), we considered the trajectory to have passed through almost the center of the STN and implanted the DBS lead at the trajectory. If sufficient STN activity was not obtained (length less than 4.0 mm), the trajectory was assumed not to have passed through the center of the STN. Then, altered trajectories were followed until sufficient STN activity (length more than 4.0 mm) was obtained. Typically, the next trajectory was undertaken by moving 2 mm in any direction according to the functional mapping of the first trajectory. The activity of the thalamus above the STN, the shape of the STN, and the approach angle were considered in determining the direction of the trajectory.
Short STN activity with an absence of thalamic activity above the STN suggested that the trajectory was too anterior and/or lateral. On the other hand, short STN with obvious thalamic activity above the STN suggested that the trajectory was too posterior and/or medial (Figs. 2 and 3). Considering the shape of the STN, if the trajectory was too anterior and/or lateral, STN activity was obtained in the upper part of the STN. If the trajectory was too posterior and/ or medial, STN activity was obtained in the lower part of the STN. Regarding the approach angle, if the approach was from a more lateral and/or anterior entry point, the trajectory would miss the thalamus. If the approach was from a more medial and/or posterior entry point, the trajectory obtained much thalamic activity. The entry point was different in each patient because it was determined such that the trajectory avoided the cortical vein, the cortical sulci and the lateral ventricle.
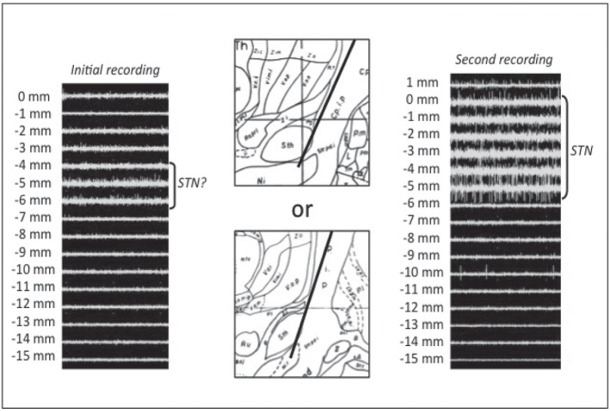
Fig. 2.
Sample case 1. In initial recording, slight subthalamic nucleus (STN) activity with no thalamic activity was obtained (left). This trajectory seemed to be too anterior (center top) or too lateral (center bottom). We moved the trajectory 2 mm posteriorly. Consequently, sufficient STN activity was obtained in the second recording (right).
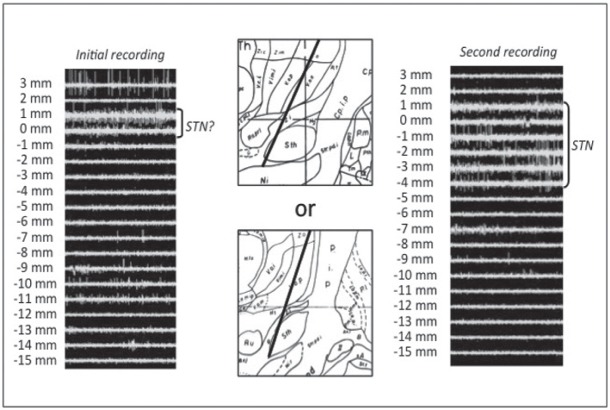
Fig. 3.
Sample case 2. In initial recording, some thalamic activity and slight subthalamic nucleus (STN) activity near the target were obtained (left). This trajectory seemed to be too posterior (center top) or too medial (center bottom). In this case, the third ventricle was markedly dilated, and therefore we moved the trajectory 2 mm laterally. Consequently, sufficient STN activity was obtained in the second recording (right).
III. DBS lead and implantable pulse generator implantation
After physiological refinement of the target, quadripolar DBS electrode (Activa 3389; Medtronic) was implanted through the guiding cannula under fluoroscopic guidance. The electrode was placed as the most distant contact (contact 0) placed at the bottom of the STN. Then, test stimulation was performed to assess symptom relief and to evaluate the threshold for adverse effects. The patient was asked to perform various tasks such as finger tapping and was checked for cogwheel rigidity on the affected side. When the DBS lead was placed close to the corticospinal tract, medial lemniscus, or oclomotor fibers, adverse effects such as muscle contraction, paresthesia, or eye movement (double vision) might be observed, respectively.15) If these adverse effects occured at a low threshold, the lead should be replaced to a more suitable position. Afterward, the lead was fixed using the burr hole ring and cap of the DBS system under fluoroscopic guidance.
Subsequently, the patient was put under general anesthesia and the implantable pulse generator (IPG) (Soletra; Medtronic) was placed in the infraclavicular subcutaneous pocket. The extension wire was tunneled subcutaneously and connected to the IPG.
IV. Adjusting stimulation parameter
Electrical stimulation began a few days after surgery. Stimulation parameters were adjusted to produce maximal clinical benefit for cardinal PD symptoms without side effects. Preferably, a monopolar electrode setting was used, unless stimulator-induced side effects required a more focal bipolar stimulation paradigm. Stimulation parameters were 90 μsec of pulse width, 130 Hz of pulse rate, and 2–3V of amplitude. After surgery, dopaminergic medication was initially reduced by approximately 50% and then further adjusted based on stimulation-induced improvements of PD symptoms. Post DBS evaluations of motor function were performed after initial adjustment of stimulation and medication (usually 1 month after surgery).
Results
I. Surgical outcomes and hemorrhagic complications
Table 1 shows initial outcomes in this consecutive series of bilateral STN DBS. As expected, bilateral STN DBS yielded marked improvement in the Unified Parkinson’s Disease Rating Scale Part III motor score (p < 0.001), dyskinesia/fluctuation score (p < 0.001), and reduced requirement of dopaminergic medication (p < 0.001) in most patients. These results seem to validate our STN DBS surgery.
Table 1.
Initial clinical outcomes of bilateral STN DBS in 221 patients
| Pre-DBS | Post-DBS | p value | |
|---|---|---|---|
| UPDRS III motor score | 17.0 ± 12.9 (med-on) | 12.2 ± 9.3 (med-on) | <0.001 |
| 36.1 ± 15.4 (med-off) | |||
| Dyskinesia/fiuctuation score (UPDRS item 32–39) | 6.0 ± 2.8 | 1.1 ± 1.7 | <0.001 |
| Medication (LEDD, mg) | 594 ± 208 | 236 ± 150 | <0.001 |
Values are expressed as mean ± standard deviation. The Wilcoxon signed-rank test was used for comparison between preand post-DBS values.
DBS: deep brain stimulation, LEDD: levodopa-equivalent daily dosage, STN: subthalamic nucleus, UPDRS: Unified Parkinson’s Disease Rating Scale Part III.
Two patients (0.9%) had symptomatic intracerebral hemorrhages (ICHs) at the site of the lead implantation. In one case, hemorrhage was identified by unexpected electrical silence during MER and slight bleeding from the cannula. The procedure was interrupted and emergency computed tomography confirmed ICH. We had already implanted DBS lead successfully in one side and abandoned the implantation in the affected side. In the other case, hemorrhage was identified after implantation of the whole DBS system. This patient showed hemiparesis after recovery from general anesthesia for IPG implantation. The number of microelectrode tracts was two in both cases. These hematomas did not require evacuation but caused hemiparesis, and these patients mostly recovered with rehabilitation. One patient showed prolonged hemispatial neglect. Fortunately, cardinal motor symptoms of PD significantly improved with pallidotomy-like effect by hematoma without stimulation in both patients.
II. Result of MER and the final direction of lead placement
The number of trajectories used to obtain sufficient activity of the STN was one in 348 sides (79.0%), two in 80 sides (18.2%), three in 6 sides (1.4%), four in 5 sides (1.1%), and five in 1 side (0.2%) (Table 2). In most sides (97.2%), sufficient STN activity was obtained with minimal (one or two) trajectories. In one side, we could not obtain sufficient STN activity even with seven trajectories and abandoned DBS lead placement in that side. The mean number of trajectories to obtain sufficient STN was 1.25. There was no obvious difference between the first and second side in the mean number of trajectories (1.26 in the first side and 1.24 in the second side). The mean length of STN activity in the final trajectory was 5.41 ± 0.71 mm.
Table 2.
Number of trajectories to obtain sufficient subthalamic nucleus
| Number of trajectory | First side (%) | Second side (%) | Total (%) |
|---|---|---|---|
| 1 | 177 (80.1) | 171 (78.1) | 348 (79.0) |
| 2 | 36 (16.3) | 44 (20.1) | 80 (18.2) |
| 3 | 3 (1.4) | 3 (1.4) | 6 (1.4) |
| 4 | 4 (1.8) | 1 (0.5) | 5 (1.1) |
| 5 | 1 (0.5) | 0 (0) | 1 (0.2) |
Altered trajectories were required to obtain sufficient STN activity in 92 sides (21.0%). The final direction of trajectory movement was posterior in 68 sides (73.9%), anterior in 12 sides (13.0%), lateral in 5 sides (5.4%), medial in 4 sides (4.3%), anterolateral in 2 sides (2.2%), and posteromedial in 1 side (1.1%) (Table 3). Anterior–posterior moves rather than medial–lateral moves were more efficient for obtaining sufficient STN activity in most sides. Lateral moves were particularly required in patients with dilatation of the third ventricle (Fig. 3). Significant brain shift with intracranial air caused by flow out of the cerebrospinal fluid during the second side procedure necessitated posterior moves in 18 cases (Fig. 4)
Table 3.
Final direction of movement in altered trajectory
| Final direction of movement | First side (%) | Second side (%) | Total (%) |
|---|---|---|---|
| Posterior | 33 (75.0) | 35 (72.9) | 68 (73.9) |
| Anterior | 4 (9.1) | 8 (16.7) | 12 (13.0) |
| Lateral | 1 (2.3) | 4 (8.3) | 5 (5.4) |
| Medial | 3 (6.8) | 1 (2.1) | 4 (4.3) |
| Anterolateral | 2 (4.5) | 0 (0) | 2 (2.2) |
| Posteromedial | 1 (2.3) | 0 (0) | 1 (1.1) |
Fig. 4.
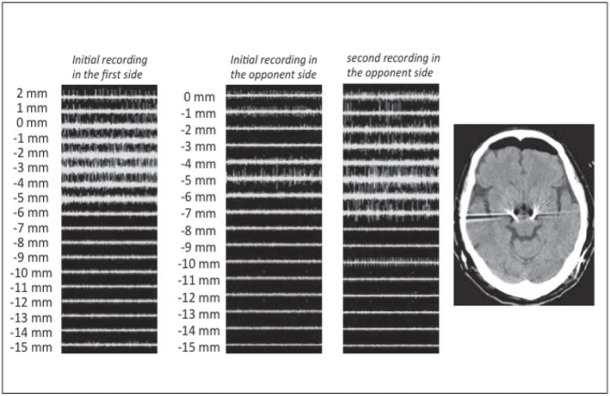
Sample case 3. In the first side, sufficient subthalamic nucleus (STN) activity was obtained in initial recording and lead was successfully placed (left). In the second side, only slight STN activity was obtained in the initial recording (center left) and was needed posterior move to obtain sufficient STN activity in the second recording (center right). Posterior brain shift seemed to occur by outflow of cerebrospinal fluid during the first side surgery. Postoperative computed tomography demonstrated significant intracranial air collection in the frontal region (right).
Discussion
Surgical procedure for STN DBS varies across centers.9) Some groups avoid using MER for placement of the DBS lead in the STN because recent targeting methods using MRI accurately indicate position of the STN3,5) and use of MER may increase risk of ICH. However, physiological refinement by MER is the gold standard for identifying STN and its borders. The optimal region for STN stimulation might be missed due to individual anatomical variations. In fact, in our series, STN activity was absent or slight in the first MER in some patients. Therefore, physiological refinement with MER seems to be essential before implantation of the DBS lead.
In most centers, the MER criterion for DBS lead implantation is the length of STN.10) STN recording of more than 4 mm with good clinical effect without adverse effect by test stimulation seems to be feasible.15) In this study, we retrospectively analyzed the results of MER in STN surgery. A sufficient length of STN activity (mean length = 5.41 mm) was confirmed with a minimum number of trajectories (mean number = 1.25) in MER, and a good clinical outcome was obtained in most cases. These results seem to validate our surgical procedure with single tract MER. Recently, multiple simultaneous MER using multiple electrode holder is employed in some centers. It is controversial whether single tract MER or multiple tract MER is more advantageous. Amirnovin et al.2) demonstrated that the average STN length of the trajectory selected for the DBS electrode in the multiple tract MER method was 5.6 ± 0.4 mm on the left and 5.7 ± 0.4 mm on the right. These values are almost equivalent to our result in single tract MER. The STN is accurately hit by proper targeting with MRI in most cases. Even if the center of the STN is missed, subsequent trajectory guided by precise interpretation of MER findings will hit the STN with minimum trajectories. Chang et al.7) also asserted that the accuracy of electrode positioning appears to be acceptable under single tract MER. Temel et al. compared single tract MER with multiple tract MER. There were no significant differences of the STN length between single and multiple tract MER. They demonstrated that multiple MER resulted in better motor outcome but deterioration in neuropsychological function. More extensive microlesion from microelectrodes could be a possible explanation for the deterioration.20)
As the target coordinates of the second side were determined based on the MER results of the first side, the number of trajectories in the second side should be theoretically much fewer. However, the number of trajectories did not differ between the first and the second side. This is attributable to the brain shift during surgery described afterward.
Regarding hemorrhagic complication, two patients had symptomatic ICH in our series. The number of microelectrode tracts was two in both cases. In an early report from the DBS for PD study group, the number of microelectrode passes used to determine target location correlated with the risk of hemorrhage (2.9 passes in patients without hemorrhage versus 4.1 passes in patients with hemorrhage).9) Therefore, we recommend a minimum number of microelectrode insertions.
Among 21% of sides, which required altered trajectories, anterior–posterior moves rather than medial–lateral moves were more effective for obtaining sufficient STN activity. This fact indicates that individual anatomical variations of the STN tend to deviate in the anterior–posterior direction. Anterior–posterior moves rather than medial–lateral moves should be attempted first in cases with insufficient recording of STN activity.
Furthermore, as demonstrated in our series, brain shift from intracranial air caused by outflow of cerebrospinal fluid during surgery seems to accelerate posterior shift of the STN. Some previous studies demonstrated this kind of brain shift during the second side procedure in bilateral surgery.4,13,14) Azmi et al.4) demonstrated that cerebral atrophy could be a potential risk factor for pneumocephalus during surgery. Obuchi et al.14) demonstrated that enlargement of the body part of the lateral ventricle was the most reliable factor for predicting a brain shift.
In our series, lateral shift of the STN was seen in some patients with dilatation of the third ventricle. Considering the shape of the midbrain, this finding represents a reasonable variation. These experiences are useful in determining the initial target localization in subsequent cases. For example, in cases with remarkable cerebral atrophy, initial coordinates of the target in the second side should be shifted a little bit to the posterior. This kind of strategy might further reduce the number of trajectories needed to obtain sufficient STN activity in MER.
Conclusions
In STN surgery, sufficient STN activity is obtained with minimum trajectories by proper targeting and precise interpretation of MER findings, even using the single tract method. Anterior–posterior moves rather than medial–lateral moves should be attempted first in cases with insufficient recording of STN activity.
References
- 1). Abosch A, Hutchison WD, Saint-Cyr JA, Dostrovsky JO, Lozano AM: Movement-related neurons of the subthalamic nucleus in patients with Parkinson disease. J Neurosurg 97: 1167– 1172, 2002. [DOI] [PubMed] [Google Scholar]
- 2). Amirnovin R, Williams ZM, Cosgrove GR, Eskandar EN: Experience with microelectrode guided subthalamic nucleus deep brain stimulation. Neurosurgery 58 ( Suppl 1): ONS96– 102, 2006. [DOI] [PubMed] [Google Scholar]
- 3). Andrade-Souza YM, Schwalb JM, Hamani C, Eltahawy H, Hoque T, Saint-Cyr J, Lozano AM: Comparison of three methods of targeting the subthalamic nucleus for chronic stimulation in Parkinson's disease. Neurosurgery 62 ( Suppl 2): 875– 883, 2008. [DOI] [PubMed] [Google Scholar]
- 4). Azmi H, Machado A, Deogaonkar M, Rezai A: Intracranial air correlates with preoperative cerebral atrophy and stereotactic error during bilateral STN DBS surgery for Parkinson's disease. Stereotact Funct Neurosurg 89: 246– 252, 2011. [DOI] [PubMed] [Google Scholar]
- 5). Bejjani BP, Dormont D, Pidoux B, Yelnik J, Damier P, Arnulf I, Bonnet AM, Marsault C, Agid Y, Philippon J, Cornu P: Bilateral subthalamic stimulation for Parkinson's disease by using three-dimensional stereotactic magnetic resonance imaging and electrophysiological guidance. J Neurosurg 92: 615– 625, 2000. [DOI] [PubMed] [Google Scholar]
- 6). Benazzouz A, Breit S, Koudsie A, Pollak P, Krack P, Benabid AL: Intraoperative microrecordings of the subthalamic nucleus in Parkinson's disease. Mov Disord 17 ( Suppl 3): S145– 149, 2002. [DOI] [PubMed] [Google Scholar]
- 7). Chang WS, Kim HY, Kim JP, Park YS, Chung SS, Chang JW: Bilateral subthalamic deep brain stimulation using single track microelectrode recording. Acta Neurochir (Wien) 153: 1087– 1095, 2011. [DOI] [PubMed] [Google Scholar]
- 8). Chen SY, Lee CC, Lin SH, Hsin YL, Lee TW, Yen PS, Chou YC, Lee CW, Annie Hsieh W, Su CF, Lin SZ: Microelectrode recording can be a good adjunct in magnetic resonance image-directed subthalamic nucleus deep brain stimulation for parkinsonism. Surg Neurol 65: 253– 260; discussion 260–261, 2006. [DOI] [PubMed] [Google Scholar]
- 9). Deep-Brain Stimulation for Parkinson's Disease Study Group : Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med 345: 956– 963, 2001. [DOI] [PubMed] [Google Scholar]
- 10). Gross RE, Krack P, Rodriguez-Oroz MC, Rezai AR, Benabid AL: Electrophysiological mapping for the implantation of deep brain stimulators for Parkinson's disease and tremor. Mov Disord 21 ( Suppl 14): S259– 283, 2006. [DOI] [PubMed] [Google Scholar]
- 11). Hamid NA, Mitchell RD, Mocroft P, Westby GW, Milner J, Pall H: Targeting the subthalamic nucleus for deep brain stimulation: technical approach and fusion of pre- and postoperative MR images to define accuracy of lead placement. J Neurol Neurosurg Psychiatr 76: 409– 414, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Hutchison WD, Allan RJ, Opitz H, Levy R, Dostrovsky JO, Lang AE, Lozano AM: Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson's disease. Ann Neurol 44: 622– 628, 1998. [DOI] [PubMed] [Google Scholar]
- 13). Miyagi Y, Shima F, Sasaki T: Brain shift: an error factor during implantation of deep brain stimulation electrodes. J Neurosurg 107: 989– 997, 2007. [DOI] [PubMed] [Google Scholar]
- 14). Obuchi T, Katayama Y, Kobayashi K, Oshima H, Fukaya C, Yamamoto T: Direction and predictive factors for the shift of brain structure during deep brain stimulation electrode implantation for advanced Parkinson's disease. Neuromodulation 11: 302– 310, 2008. [DOI] [PubMed] [Google Scholar]
- 15). Pollak P, Krack P, Fraix V, Mendes A, Moro E, Chabardes S, Benabid AL: Intraoperative micro-and macrostimulation of the subthalamic nucleus in Parkinson's disease. Mov Disord 17 ( Suppl 3): S155– 161, 2002. [DOI] [PubMed] [Google Scholar]
- 16). Saint-Cyr JA, Hoque T, Pereira LC, Dostrovsky JO, Hutchison WD, Mikulis DJ, Abosch A, Sime E, Lang AE, Lozano AM: Localization of clinically effective stimulating electrodes in the human subthalamic nucleus on magnetic resonance imaging. J Neurosurg 97: 1152– 1166, 2002. [DOI] [PubMed] [Google Scholar]
- 17). Schlaier JR, Habermeyer C, Warnat J, Lange M, Janzen A, Hochreiter A, Proescholdt M, Brawanski A, Fellner C: Discrepancies between the MRI- and the electrophysiologically defined subthalamic nucleus. Acta Neurochir (Wien) 153: 2307– 2318, 2011. [DOI] [PubMed] [Google Scholar]
- 18). Starr PA, Christine CW, Theodosopoulos PV, Lindsey N, Byrd D, Mosley A, Marks WJ: Implantation of deep brain stimulators into the subthalamic nucleus: technical approach and magnetic resonance imaging-verified lead locations. J Neurosurg 97: 370– 387, 2002. [DOI] [PubMed] [Google Scholar]
- 19). Sterio D, Zonenshayn M, Mogilner AY, Rezai AR, Kiprovski K, Kelly PJ, Beric A: Neurophysiological refinement of subthalamic nucleus targeting. Neurosurgery 50: 58– 67; discussion 67–69, 2002. [DOI] [PubMed] [Google Scholar]
- 20). Temel Y, Wilbrink P, Duits A, Boon P, Tromp S, Ackermans L, van Kranen-Mastenbroek V, Weber W, Visser-Vandewalle V: Single electrode and multiple electrode guided electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. Neurosurgery 61 ( 5 Suppl 2): 346– 355, 2007. [DOI] [PubMed] [Google Scholar]
- 21). Voges J, Volkmann J, Allert N, Lehrke R, Koulousakis A, Freund HJ, Sturm V: Bilateral high-frequency stimulation in the subthalamic nucleus for the treatment of Parkinson disease: correlation of therapeutic effect with anatomical electrode position. J Neurosurg 96: 269– 279, 2002. [DOI] [PubMed] [Google Scholar]